Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1988
Peer-review started: January 16, 2020
First decision: March 5, 2020
Revised: April 1, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 26, 2020
Processing time: 124 Days and 4.4 Hours
Immune dysregulation, polyendocrinopthy, enteropathy, X-linked (IPEX) syndrome is a rare X-linked recessive disease caused by mutations in the forkhead box protein 3 (FOXP3) gene, which is a master transcriptional regulator for the development and function of CD4+CD25+ regulatory T (Treg) cells. The dysfunction of these cells leads to multiple system autoimmune diseases. We present a case of IPEX due to a mutation not reported in the literature before.
We report a male patient with IPEX syndrome who presented with refractory diarrhea and malabsorption leading to failure to thrive, as well as with hypothyroidism and nephrotic syndrome. Laboratory investigation showed increased total IgE and Treg cells, decreased free triiodothyronine (FT3) and free thyroxine (FT4), and proteinuria. Multiple dietary and supportive treatments were introduced but did not improve the diarrhea during his hospital stay. Ultimately, whole exome sequencing revealed that the patient was hemizygous for the exon 5, c.542G>A (p.Ser181Asn) mutation of the FOXP3 gene, which has not been previously reported. The patient remains on prednisone and euthyrox while awaiting hematopoietic stem cell transplantation at the time of the compilation of this case report.
We report a novel FOXP3 gene mutation involved in IPEX. A high level of suspicion should be maintained in an early-onset refractory diarrhea patient.
Core tip: Immune dysregulation, polyendocrinopthy, enteropathy, X-linked syndrome is a rare X-linked recessive disease caused by mutations in the FOXP3 gene. Patients most commonly present symptoms with refractory diarrhea, type 1 diabetes mellitus, eczema, and rarely, symptoms of nephrotic syndrome, hypothyroidism, and thrombocytopenia. Herein, we present a patient with refractory diarrhea, failure to thrive, hypothyroidism, and nephrotic syndrome who was subsequently found to have a novel mutation in the FOXP3 gene [c.542G>A (p.Ser181Asn)]. Patients with immune dysregulation, polyendocrinopthy, enteropathy, X-linked generally require supportive treatments and replacement therapy, such as steroids, immunosuppressors, or hematopoietic stem cell transplantation.
- Citation: Su N, Chen C, Zhou X, Ma GD, Chen RL, Tian C. Early-onset refractory diarrhea due to immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome associated with a novel mutation in the FOXP3 gene: A case report. World J Clin Cases 2020; 8(10): 1988-1994
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1988.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1988
Immune dysregulation, polyendocrinopthy, enteropathy, X-linked (IPEX) syndrome is a rare disorder of genetic autoimmunity caused by mutations in the forkhead box protein 3 (FOXP3) gene[1]. FOXP3 is an important transcription regulator in the development of CD4+CD25+ regulatory T (Treg) cells. FOXP3 gene mutations lead to developmental defects, differentiation disorders, and dysfunction of Tregs, which lead to serious autoimmune phenomena in multiple systems[2]. The disease mainly influences the intestine, endocrine system, and skin[2], whereas the thyroid glands, kidneys, blood cells, liver, and joints may also be influenced[3]. IPEX is an X-linked recessive disorder, and patients who have not been treated effectively in the early stage mostly die within 2 years of age[4]. The main treatment measures for patients with IPEX include supportive care, immunosuppressive treatments, and hematopoietic stem cell transplantation. The clinical manifestations of the disease were first reported in 1982 by Powell et al[5], and an increasing number of cases were subsequently reported. Here, we report a male patient with IPEX syndrome who had refractory diarrhea, failure to thrive, hypothyroidism, and nephrotic syndrome and who was hemizygous in the FOXP3 gene [c.542G>A (p.Ser181Asn)].
A 13-month-old male patient was admitted to the hospital in June 2019 due to chronic diarrhea and malnutrition.
The patient’s symptoms started a year ago with recurrent diarrhea and malnutrition. His diarrhea had been worsened the last 4 h (10 watery bowel movements).
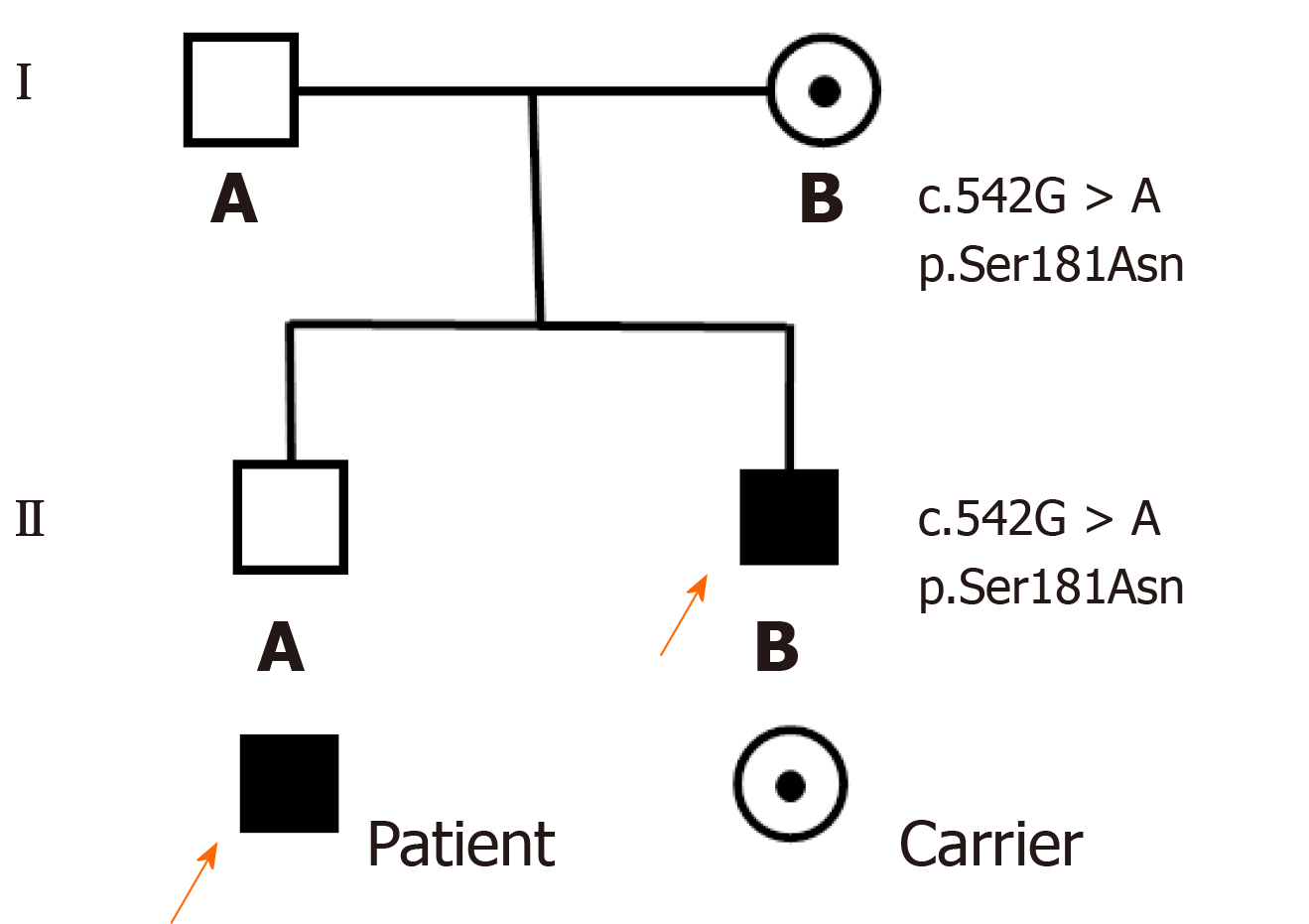
The patient is the second live-born child of nonconsanguineous and healthy parents (Figure 1) and was born at 35 wk of gestation by spontaneous vaginal delivery. The newborn had a birth weight of 2.15 kg and was hospitalized as a premature infant. The family history was negative for inherent diseases.
At the age of 1 mo, the patient had refractory diarrhea (up to 10-15 watery bowel movements per day) and scattered eczema. Therefore, he was hospitalized on several occasions at a local hospital and given hydrolyzing protein formula milk and supportive care to improve gastrointestinal symptoms. The eczema gradually disappeared in May after birth, but diarrhea did not significantly improve.
Upon admission to the hospital, the patient had a weight of 6.4 kg, head circumference of 40.5 cm, and length 70 cm, failure to thrive, and moderate-severe dehydration. Abdominal subcutaneous fat thickness was less than 4 mm, and bowel sounds were active, approximately 7 per minute.
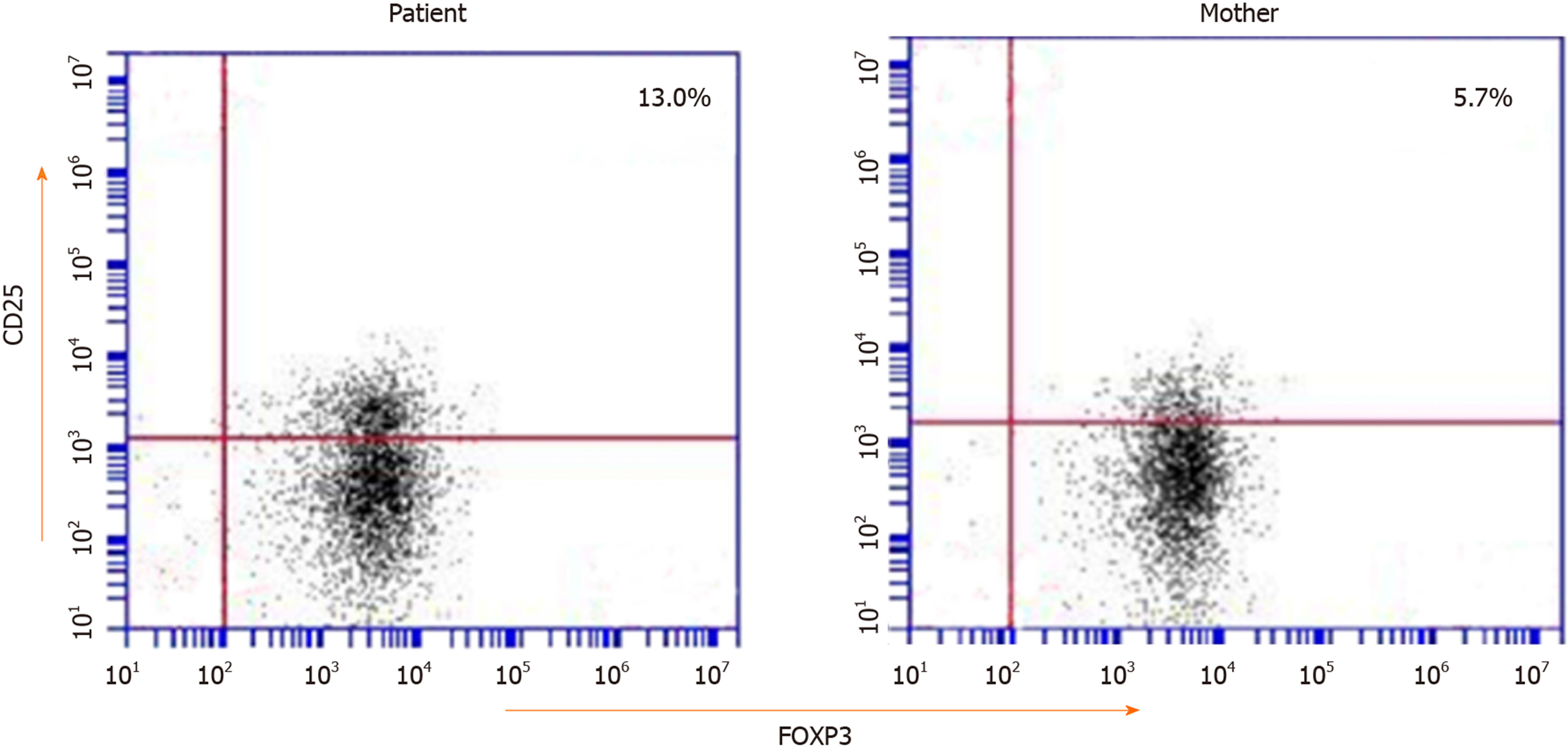
The laboratory results showed a normal eosinophil count and hemoglobin concentration; a high white blood cell count (23.25 × 109/L); normal immunoglobulin A, IgG, and IgM levels but markedly elevated IgE levels (1970 IU/mL); high triglyceride (3.74 mmol/L); and decreased complement C3 (0.25 g/L), complement C4 (0.1 g/L), and albumin (29.1 g/L). The endocrine test showed normal antithyroid peroxidase (aTPO), antithyroglobulin (aTG), and thyroid stimulating hormone (TSH), and high free triiodothyronine (FT3, 2.20 pmol/L) and free thyroxine (FT4, 10.49 pmol/L) (Table 1). Multiple urinalyses indicated proteinuria (+ to +++). Multiple stool analyses were unremarkable. The patient was allergic to eggs, peanuts, and milk. Endoscopy (gastroscope and colonoscopy) found duodenal anterior wall erosion, and the immunohistochemical staining for cytomegalovirus (CMV) and in situ hybridization for EBV-encoded small RNA (EBER) were negative. Flow cytometry showed a slightly higher proportion of CD25+FOXP3+ Tregs in CD4+ T cells (13.0%) (Figure 2).
| Blood index | Admission | Reference range |
| WBC (× 109/L) | 23.25 | 3.5-9.5 |
| Eosinophils (× 109/L) | 0.13 | 0.02-0.52 |
| HGB (g/L) | 112 | 110-160 |
| ALB (g/L) | 29.1 | 40-55 |
| IgA (g/L) | 0.61 | 0.14-1.14 |
| IgG (g/L) | 9.12 | 3.82-10.58 |
| IgM (g/L) | 0.67 | 0.4-1.28 |
| IgE (IU/mL) | 1.970 | 0-60 |
| Triglyceride (mmol/L) | 3.74 | 0.56-1.70 |
| Complement C3 (g/L) | 0.25 | 0.8-1.5 |
| Complement C4 (g/L) | 0.1 | 0.12-0.4 |
| TSH (mIU/L) | 0.931 | 0.38-7.31 |
| FT3 (pmol/L) | 2.20 | 4.1-7.42 |
| FT4 (pmol/L) | 10.49 | 14.45-22.74 |
| aTPO (U/mL) | 30.90 | < 60 |
| aTG (U/mL) | 20.50 | < 60 |
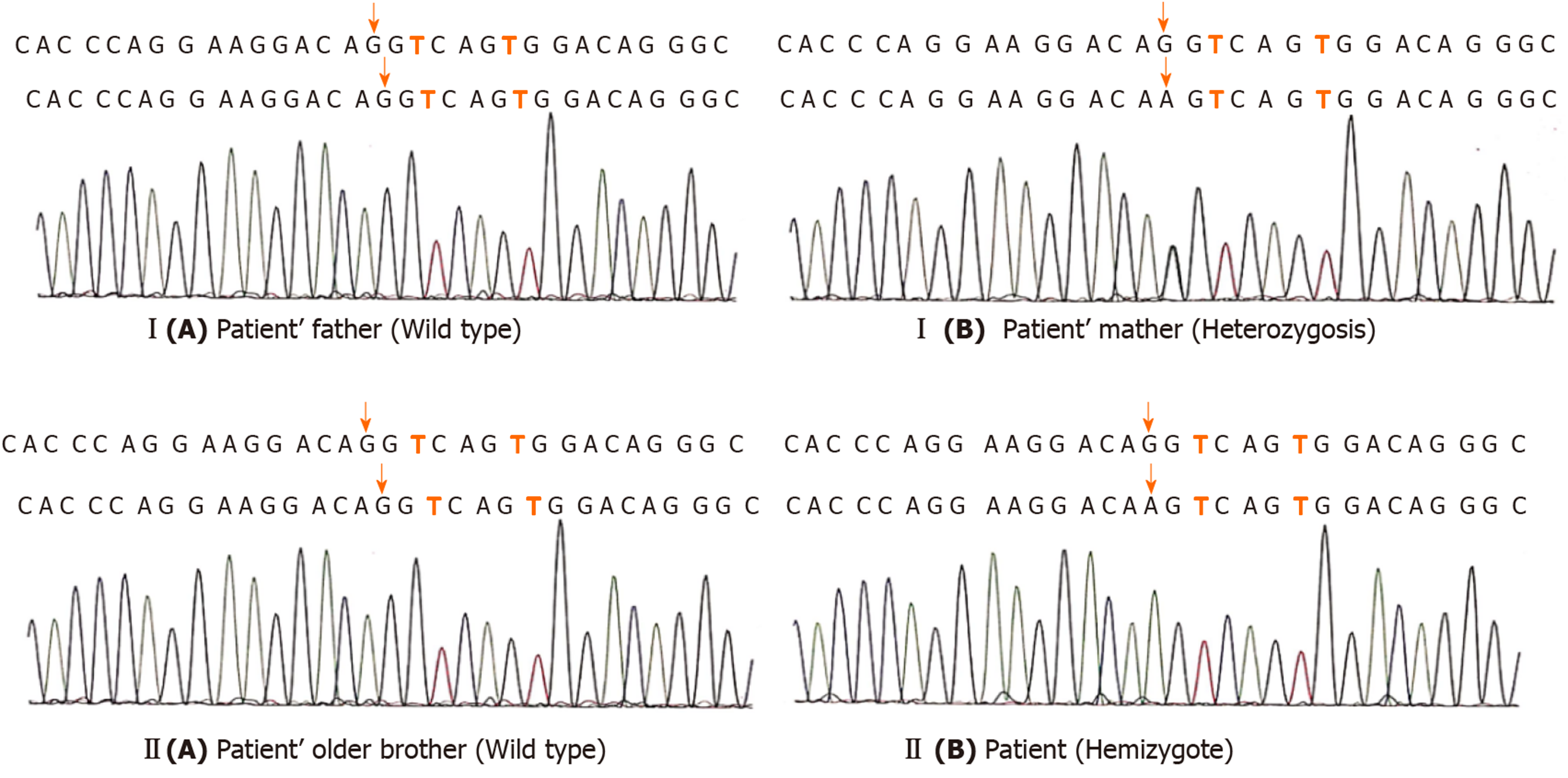
The patient had early refractory diarrhea, malabsorption, high immunoglobulin E, hypothyroidism, and nephrotic syndrome. The diagnosis of IPEX was confirmed by whole exome sequencing, which revealed a pathogenic site in exon 5, c.542G>A (p.Ser181Asn) mutation of the FOXP3 gene. Subsequently, the same heterozygous mutation was found in the mother, who was a carrier, but his father and older brother were normal (Figure 3). The family pedigree of the patient is shown in Figure 1.
The final diagnosis of the presented case was IPEX syndrome, hypothyroidism, and nephrotic syndrome.
The patient was initially diagnosed with refractory diarrhea, malabsorption, hypothyroidism, and nephrotic syndrome. He was treated with anti-infection medications, total parenteral nutrition, prednisone (5 mg/d), and euthyrox (37.5 µg/d). After 10 d of this treatment, he had 4–6 watery bowel movements per day but was not significantly gaining weight. Subsequently, the diagnosis of IPEX was confirmed by whole exome sequencing, and a scheduled immunosuppressor treatment was started. However, the family thought that these drugs had severe side effects and refused this type of treatment. Therefore, the patient was treated with prednisone, euthyrox, and other supportive cares.
Since the diagnosis of IPEX syndrome, the patient has been regularly treated with prednisone and euthyrox. Instead of getting better, his condition is slightly worse. He was hospitalized for refractory diarrhea (up to 15-20 watery bowel movements per day) and anasarca, and was discharged after correction of electrolyte disorder and dehydration therapy. At present, he is awaiting hematopoietic stem cell transplantation.
The typical symptoms of IPEX syndrome present as early refractory diarrhea, type 1 diabetes mellitus, and eczema[2]. The enteropathy of IPEX syndrome is present in virtually all affected individuals. The main manifestation of the patients was watery diarrhea and sometimes mucoid or bloody diarrhea[2]. Dermatitis is most frequently eczematous[6] and can also be psoriasiform[7] and ichthyosiform[8]. Endocrine diseases are common in type 1 diabetes mellitus, most of which are newborn[9], but hypothyroidism has also been reported[3]. Rare symptoms such as thrombocytopenia, hepatitis, pneumonia, and nephrotic syndrome have also been reported[3,10]. In this report, we present an early-onset IPEX syndrome case with chronic diarrhea as the first symptom at the age of 1 mo, as well as hypothyroidism and nephrotic syndrome. The patient received anti-infection therapy, total parenteral nutrition, and other supportive care more than five times after admission in 1 year. However, diarrhea was not well controlled. Finally, the patient was diagnosed with IPEX syndrome associated with a novel FOXP3 gene mutation [c.542G>A (p.Ser181Asn)] by whole exome sequencing.
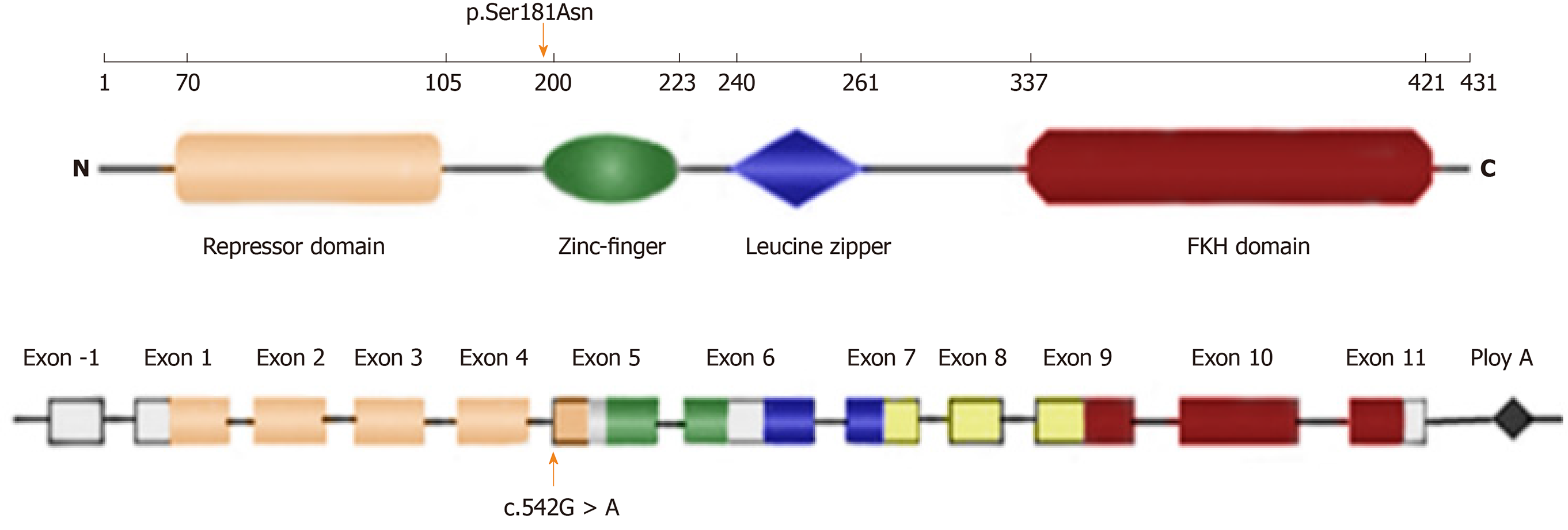
The human FOXP3 gene is located on the X chromosome (Xq11.23–Xq13.3) and consists of 11 coding exons plus one upstream non-coding exon[11]. The gene encodes a protein of 431 amino acids, which contains a repressor domain, a zinc-finger (ZF) domain, a leucine zipper (LZ) domain, and a forkhead (FKH) domain (Figure 4). Ziegler et al[12] found that there were two functional domains within the N-terminal of FOXP3, one between amino acids 67 and 132 and the other between amino acids 135 and 198, which are functional as transcriptional repressors; the latter is especially required for inhibition of nuclear factor of activated T cells (NFAT)-mediated transcription. NFAT is an important transcription factor of cytokine gene expression. In the promoters of cytokine genes, it was found that the NFAT site potentially overlapped or was adjacent to the FKH-binding site[12]. Therefore, FOXP3 may play an immunosuppressive function by competing for the DNA-binding sites of NFAT or directly inhibiting the negative feedback of NFAT activity to regulate the expression of cytokine genes[13,14]. Therefore, this mutation leads to impaired NFAT transcriptional regulatory activity by altering the binding sites to DNA or inhibiting cytokine gene expression and eventually is responsible for this disease.
The FOXP3 gene is a master regulator of Tregs[2]. If FOXP3 mutation leads to this protein being functionally defective, it is unable to sustain Treg development[15]. Therefore, most IPEX patients had decreased percentages of CD25+FOXP3+ Tregs in CD4+ T cells. However, the patient had increased percentages in this study. In previous reports, in addition to the natural Treg cells in the thymus, peripheral CD4+CD25-cells can also develop into Tregs with inhibitory function by stimulation of anti-CD3 and anti-CD28[16] or by retroviral vector or transgene expression. Bacchetta et al[17] found that the ratio of CD4+CD25+ Tregs in peripheral blood mononuclear cells (PBMCs) can appear normal, but their capacity to suppress was impaired, which indicates that the Foxp3 mutation could not affect Treg development but affected suppression function. Therefore, the function of FOXP3 is not restricted to Tregs but it could also play a role outside the Treg subset. It has been reported in the literature that the percentage of CD4+CD25+ FOXP3+ Tregs in CD4+ T cells or the percentage of FOXP3 expressing CD4+ cells in children with IPEX is high (for example, mutation sites were p.M370I[18], p.E323K[19], and p.S390N[19]). Therefore, we speculate that the high ratio of CD25+ Tregs in our patient may be related to other regulatory factors.
IPEX is a rare X-lined recessive disease with multiple system involvement, especially enteropathy and refractory diarrhea, caused by mutations in the FOXP3 gene. The onset of symptoms is as early as the first few months of life, and patients usually require supportive and alternative treatments. In the differential diagnosis of any patient with unexplained and intractable diarrhea, the disease should be considered and eventually confirmed by gene testing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishizawa K S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2489] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 2. | Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 239] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 3. | Pogoriler J, Kamin D, Goldsmith JD. Pediatric non-Helicobacter pylori atrophic gastritis: a case series. Am J Surg Pathol. 2015;39:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Chen CA, Chung WC, Chiou YY, Yang YJ, Lin YC, Ochs HD, Shieh CC. Quantitative analysis of tissue inflammation and responses to treatment in immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, and review of literature. J Microbiol Immunol Infect. 2016;49:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 297] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Owen CJ, Jennings CE, Imrie H, Lachaux A, Bridges NA, Cheetham TD, Pearce SH. Mutational analysis of the FOXP3 gene and evidence for genetic heterogeneity in the immunodysregulation, polyendocrinopathy, enteropathy syndrome. J Clin Endocrinol Metab. 2003;88:6034-6039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Nieves DS, Phipps RP, Pollock SJ, Ochs HD, Zhu Q, Scott GA, Ryan CK, Kobayashi I, Rossi TM, Goldsmith LA. Dermatologic and immunologic findings in the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Arch Dermatol. 2004;140:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Baud O, Goulet O, Canioni D, Le Deist F, Radford I, Rieu D, Dupuis-Girod S, Cerf-Bensussan N, Cavazzana-Calvo M, Brousse N, Fischer A, Casanova JL. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75-R81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 676] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 10. | Park E, Chang HJ, Shin JI, Lim BJ, Jeong HJ, Lee KB, Moon KC, Kang HG, Ha IS, Cheong HI. Familial IPEX syndrome: different glomerulopathy in two siblings. Pediatr Int. 2015;57:e59-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1380] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 12. | Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 752] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 13. | Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672-37679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 400] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138-5143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 15. | Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol. 2012;4:a007021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 840] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 17. | Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | An YF, Zhao XD, Xu F, Yang XQ. A noveI missense mutation of FoxP3 causes immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome in a Chinese child. Zhonghua Erke Zazhi. 2009;47:824-828. [DOI] [Full Text] |
| 19. | Gambineri E, Ciullini Mannurita S, Hagin D, Vignoli M, Anover-Sombke S, DeBoer S, Segundo GRS, Allenspach EJ, Favre C, Ochs HD, Torgerson TR. Clinical, Immunological, and Molecular Heterogeneity of 173 Patients With the Phenotype of Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX) Syndrome. Front Immunol. 2018;9:2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |