Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1878
Peer-review started: January 20, 2020
First decision: March 18, 2020
Revised: March 19, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: May 26, 2020
Processing time: 125 Days and 23.3 Hours
Diabetes is a clinically common chronic disease, and its incidence has been increasing in recent years. Diabetes is believed to accelerate the process of atherosclerosis in patients, and abnormal endothelial function is an important factor leading to diabetic kidney damage.
To investigate the efficacy of ligliptin in the treatment of type 2 diabetes mellitus (T2DM) with early renal injury and its effect on serum endogenous hydrogen sulfide (H2S), endothelial cell particles, and endothelial function.
From January 2018 to April 2019, 110 patients with T2DM and early kidney injury treated at our hospital were divided into an observation group (receiving ligliptin treatment, n = 54) and a control group (receiving gliquidone therapy, n = 56). Blood glucose and renal function before and after treatment were compared between the two groups.
The differences in fasting blood glucose, 2 h blood glucose, and glycated hemoglobin were not statistically significant between the two groups after treatment. The urinary albumin excretion rate after treatment in the ligliptin group was 70.32 ± 11.21 µg/min, which was significantly lower than that of the gliquidone group (P = 0.000). Serum endogenous H2S and endothelial cell microparticles of the ligliptin treatment group were 40.04 ± 8.82 mol/L and 133.40 ± 34.39, respectively, which were significantly lower than those of the gliquidone treatment group (P = 0.000 for both); endothelin-dependent diastolic function and nitric oxide after treatment in the ligliptin group were 7.98% ± 1.22% and 190.78 ± 30.32 mol/L, significantly higher than those of the gliquidone treatment group (P = 0.000 for both).
Ligliptin treatment of T2DM with early renal injury has the same glucose-lowering effect as gliquidone treatment. Ligliptin treatment has a better effect and it can significantly improve the renal function and vascular endothelial function of patients, and reduce serum endogenous H2S and endothelial cell particle levels.
Core tip: At present, diabetes is believed to accelerate the process of atherosclerosis in patients, and abnormal endothelial function is an important factor leading to diabetic kidney damage. The dipeptidyl peptidase 4 inhibitor ligliptin belongs to intestinal insulinotropic drugs, which can effectively reduce blood sugar. In order to further confirm the effect and specific mechanism of ligliptin application in patients with type 2 diabetes with kidney injury, this study compared the effects of ligliptin and gliquidone in this population.
- Citation: Zhang J, Du YL, Zhang H, Sui H, Hou WK. Ligliptin for treatment of type 2 diabetes mellitus with early renal injury: Efficacy and impact on endogenous hydrogen sulfide and endothelial function. World J Clin Cases 2020; 8(10): 1878-1886
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1878.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1878
Diabetes is a clinically common chronic disease, and its incidence has been increasing in recent years. At present, nearly 100 million people in China have suffered from this disease. As the disease progresses, type 2 diabetes (T2DM) can cause kidney function damage and abnormal glucose and lipid metabolism in the body, which will cause changes in glomerular filtration in the kidney, affect renal tissue metabolism, and result in excessive formation of glycosylation products and severe renal failure[1].
At present, diabetes is believed to accelerate the process of atherosclerosis in patients, and abnormal endothelial function is an important factor leading to diabetic kidney damage. The dipeptidyl peptidase 4 inhibitor ligliptin belongs to intestinal insulinotropic drugs, which can effectively reduce blood sugar. It has certain effects on the cardiovascular system and does not increase adverse reactions. The specific mechanism may involve improving vascular endothelial function, but clinical reports are relatively rare[2].
In order to further confirm the effect and specific mechanism of ligliptin application in patients with T2DM with kidney injury, this study compared the effects of ligliptin and gliquidone in this population.
A total of 110 patients with T2DM and early kidney injury treated at our hospital from January 2018 to April 2019 were selected. The inclusion criteria were as follows: T2DM met the criteria established by the World Health Organization; the urinary albumin excretion rate (UAER) was 20-200 μg/min; no urinary tract infection, acute and chronic nephritis, or other kidney diseases; informed consent was obtained from the patients and their families. The exclusion criteria were: Concomitance with other diseases such as malignant tumors, immune system diseases, hematological diseases, and cardiac insufficiency; there was a history of taking hormonal drugs, immunomodulators, and nephrotoxic drugs within 1 mo before the study.
According to the treatment regimen, they were divided into a ligliptin group (n = 54) and a gliquidone group (n = 56). There was no significant difference in general information between the two groups of patients, as shown in Table 1.
| Ligliptin treatment group (n = 54) | Gliquidone treatment group (n = 56) | P value | |
| Gender: Male/female | 30/24 | 33/23 | 0.721 |
| Age (yr) | 50.38 (7.82) | 49.72 (9.10) | 0.685 |
| Duration of diabetes (yr) | 7.80 (1.12) | 8.04 (1.40) | 0.324 |
| BMI (kg/m2) | 23.40 (3.11) | 22.74 (2.87) | 0.250 |
Ligliptin 5 mg (Boehringer Ingelheim; drug approval number: National Drug Standard J20171087; drug batch number: 20180214) was administered before breakfast once a day, and the effects were observed after continuous treatment for 4 mo; gliquidone 30 mg (Beijing Wanhui Shuanghe Pharmaceutical Co., Ltd.; drug approval number: National Drug Standard H10940258; drug batch number: 20180116) was administered before the three meals daily, and the effects were observed after continuous treatment for 4 mo.
Fasting venous blood (5 mL) was collected from patients before and 4 mo after treatment, and serum was separated at 2000 rpm and frozen at -80 °C for examination. Fasting blood glucose (FBG), 2 h blood glucose (2hPG), UAER, and blood urea nitrogen (BUN) were determined by continuous monitoring method using the H-800 biochemical analyzer from Japan Elekko Corporation. Cystatin C (CysC) was measured by particle-enhanced immunoturbidimetry. Glycated hemoglobin (HbA1c) was measured by ion-exchange high-pressure liquid chromatography. Endothelin-dependent diastolic function (FMD), plasminogen activator inhibitor-1 (PAI-1), and nitric oxide (NO) were measured by enzyme-linked immunosorbent assay. Serum endogenous H2S was determined with a spectrophotometer. For determination of endothelial cell microparticles, they were mixed with 50 μL of platelet-depleted plasma and 4 μL of mouse anti-human CD31-phycoerythrin fluorescein and mouse anti-human CD42b-fluorescein isothiocyanate. The buffer was diluted and analyzed by flow cytometry, and standard microspheres with a diameter of 1.0 μm were gated at the forward angle. The diameter of the collected particles was < 1.0 μm. Mouse anti-human CD31-phycoerythrin fluorescein and mouse anti-human CD42b -An example of fluorescein isothiocyanate ratio < 1.0 μm in diameter is defined as endothelial cell microparticles.
Data analyses were performed using SPSS22.0 software. FPG, HbA1c, and other data are expressed as the mean ± SD. Differences between groups were compared using independent sample t-tests. Gender and other data were compared using χ2 test. P < 0.05 was considered statistically different.
FBG, 2hPG, and HbA1c after treatment were significantly lower than those before treatment in both groups (P = 0.000 for all). There was no significant difference in FBG, 2hPG, or HbA1c between the two groups (P = 0.919, 0.876, and 0.760, respectively; Table 2).
| Ligliptin treatment group | Gliquidone treatment group | P value | |
| Number of cases | 54 | 56 | |
| FBG (mmol/L) | |||
| Before treatment | 8.90 (1.28) | 8.78 (1.30) | 0.627 |
| After treatment | 7.13 (1.05)a | 7.15 (1.01)a | 0.919 |
| 2hPG (mmol/L) | |||
| Before treatment | 12.05 (1.43) | 12.10 (1.51) | 0.859 |
| After treatment | 8.15 (1.65)a | 8.20 (1.70)a | 0.876 |
| HbA1c (%) | |||
| Before treatment | 7.99 (1.40) | 7.94 (1.31) | 0.847 |
| After treatment | 7.00 (1.22)a | 6.93 (1.18)a | 0.760 |
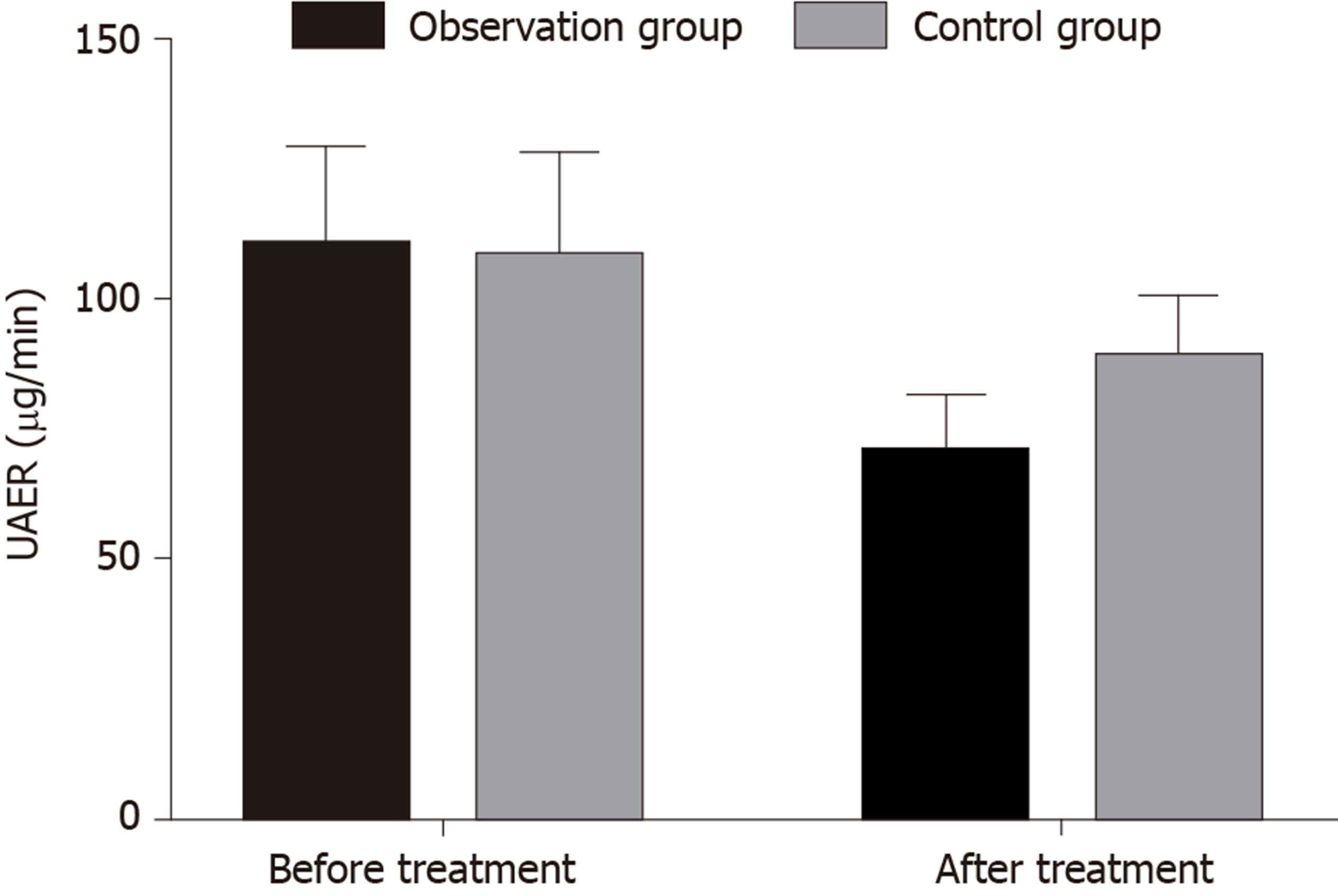
UAER, BUN, and CysC after treatment were significantly lower than those before treatment in both groups (P = 0.000 for all). UAER, BUN, and CysC after treatment were significantly lower in the ligliptin treatment group than in the gliquidone treatment group (P = 0.000 for all; Table 3 and Figure 1).
| Ligliptin treatment group | Gliquidone treatment group | P value | |
| Number of cases | 54 | 56 | |
| UAER (μg/min) | |||
| Before treatment | 110.21 (18.82) | 107.82 (20.11) | 0.522 |
| After treatment | 70.32 (11.21)a | 88.42 (12.27)a | 0.000 |
| BUN (mmol/L) | |||
| Before treatment | 6.10 (1.12) | 6.05 (1.09) | 0.813 |
| After treatment | 5.77 (1.04)a | 5.79 (1.21)a | 0.926 |
| Cr (μmol/L) | |||
| Before treatment | 110.38 (15.56) | 108.82 (18.81) | 0.637 |
| After treatment | 108.02 (12.38) | 107.22 (11.87) | 0.730 |
| CysC (mg/L) | |||
| Before treatment | 1.74 (0.30) | 1.78 (0.33) | 0.508 |
| After treatment | 1.65 (0.33)a | 1.67 (0.35)a | 0.759 |
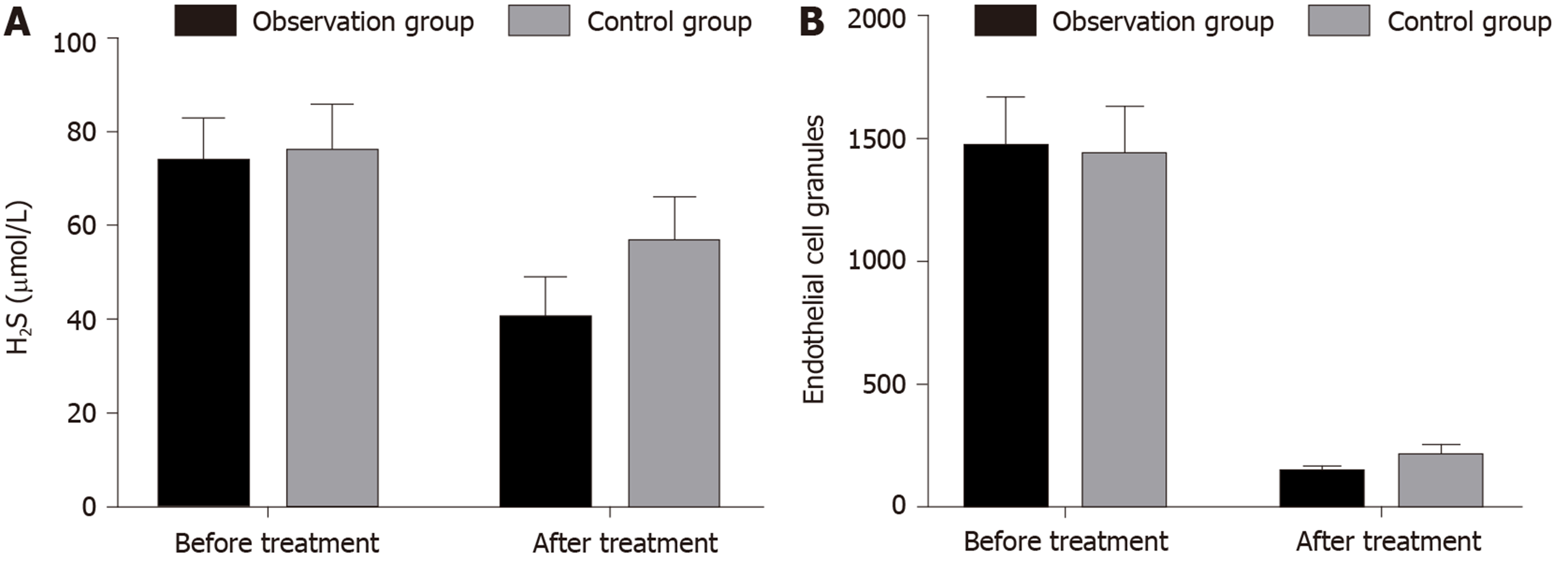
After treatment, the endogenous H2S and endothelial cell microparticles in the serum were decreased in the ligliptin treatment group and the gliquidone treatment group compared with those before treatment (P = 0.000 for all). After treatment, serum endogenous H2S and endothelial cell microparticles were significantly lower in the ligliptin treatment group than in the gliquidone treatment group (P = 0.000 for both; Table 4 and Figure 2).
| Ligliptin treatment group | Gliquidone treatment group | P value | |
| Number of cases | 54 | 56 | |
| H2S (μmol/L) | |||
| Before treatment | 73.39 (9.28) | 75.58 (10.16) | 0.241 |
| After treatment | 40.04 (8.82)a | 56.12 (9.90)a | 0.000 |
| Endothelial cell granules | |||
| Before treatment | 1459.28 (210.22) | 1430.04 (201.78) | 0.458 |
| After treatment | 133.40 (34.39)a | 202.32 (50.43)a | 0.000 |
The FMD, PAI-1, and NO of the ligliptin group and the gliquidone group were improved after treatment (P = 0.000 for all). FMD and NO were significantly higher in the ligliptin treatment group than in the gliquidone treatment group (P = 0.000 for both; Table 5 and Figure 3).
| Ligliptin treatment group | Gliquidone treatment group | P value | |
| Number of cases | 54 | 56 | |
| FMD (%) | |||
| Before treatment | 6.33 (1.21) | 6.40 (1.16) | 0.757 |
| After treatment | 7.98 (1.22)a | 7.10 (1.13)a | 0.000 |
| PAI-1 (ng/mL) | |||
| Before treatment | 2.45 (0.43) | 2.51 (0.40) | 0.450 |
| After treatment | 2.03 (0.56)a | 2.06 (0.61)a | 0.789 |
| NO (μmol/L) | |||
| Before treatment | 154.42 (32.19) | 150.03 (30.03) | 0.461 |
| After treatment | 190.78 (30.32)a | 168.20 (29.37)a | 0.000 |
T2DM is a common endocrine metabolic disease. The improvement of Chinese people's living standards and changes in lifestyles have caused the rising incidence of diabetes in China, which has become a public health problem that seriously endangers public health. Due to a variety of chronic complications of diabetes, some patients will have microvascular and macrovascular pathological changes, which seriously affect the quality of life and physical and mental health of patients[3].
Kidney injury is one of the most common complications of diabetes. Nearly 2/5 of end-stage renal diseases are caused by diabetes. Patients with kidney injury have early mesangial hyperplasia, thickened glomerular basement membrane, and severe changes in glomerulosclerosis. The pathological analysis found that the glomerulus volume of patients with diabetic nephropathy (DN) increased, and mesangial cells showed proliferative changes, accompanied by obvious inflammatory cell infiltration[4,5]. At present, it is clinically believed that there are many mechanisms by which diabetes causes kidney damage. Due to non-enzymatic glycosylation, the formation of macromolecular glycosylation end products is too much, and protein kinase activation leads to increased polyol pathway activity and oxidative stress that can cause kidney damage[6]; on the other hand, hyperglycemia induces the proximal tubules of the kidney to secrete inflammatory factors and a variety of cytokines, leading to the process of renal interstitial fibrosis, which promotes fibrosis and an increase in the number of mediators of epithelial mesenchymal transdifferentiation[7]. At the same time, the hyperglycemic state of the cells will cause the activation of aldose reductase. Thus, glucose is converted into sorbitol, fructose is formed in the human body and unable to pass through the biofilm, and the osmotic pressure in the cell increases. As a result, edema of cells occurs, sodium-potassium-ATP enzyme activity in the body decreases, and endothelial cells are damaged by hypoxia, which leads to the continuous progression of DN[8]. In addition, disorders of human fat metabolism can also cause glomerulosclerosis. Changes in the structure of adipose-resistant acids, increased secretion of vasoconstriction active substances, increased glomerular capillary pressure, and changes in glomerular hemodynamics can accelerate the continuous progression of DN[9,10]. At present, clinical treatment of DN is not radical. The method is mainly targeted treatment to delay the exacerbation of the disease, so for DN, on the one hand, it is necessary to control the patient's blood glucose and blood pressure while cooperating with sports, diet, and other conventional treatments to reduce the accumulation process of glomerular extracellular matrix and regulate the glomerular blood. Hydrodynamic changes reduce the damage of cells by inflammatory factors with the goal of reducing proteinuria formation in DN[11].
Gliquidone is a second-generation oral sulfonylurea hypoglycemic agent that can bind to specific receptors on the islet β-cell membrane and induce the production of appropriate amounts of insulin to reduce blood glucose concentration, but the advantage in terms of renal protection is not obvious. Gliptin is used in the treatment of hypoglycemic patients with T2DM and kidney injury. The drug is a dipeptidyl peptidase-IV inhibitor. Dipeptidyl peptidase-IV is a peptidase with special uses in the human body, which can quickly reduce the concentration of human glucagon-like peptide-1. Glucagon-like peptide-1 levels are extremely low when the body does not eat under normal conditions. It will rise rapidly within a few minutes after eating, accelerate human insulin secretion, and reduce blood sugar after meals. However, the blood glucose level is relatively low and its hypoglycemic effect is reduced, thus reducing the occurrence of hypoglycemic events[12]. This study showed that FBG, 2hPG, and HbA1c were lower in both the ligliptin group and the gliquidone group after treatment than those before treatment. However, there was no statistically significant difference between the two groups, indicating that the effect of ligliptin in improving blood glucose reduction in patients with diabetes and kidney injury is comparable to that of gliquidone and that it is relatively safe. This study also found that UAER, BUN, and CysC were lower in both the ligliptin-treated group and the gliquidone-treated group compared with those before treatment; the UAER of the ligliptin-treated group was significantly lower than that of the gliquidone-treated group, indicating that ligliptin is superior to gliquidone in improving renal function in patients with diabetes and kidney injury. CysC is a non-glycosylated basic protein, and it is generally catabolized by epithelial cells in the glomerular capillaries. The concentration in the body is maintained at a certain level, and its concentration change indirectly reflects the degree of renal function impairment[13,14]. This is mainly because ligliptin can inhibit the degradation of glucagon-like peptide-1 and inhibit intercellular adhesion molecule-1, thus reducing macrophage filtration, oxidative stress, and the incidence of DN[15]. Animal experiments have found that the administration of ligliptin in a mouse model can reduce proteinuria and kidney histological changes, and at the same time, it can elevate levels of stromal cell-derived factor-1α in the body are repaired by carrier cells to the site of tissue damage and exert renal protection[16].
Endothelial dysfunction is an important cause of kidney injury. Both NO and PAI-1 can be used to evaluate changes in endothelial function. The former is a protective and powerful vasodilating substance and an important indicator of endothelial progenitor cell function, while the latter is tissue in plasma, an important biological inhibitor of type plasminogen activator. Elevated concentrations of PAI-1 are closely related to hyperglycemia-related endothelial dysfunction and accelerated atherosclerosis[17]. H2S are formed in many mammals and have Physiological regulation that is generally formed by cysteine under the catalysis of cystathionine-b-synthetase and cystathionine-1-lyase, which is produced by H2S in pancreatic tissue and islet β cells And its synthetase expression[18]. In this study, serum endogenous H2S and endothelial cell microparticles in the ligliptin-treated and gliquidone-treated groups were lower than those before treatment. In addition, serum endogenous H2S and endothelial cell microparticles in the ligliptin treatment group were significantly lower than those in the gliquidone treatment group. The FMD, PAI-1, and NO in both groups were improved after treatment; the FMD and NO were significantly higher in the ligliptin treatment group after treatment, suggesting that ligliptin is superior to gliquidone in improving endothelial cell damage in patients with diabetes mellitus and kidney injury. This is mainly because ligliptin can increase the concentration of glucagon-like peptide-1, by increasing adiponectin and reducing the concentration of nitric oxide synthase inhibitor L-dimethylarginine, the level of nitric oxide can be increased, and at the same time, it can be related to the resistance to peroxynitrite and autophagy[19,20].
This study confirmed that ligliptin has a certain effect in reducing blood glucose in patients with T2DM, which is similar to previous studies, and found that it is superior to gliquidone in improving renal damage and regulating vascular endothelial function in patients. H2S, an important indicator of endothelial cell function, has rarely been observed in clinical studies in the past. Ligliptin can delay the progression of T2DM with kidney injury to a certain extent, providing clinical benefits. A new treatment idea was given in this study, but the follow-up time was short, the number of patients enrolled was small, and there may be measurement errors that have affected the accuracy of the results. Therefore, it is necessary to expand the sample size and conduct long-term follow-up studies to confirm our findings.
To sum up, ligliptin has a better effect on T2DM with early renal injury, and it can significantly improve patients' renal function and vascular endothelial function, and reduce serum endogenous H2S and endothelial cell particle levels.
Diabetes is believed to accelerate the process of atherosclerosis in patients, and abnormal endothelial function is an important factor leading to diabetic kidney damage.
The dipeptidyl peptidase 4 inhibitor ligliptin can effectively reduce blood sugar. It has certain effects on the cardiovascular system and does not increase adverse reactions.
This study aimed to investigate the efficacy of ligliptin in the treatment of type 2 diabetes mellitus (T2DM) with early renal injury and its effect on serum endogenous hydrogen sulfide, endothelial cell particles, and endothelial function.
Totally 110 patients with T2DM and early kidney injury were divided into either an observation group to receive ligliptin treatment or a control group to receive gliquidone therapy. Blood glucose and renal function were compared between before and after treatment and between the two groups.
The differences in fasting blood glucose, 2 h blood glucose, and glycated hemoglobin were not statistically significant between the two groups after treatment. The urinary albumin excretion rate after treatment in the ligliptin group was significantly lower than that of the gliquidone group. Serum endogeneous H2S and endothelial cell microparticles of the ligliptin treatment group were significantly lower than those of the gliquidone treatment group; endothelin-dependent diastolic function and nitric oxide after treatment in the ligliptin group were significantly higher than those of the gliquidone treatment group.
Ligliptin treatment of T2DM with early renal injury has the same glucose-lowering effect as gliquidone treatment. Ligliptim treatment has a better effect and can significantly improve the renal function and vascular endothelial function of patients, and reduce serum endogenous hydrogen sulfide and endothelial cell particle levels.
The follow-up time in this study was short, the number of patients enrolled was small, and there may be measurement errors that have affected the accuracy of the results. Therefore, it is necessary to expand the sample size and conduct long-term follow-up studies to confirm our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bello BL, Kandulski A, Liem S S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Zhang L, Gao XF, Wang YH. [The expression of chemerin and the influence of sitagliptin on its expression in non-alcoholic fatty liver disease rats complicated with prediabetes]. Zhonghua Yi Xue Za Zhi. 2018;98:2407-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Chihara A, Tanaka A, Morimoto T, Sakuma M, Shimabukuro M, Nomiyama T, Arasaki O, Ueda S, Node K. Differences in lipid metabolism between anagliptin and sitagliptin in patients with type 2 diabetes on statin therapy: a secondary analysis of the REASON trial. Cardiovasc Diabetol. 2019;18:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Sekula P, Caputo A, Dunant A, Roujeau JC, Mockenhaupt M, Sidoroff A, Schumacher M. An application of propensity score methods to estimate the treatment effect of corticosteroids in patients with severe cutaneous adverse reactions. Pharmacoepidemiol Drug Saf. 2010;19:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Pratley RE, Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007;23:919-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Al Drees A, Salah Khalil M, Soliman M. Histological and Immunohistochemical Basis of the Effect of Aminoguanidine on Renal Changes Associated with Hemorrhagic Shock in a Rat Model. Acta Histochem Cytochem. 2017;50:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | de Souza Oliveira MA, Dos Santos TOC, Monte JCM, Batista MC, Pereira VG, Dos Santos BFC, Santos OFP, de Souza Durão M. The impact of continuous renal replacement therapy on renal outcomes in dialysis-requiring acute kidney injury may be related to the baseline kidney function. BMC Nephrol. 2017;18:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Muratsubaki S, Kuno A, Tanno M, Miki T, Yano T, Sugawara H, Shibata S, Abe K, Ishikawa S, Ohno K, Kimura Y, Tatekoshi Y, Nakata K, Ohwada W, Mizuno M, Miura T. Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes. Sci Rep. 2017;7:5311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Sun J, Zhang M, Lin Y, Fang L, Fang X, Mai W, Yin Z. The significance of combined detection of CysC, urinary mAlb and β2-MG in diagnosis of the early renal injury in pregnancy-induced hypertension syndrome. Saudi J Biol Sci. 2019;26:1982-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cortes AL, Gonsalez SR, Rioja LS, Oliveira SSC, Santos ALS, Prieto MC, Melo PA, Lara LS. Protective outcomes of low-dose doxycycline on renal function of Wistar rats subjected to acute ischemia/reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Al-Jobori H, Daniele G, Cersosimo E, Triplitt C, Mehta R, Norton L, DeFronzo RA, Abdul-Ghani M. Empagliflozin and Kinetics of Renal Glucose Transport in Healthy Individuals and Individuals With Type 2 Diabetes. Diabetes. 2017;66:1999-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Mavrakanas TA, Aurian-Blajeni DE, Charytan DM. Early versus late initiation of renal replacement therapy in patients with acute kidney injury: a meta-analysis of randomised clinical trials. Swiss Med Wkly. 2017;147:w14507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | MarquezZampiva MM, de Paula H, Cheah CW, Neto TPT, Nakamura KK, Ueda J, Nassar PO, Nassar CA. Clinical Evaluation of Obesity In Patients with Type 2 Diabetes Mellitus after Periodontal Treatment: A Comparative Study. J Int Acad Periodontol. 2019;21:132-138. [PubMed] |
| 13. | Eriguchi M, Lin M, Yamashita M, Zhao TV, Khan Z, Bernstein EA, Gurley SB, Gonzalez-Villalobos RA, Bernstein KE, Giani JF. Renal tubular ACE-mediated tubular injury is the major contributor to microalbuminuria in early diabetic nephropathy. Am J Physiol Renal Physiol. 2018;314:F531-F542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Arsalan M, Ungchusri E, Farkas R, Johnson M, Kim RJ, Filardo G, Pollock BD, Szerlip M, Mack MJ, Holper EM. Novel renal biomarker evaluation for early detection of acute kidney injury after transcatheter aortic valve implantation. Proc (Bayl Univ Med Cent). 2018;31:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ng CF, Luke S, Yee CH, Chu WC, Wong KT, Yuen JW. A Prospective Randomized Study Comparing the Effect of Different Kidney Protection Treatment Protocols on Acute Renal Injury After Extracorporeal Shockwave Lithotripsy. J Endourol. 2017;31:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Patschan D, Schwarze K, Henze E, Patschan S, Scheidemann R, Müller GA. Fibrate treatment of eEOCs in murine AKI. J Nephrol. 2014;27:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Harrill AH, Lin H, Tobacyk J, Seely JC. Mouse population-based evaluation of urinary protein and miRNA biomarker performance associated with cisplatin renal injury. Exp Biol Med (Maywood). 2018;243:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Sibiya N, Ngubane P, Mabandla M. The Ameliorative Effect of Pectin-Insulin Patch On Renal Injury in Streptozotocin-Induced Diabetic Rats. Kidney Blood Press Res. 2017;42:530-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Nezu M, Souma T, Yu L, Suzuki T, Saigusa D, Ito S, Suzuki N, Yamamoto M. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney Int. 2017;91:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Trenchevska O, Nelson RW, Nedelkov D. Mass spectrometric immunoassays for discovery, screening and quantification of clinically relevant proteoforms. Bioanalysis. 2016;8:1623-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |