Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1979
Peer-review started: February 20, 2020
First decision: March 5, 2020
Revised: April 11, 2020
Accepted: April 17, 2020
Article in press: April 17, 2020
Published online: May 26, 2020
Processing time: 94 Days and 20.6 Hours
Heterotopic pancreas is a common lesion found in the gastrointestinal tract and is usually considered a benign disease. Reports of malignant change of heterotopic pancreas are scarce.
A 44-year-old Chinese female underwent a gastroscopy to assess abdominal distension that had persisted for 2 months. A protruding lesion in the gastric antrum was revealed but no malignant tissue was found in the biopsy specimen. The patient's symptom persisted and progressed to repeated vomiting. Endoscopy after 4 months revealed obstruction of the gastric outlet caused by the protruding lesion. A distal gastrectomy was performed. Histopathological examination of the surgical specimen showed the malignant transformation of aberrant pancreas in the stomach. Chemotherapy consisting of folinic acid, fluorouracil, and oxaliplatin was administered for three cycles, and was changed to gemcitabine monotherapy because of adverse effects and increased serum tumor marker levels. The patient remained asymptomatic during a 12-month follow-up.
Pancreatic heterotopy should be considered as source of a potentially malignant lesion, and early treatment or close monitoring for aberrant pancreas is recommended.
Core tip: Heterotopic pancreas is usually considered a benign disease. We here present a rare case of heterotopic pancreas in the stomach with malignant change in a middle-aged female patient. Endoscopy revealed a protruding lesion in the gastric antrum which caused gastric outlet obstruction. The malignant transformation was not diagnosed until the lesion was excised surgically and examined by pathology. This case emphasizes the importance of early treatment or close monitoring for aberrant pancreas to avoid the potential cancerization and highlights that a protruding lesion in the stomach should prompt suspicion of a malignant pancreatic heterotopia.
- Citation: Xiong Y, Xie Y, Jin DD, Wang XY. Heterotopic pancreas adenocarcinoma in the stomach: A case report and literature review. World J Clin Cases 2020; 8(10): 1979-1987
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1979.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1979
Heterotopic pancreas, also known as ectopicor aberrant pancreas, is defined as pancreatic tissue lying outside its normal location and lacking anatomic or vascular connections with the pancreas[1]. The majority of heterotopic pancreatic masses are small (< 1 cm) and the patients appear to be asymptomatic[2]. The frequency of this disease has been reported to be 0.5% in upper abdominal operations and 0.55%-13.7% at autopsy[3,4]. It has been observed in areas such as the gastrointestinal tract[5-7], biliary duct[8], liver[9], spleen[10], and mesentery[11]. The most common location of heterotopic pancreas is the stomach and the lesion is usually discovered by endoscopy, appearing as a submucosal mass with an endoluminal growth pattern[12].
Although heterotopic pancreatic tissue is relatively common in the stomach, malignancy arising within it is extremely rare, with only 16 well-documented cases being reported in the English language literature[4,13-27]. Herein, we present a case of this rare disease and review the series of other case reports describing malignant transformation of aberrant pancreas in the stomach indexed in the PubMed database (up to December 2019). To our knowledge, our case is the first of its kind reported in a Chinese patient with malignant aberrant pancreas in the stomach; our literature review provides a better understanding of the clinicopathological features, treatment and prognosis of this rare clinical entity.
A 44-year-old female presented to our department with symptoms of intermittent abdominal distension for 6 months and repeated vomiting over the last 3 days.
Because of the abdominal distension, the patient presented to a local hospital for assessment and underwent a gastroscopy 2 months later. The gastroscopy revealed a protruding lesion in the gastric antrum, with its distal margin invading the pyloric ring. Since no malignant tissue was found in the biopsy specimen obtained from the lesion, the patient was treated with acid-suppressive and mucosa-protecting drugs. At 2 months later, gastroscopic re-examination was carried out at the same local hospital and showed gastric retention and pyloric stenosis. An ultrasound gastroscopy was then performed and detected significant thickening of the gastric antrum wall, with a heterogenous hypoechoic lesion where the layered structure had disappeared.
As the abdominal distension symptom persisted and progressed to repeated vomiting, the patient came to our hospital for further evaluation.
The patient had no other significant medical history. She denied history of hypertension, diabetes, coronary heart disease, and any other chronic disease.
The patient had no significant personal and family history.
Physical examination showed no abnormality.
The results of complete blood count and routine biochemical investigations were unremarkable. Blood tumor marker tests showed elevated level of carbohydrate antigen 72-4 (CA724) (50.1 kU/L; normal range: 0-6.9 kU/L) but normal levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA199).
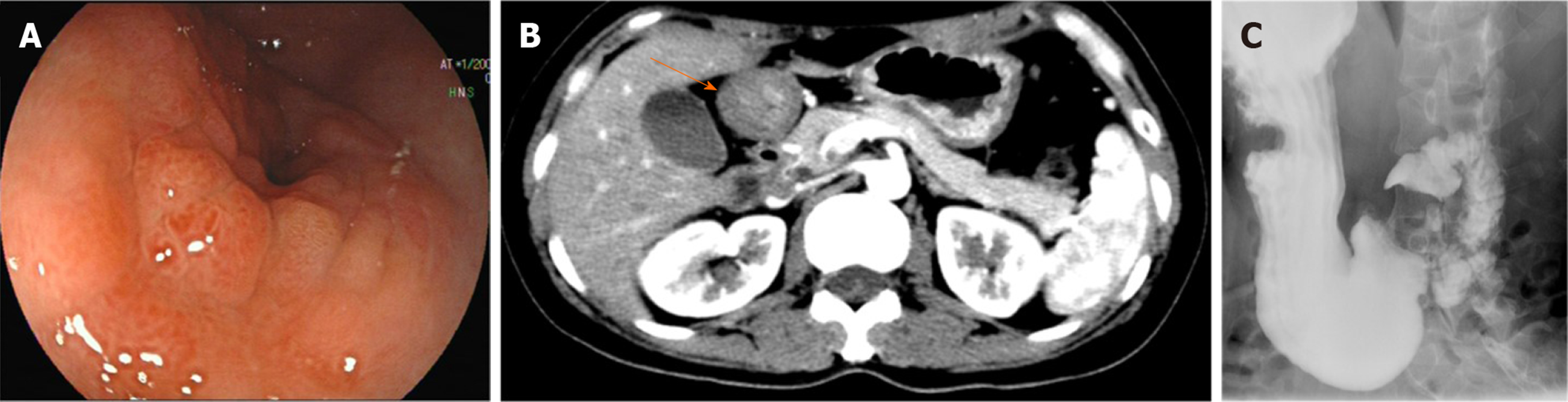
Gastroscopy showed that the antrum mucosa was congestive and edematous, and its part near the pylorus appeared rough, with a nodular protruding lesion causing obstruction of the gastric outlet (Figure 1A). Endoscopic passage through the site was difficult, and only an ultrathin electronic gastroscope could pass to the duodenum. The duodenum bulb and descending duodenum appeared unremarkable. Biopsies were not representative for a neoplastic lesion. Abdominal computed tomography showed marked wall thickening of the antrum, which was enhanced by contrast agent (Figure 1B), while the remainder of the abdomen, including the pancreas, was unremarkable. Barium meal examination showed filling defect at the antrum and stenosis of the lumen (Figure 1C).
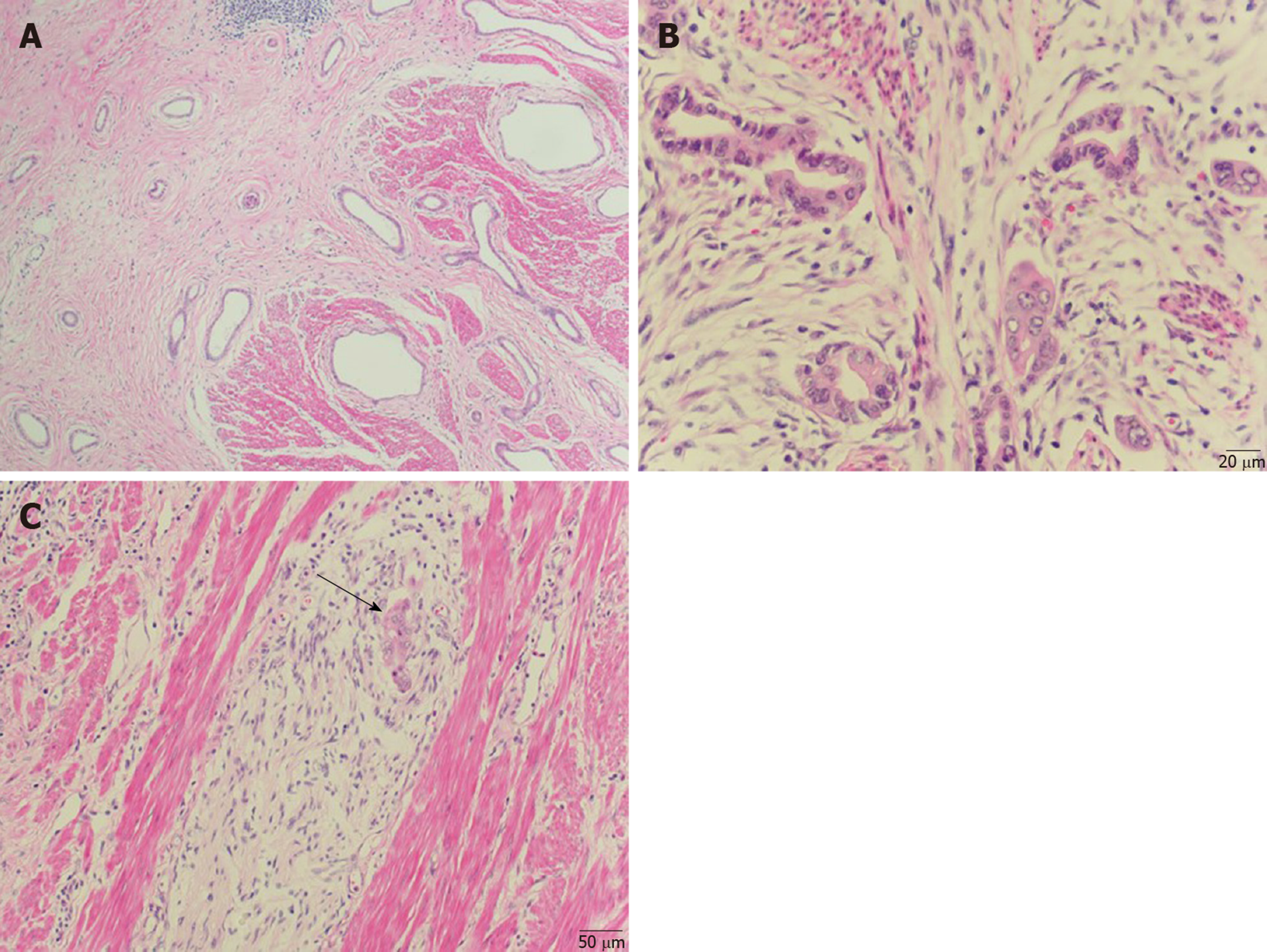
A distal gastrectomywas performed. Gross examination of the surgically resected specimen revealed that the gastric pylorus was thickened and rigid. Histopathological examination confirmed the presence of a well-differentiated adenocarcinoma and the coexisting presence of pancreatic heterotopia composed of ducts (Heinrich type III) (Figure 2A and B), thus supporting the diagnosis of a malignancy arising from pancreatic heterotopia in the stomach. The lesions developed predominantly in the submucosal layer and infiltrated the muscular layer. The lesions also invaded and broke through the serosal membrane and invaded bundles of nerves (Figure 2C). The resected margins were free of tumor tissue.
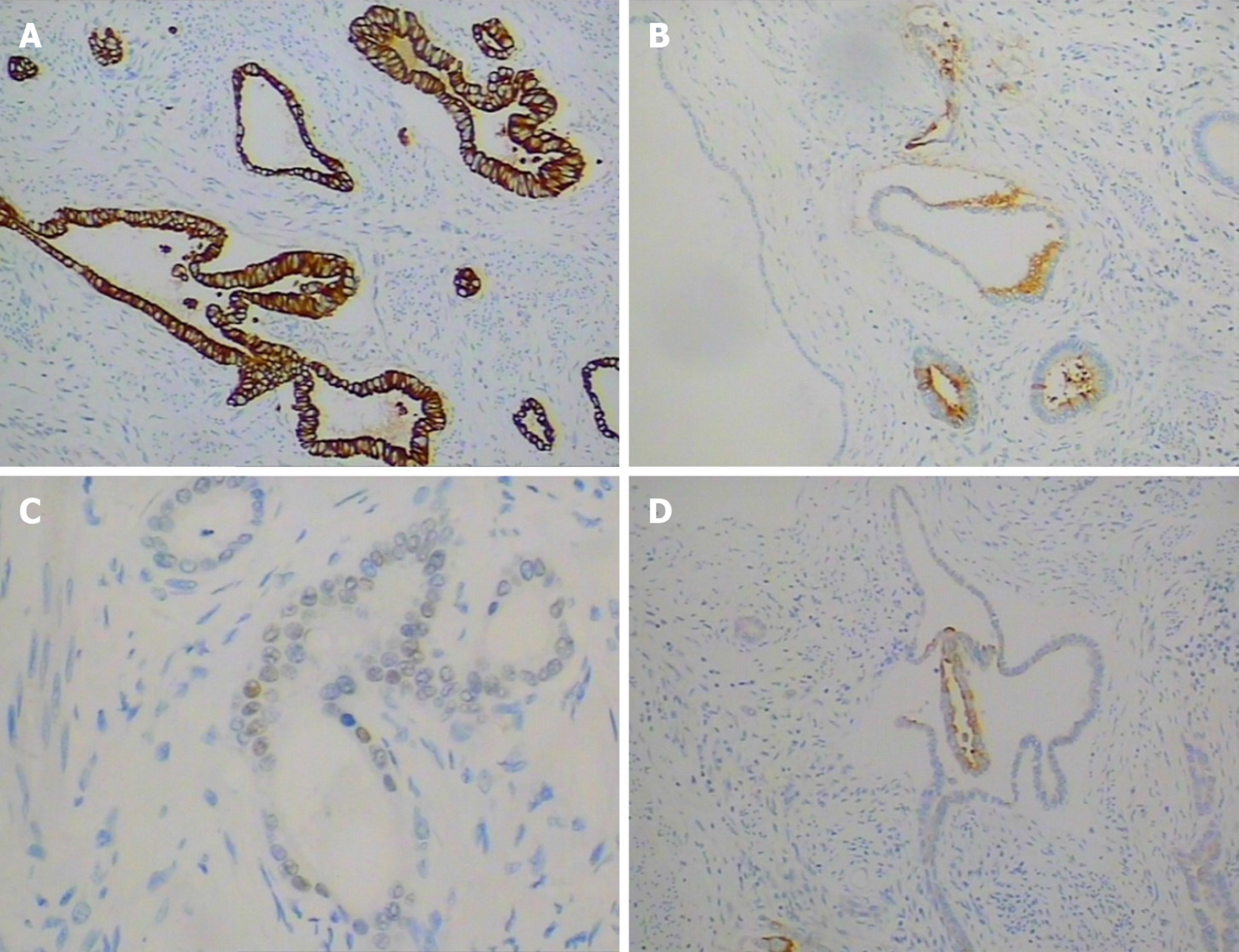
None of the 20 identified lymph nodes was positive. Immunohistochemistry analysis of the cancer cells showed positivity for cytokeratin 7 (Figure 3A), cytokeratin, CEA (Figure 3B), CDX-2 (Figure 3C), cytokeratin20 (partially) (Figure 3D), P53 (about 10%) and Ki-67 (about 7%), and negativity for Her-2.
The final diagnosis was malignant transformation of aberrant pancreas in the stomach.
At 1 month after surgery, the patient received chemotherapy using the combination FOLFOX6 regimen (folinic acid, fluorouracil, and oxaliplatin), which is a protocol for treatment of resected stomach cancer. However, after three cycles, adverse effects (e.g., hepatic and mucocutaneous damage) and an increased serum level of CA724 (107 kU/L) were observed. The chemotherapy was switched to gemcitabine, which is recommended in pancreatic cancer, and the CA724 serum level decreased gradually (19 kU/L).
The follow-up course was uneventful at 12 months postoperatively.
Heterotopic pancreas is often found incidentally during surgery or endoscopic examination and is clinically silent and benign. Malignant transformation of the heterotopic pancreas tissue is extremely rare, thus it is usually neither suspected nor included in the diagnostic work-up of different subepithelial tumors. Most cases are diagnosed by pathological findings after surgery. Guillouet al[28] has proposed the following three criteria for the diagnosis of carcinoma arising from a heterotopic pancreas: (1) The tumor must be within or near the heterotopic pancreatic tissue; (2) A direct transition between the pancreatic structures and carcinoma must be present; and (3) The non-neoplastic heterotopic pancreatic tissue must comprise, at least, fully developed acini and/or ductal structures. The lesion in the present case was compatible with all the three criteria.
A search of the English and Chinese language literature revealed 16 reports of well-documented cases of neoplasms arising in the heterotopic pancreas in the stomach, which met the three diagnostic criteria mentioned above (Table 1)[4,13-27]. Including the case reported herein, the patients’ ages ranged from 31 to 85 years (56.7 years, on average). There was no sex predilection. Except for two patients who were asymptomatic, the others had common symptoms, including epigastralgia, dyspepsia, and vomiting. With respect to tumor markers, excluding eight cases that were not described in detail, 33% of the remaining cases had increased CA199 or CEA levels, and increased CA724 level was reported only in our case. Most of the tumors are located in the antropyloric region (70%), which is likely to produce gastric outlet obstruction. A subepithelial tumor-like appearance is most frequently observed, followed by a stenotic or ulcerated appearance.
| Ref. | Sex, age (yrs) | Location | Appearance | Clinical symptoms | Heinrich type | Outcome (time after treatment) | Size in cm | Tumor markers |
| Goldfarb et al[15], 1962 | F, 55 | Pylorus | Stenotic with ulceration | Abdominal pain, weight loss, vomiting | I | Recurrence (6 yr) | 6.0 | NR |
| Tanimura et al[16], 1979 | F, 55 | Antrum | Submucosal tumor | GI bleeding, gastric discomfort, lumbago, pain in the right leg | II | Death (1 mo) | 0.5× 2 × 2 | NR |
| Ura et al[17], 1997 | F, 60 | Lesser curvature | Extramural mass | Asymptomatic | I | NR | 1.8 × 1.3 | CEA and CA199 were normal |
| Osanai et al[14], 2001 | F, 57 | Lesser curvature | Protruding lesion with a central ulcer | Epigastric discomfort and periodic nausea | II | Death (13 mo) | 12.5 × 9 | NR |
| Halkic et al[18], 2001 | M, 60 | Esophagogastric junction | Stenotic, ulcerated | Epigastric pain, dysphagia, weight loss | I | Death (4 mo) | 6×4.5×4 | NR |
| Jeong et al[19], 2002 | M, 64 | Antrum | Subepithelial tumor, stenotic | Dyspepsia, vomiting | I | Uneventful (1 yr) | 3×3 × 1.5 | NR |
| Song et al[4], 2004 | M, 35 | Antrum | Submucosal tumor | Asymptomatic | III | Uneventful (5 mo) | 2×1.7×1.2 | CEA, CA199 and CA724 were normal |
| Chetty et al[20], 2004 | M, 85 | Antrum | Ulcer | Dyspepsia, increase in stool frequency | I | Uneventful (1 mo) | 1.7×1.7×0.9 | NR |
| Emerson et al[21], 2004 | M, 52 | NR | Stenotic | Epigastric, left upper quadrant pain, emesis, and bloating | III | Uneventful (9 mo) | 4.0×2.5×1.5 | NR |
| Matsuki et al[13], 2005 | F, 58 | Prepyloric region | Gastric outlet obstruction | Vomiting | II | Metastasis (1.5 yr) | NR | CEA and CA199 were normal |
| Kimura et al[22], 2008 | F, 31 | Pyloric ring | Submucosal tumor | Epigastralgia | II | NR | 5.0×2 | CA199: 660 U/mL; CA125: 40.8 U/mL |
| Papaziogas et al[23], 2008 | F, 56 | Antrum | Ulcerated lesion | Epigastric pain, nausea and vomiting | III | Uneventful (6 mo) | 2× 1.2 | CEA, AFP, CA199, CA-125 and CA-724 were normal |
| Okamoto et al[24], 2012 | F, 74 | Middle corpus | Submucosal tumor | Epigastralgia | I | Uneventful (11 yr) | 2.0 × 2.0 × 2.0 | CEA: 8.7 ng/mL, CA199: 287.4 U/mL |
| Lemaire et al[25], 2014 | M, 60 | Lesser curvature | NR | Dyspepsia and epigastric heaviness | NR | Disease-free (4 yr) | 7.5×4.4 | CA199: 40 U/mL |
| Endo et al[26], 2014 | M, 73 | Antrum | Submucosal tumor | Epigastric pain and abdominal fullness | I | Metastasis (2 yr) | 3.2×2.4 | CEA and CA199 were normal |
| Priyathersini et al[27], 2017 | M, 45 | Antrum | Submucosal mass | Early satiety, vomiting, and constipation | II | Uneventful (12 mo) | 5×4.3×3.5 | NR |
| Present case | F, 44 | Antrum | Submucosal tumor, stenotic | Abdominal distension, vomiting | III | Disease-free (9 mo) | 3×4 | CA724: 50.1 kU/L, CEA and CA199 were normal |
The diagnosis of malignant transformation in the heterotopic pancreas in the stomach may be difficult by endoscopic biopsy because of the intramural location of the mass and the delayed involvement of the overlying mucosa[29]. However, distinction between benign and malignant lesion is critical for patient management. It has been suggested that asymptomatic heterotopic pancreas of less than 2 cm can be monitored without specific therapy. In our review of well-documented cases, the sizes of malignant heterotopic pancreas in the stomach ranged from 1.7 cm to 12.5 cm, with a mean of 4.2 cm. However, in one patient, the tumor was only 0.5 cm × 2 cm ×2 cm in size but with extensive metastasis to the bone[16]. Actually, 37.5% of these cases had a tumor of no more than 2 cm. In our case, the tumor appeared as a protruding lesion, causing stenosis and with irregular shape or unclear boundary, making it difficult to accurately measure its size. Therefore, we suggest early treatment for heterotopic pancreas to prevent progression to carcinogenesis, especially when the lesion size is more than 1 cm or if there are signs of obstruction, ulceration or weight loss. Endoscopic ultrasound (EUS) was performed in our patient and other 4 patients in the literature[13,17,25,26]. In the case reported by Ura et al[17], EUS showed a swollen perigastric lymph node and changes in mass shape and size during dynamic monitoring, which strongly indicated a malignant lesion. EUS in other cases including ours showed non-specific features such as heterogenous hypoechoic lesion or thickness of the gastric wall. So using single EUS to detect the lesion itself can hardly predict the histologic diagnosis, but dynamic monitoring and detecting for perigastric lymph nodes by EUS could help distinguishing malignant and benign lesions. As for the interval of endoscopic examination for surveillance of malignant change of heterotopic pancreas, there is still no consensus. Dynamic monitoring by endoscopy was only reported in one case[17]. In this case, a mass of 1.8 cm × 1.3 cm was detected by EUS and it showed no change 1 year later, but 2 years after the second examination the mass was found to have increased to 3.3 cm × 3.0 cm and ultimately diagnosed as an invasive adenocarcinoma extending to the peritoneal surface with lymph node metastases. This case suggests that the interval of endoscopic surveillance of malignant change of heterotopic pancreas should be less than 2 years. Positron emission tomography (PET) has an important role in clinical imaging diagnosis of malignancy. PET findings were reported in only one case[25]. In this case, PET scan showed hot spots in gastric wall which indicated a malignant lesion. As the lesion size was large (7.5 cm × 4.4 cm), whether PET scan is helpful to diagnose small malignant lesion still need to be further studied.
Histologically, malignant heterotopic pancreas in the stomach has been classified predominantly as adenocarcinomas, with only one report of neuroendocrine carcinoma[20]. The Heinrich classification divides pancreatic heterotopia into three types, with type I encompassing typical pancreatic tissue with acini, ducts and islet cells, type II encompassing only exocrine components with numerous acini, few ducts and no islet cells, and type III encompassing numerous ducts, few to no acini, and no islet cells[13]. The modification by Gaspar et al[30] added a fourth type (type IV), comprising pure endocrine heterotopia containing only islet cells. In the majority of pancreatic heterotopia cases with documented classification, the malignancy arose within a type I heterotopia (46.7%). The next most frequent type was II (33.3%). Our case arose within a type III heterotopic pancreas, which is the least common background for the origin of adenocarcinoma in heterotopic pancreas.
There is no clear evidence of the efficacy of chemotherapy for malignant heterotopic pancreas in the stomach, and the type of adjuvant treatment is not clear because of the small number of reported cases. In our literature review, chemotherapy regimen was reported for only two cases, with one having used gemcitabine[25] and the other having used S-1 and cisplatin[26]. We selected the FOLFOX6 regimen based on its use in treatment of resected stomach cancer; however, the patient’s serum level of CA724 increased after receiving three cycles of the chemotherapy. We then changed the treatment to gemcitabine monotherapy based on its use in primary pancreatic cancer, and the serum level of CA724 decreased gradually. While gemcitabine seems to be effective in our case, more cases need to be investigated to provide strong evidence to support its use in these rare cases.
The prognosis of malignancies arising from heterotopic pancreas in the stomach is also unknown. In our literature review, 28.6% of the patients with a description of the clinical course were alive without evidence of recurrence at least 1 year following resection of the malignant heterotopic pancreas. In addition, 21.4% of the patients died from cancer-related complications, and 42.9% of the patients had metastasis to other organs at the time of diagnosis or during the follow-up period. These observations suggest that the prognosis may be poor.
We have described a rare case of adenocarcinoma arising from heterotopic pancreas in the stomach. It should be noted that heterotopic pancreas is a source of potentially malignant lesion and endoscopic features of its malignant change in the stomach are nonspecific, so we suggest early treatment or close monitoring of the heterotopic pancreas. The rarity of this condition makes the treatment a herculean challenge, and more detailed and in-depth studies on this topic are required.
The authors thank Mr. Yong-Jian Deng, Department of Pathology, Nanfang Hospital of Southern Medical University, for his technical assistance for this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuwai T, Lambrecht NW, Telkes G S-Editor: Wang JL L-Editor: MedE-Ma JY E-Editor: Liu JH
| 1. | Goodarzi M, Rashid A, Maru D. Invasive ductal adenocarcinoma arising from pancreatic heterotopia in rectum: case report and review of literature. Hum Pathol. 2010;41:1809-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Sun X, Gold JS, Sun Q, Lv Y, Li Q, Huang Q. Heterotopic pancreas: a clinicopathological study of 184 cases from a single high-volume medical center in China. Hum Pathol. 2016;55:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 3. | Herold G, Kraft K. Adenocarcinoma arising from ectopic gastric pancreas: two case reports with a review of the literature. Z Gastroenterol. 1995;33:260-264. [PubMed] |
| 4. | Song DE, Kwon Y, Kim KR, Oh ST, Kim JS. Adenocarcinoma arising in gastric heterotopic pancreas: a case report. J Korean Med Sci. 2004;19:145-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Ulrych J, Fryba V, Skalova H, Krska Z, Krechler T, Zogala D. Premalignant and malignant lesions of the heterotopic pancreas in the esophagus: a case report and review of the literature. J Gastrointestin Liver Dis. 2015;24:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lee MJ, Chang JH, Maeng IH, Park JY, Im YS, Kim TH, Han SW, Lee DS. Ectopic pancreas bleeding in the jejunum revealed by capsule endoscopy. Clin Endosc. 2012;45:194-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jacobz A, Nawaz A, Matta H, Al Salem A. Intussusception secondary to isolated heterotopic pancreas of the ileum: case report and review of the literature. Ann Saudi Med. 2002;22:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Sumiyoshi T, Shima Y, Okabayashi T, Kohsaki T, Kigi A, Iwata J, Kozuki A, Tokumaru T, Nishimori I, Morita S. Heterotopic pancreas in the common bile duct, with a review of the literature. Intern Med. 2014;53:2679-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Yan ML, Wang YD, Tian YF, Lin Y. Adenocarcinoma arising from intrahepatic heterotopic pancreas: a case report and literature review. World J Gastroenterol. 2012;18:2881-2884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Elsayes KM, Narra VR, Lewis JS, Abu El Abbas HA, Brown JJ. MRI of heterotopic pancreatic tissue in the spleen. AJR Am J Roentgenol. 2005;185:816-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Seo N, Kim JH. Characteristic CT features of heterotopic pancreas of the mesentery: "another pancreas" in the mesentery. Clin Imaging. 2014;38:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic Pancreas: Histopathologic Features, Imaging Findings, and Complications. Radiographics. 2017;37:484-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Matsuki M, Gouda Y, Ando T, Matsuoka H, Morita T, Uchida N, Kuriyama S. Adenocarcinoma arising from aberrant pancreas in the stomach. J Gastroenterol. 2005;40:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Osanai M, Miyokawa N, Tamaki T, Yonekawa M, Kawamura A, Sawada N. Adenocarcinoma arising in gastric heterotopic pancreas: clinicopathological and immunohistochemical study with genetic analysis of a case. Pathol Int. 2001;51:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Goldfarb WB, Bennett D, MonafoW. Carcinoma in Heterotopic Gastric Pancreas. Ann Surg. 1963;158:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Tanimura A, Yamamoto H, Shibata H, Sano E. Carcinoma in heterotopic gastric pancreas. Acta Pathol Jpn. 1979;29:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ura H, Denno R, Hirata K, Saeki A, Hirata K, Natori H. Carcinoma arising from ectopic pancreas in the stomach: endosonographic detection of malignant change. J Clin Ultrasound. 1998;26:265-268. [PubMed] [DOI] [Full Text] |
| 18. | Halkic N, Nordback P. Soft-tissue images. Malignant degeneration of heterotopic pancreas. Can J Surg. 2001;44:407. [PubMed] |
| 19. | Jeong HY, Yang HW, Seo SW, Seong JK, Na BK, Lee BS, Song GS, Park HS, Lee HY. Adenocarcinoma arising from an ectopic pancreas in the stomach. Endoscopy. 2002;34:1014-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 2004;87:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kimura J, Kaji M, Yamamoto SI, Maeda KI, Yabushita K, Konishi K, Hitoshi A, Akio U, Atsuo M. A case of adenocarcinoma arising from ectopic gastric pancreas with carcinomatous peritonitis. Jpn J Gastroenterol Surg. 2008;41:399-405. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Papaziogas B, Koutelidakis I, Tsiaousis P, Panagiotopoulou K, Paraskevas G, Argiriadou H, Atmatzidis S, Atmatzidis K. Carcinoma developing in ectopic pancreatic tissue in the stomach: a case report. Cases J. 2008;1:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Okamoto H, Kawaoi A, Ogawara T, Fujii H. Invasive ductal carcinoma arising from an ectopic pancreas in the gastric wall: a long-term survival case. Case Rep Oncol. 2012;5:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lemaire J, Delaunoit T, Molle G. Adenocarcinoma arising in gastric heterotopic pancreas. Case report and review of the literature. Acta Chir Belg. 2014;114:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Endo S, Saito R, Ochi D, Yamada T, Hirose M, Hiroshima Y, Yamamoto Y, Ueno T, Hasegawa N, Moriwaki T, Narasaka T, Kaneko T, Fukuda K, Suzuki H, Mizokami Y, Hyodo I. Effectiveness of an endoscopic biopsy procedure using EUS-FNA and EMR-C for diagnosing adenocarcinoma arising from ectopic pancreas: two case reports and a literature review. Intern Med. 2014;53:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Priyathersini N, Sundaram S, Senger JL, Rajendiran S, Balamurugan TD, Kanthan R. Malignant transformation in gastric pancreatic heterotopia a case report and review of the literature. J Pancreas. 2017;18:73-77. |
| 28. | Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568-571. [PubMed] |
| 29. | Yasuda K, Nakajima M, Yoshida S, Kiyota K, Kawai K. The diagnosis of submucosal tumors of the stomach by endoscopic ultrasonography. Gastrointest Endosc. 1989;35:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Gaspar Fuentes A, Campos Tarrech JM, FernándezBurgui JL, Castells Tejón E, RuízRossello J, Gómez Pérez J, ArmengolMiró J. [Pancreatic ectopias]. Rev Esp Enferm Apar Dig. 1973;39:255-268. [PubMed] |