Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1859
Peer-review started: December 30, 2019
First decision: February 20, 2020
Revised: March 26, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 26, 2020
Processing time: 146 Days and 21.6 Hours
Almost 90% of cerebral thromboembolism cases are caused by atherosclerosis. Craniocervical atherosclerosis is often observed at the carotid bifurcation and is responsible for 20%-30% of all stroke cases. The course of atherosclerotic carotid artery stenosis varies depending on the grade of stenosis and characteristics of the plaque. Carotid artery stenting (CAS) can be used as a less invasive method in patients with symptomatic and asymptomatic high-grade carotid artery stenosis. Diffusion-weighted imaging (DWI) is an effective method for detection of silent or symptomatic acute ischemic lesions that may arise due to CAS or carotid endarterectomy. The number and volume of new ischemic lesions are determined using DWI.
To evaluate the number and volume of ischemic lesions and their cerebral parenchymal and vascular distribution after CAS using DWI.
Forty-seven male (73.4%) and seventeen female (26.6%) patients (total, n = 64) aged 42-84 years (mean 67.96 ± 8.03 years) diagnosed with carotid stenosis between October 2006 and July 2012 were included in this retrospective study. Twelve of the cases (18.8%) were asymptomatic, while fifty-two (81.2%) were symptomatic. The area where the stenosis was highest was measured, and the stenosis rate was determined using the North American Symptomatic Carotid Endarterectomy Trial method. DWI of the cases was evaluated by two radiologists experienced in neuroradiology (B.A. with more than 15 years of experience, E.G. with more than 10 years of experience). Routine DWI examinations were carried out by a 1.5 T MR device 1 h before and after the operation. Since the ischemic lesions that developed in the first hour and in the follow-up period of 5-24 h were assumed to be due to CAS, all lesions within the first 24 h were considered as new ischemias.
In the present study, 39 new ischemic lesions were detected in 20 cases. The average number of new lesions after all CAS operations was 0.62. They were mostly located in the occipital lobes, followed by the frontal and parietal lobes. These new ischemic lesions were most common in the middle cerebral artery territory, followed by the posterior cerebral artery territory and middle cerebral artery-posterior cerebral artery watershed areas. New lesions were found in 31.2% (20/64) of patients, including 17 (26.5%) in ipsilateral and three (4.6%) in contralateral hemispheres. New bilateral lesions were detected in one case (1.5%). The average volume of the new ischemic lesions detected by the two observers was 1.10 cm³. The numbers of newly appearing ischemic lesions in DWI after CAS were significantly higher in cases where stenting was applied on the left side of the carotid artery and in cases where longer plaques (> 1 cm) were responsible for the narrowing in symptomatic patients. The stenosis rate was low in the group with ulcerated plaques.
New ischemic lesions due to CAS appear mostly in the main arterial territory but they may also occur in watershed areas.
Core tip: In this retrospective study, the number, volume and cerebral parenchymal and vascular distributions of new ischemic lesions were investigated after carotid artery stenting with diffusion-weighted imaging. Despite the detection of 39 newly arising ischemic lesions after the carotid artery stenting, there were no neurological findings in 15 out of 64 cases, and they were considered silent ischemias. On the other hand, symptomatic findings were detected in 5 cases after the operation. The present study revealed that new ischemic lesions appear mostly in the main arterial territory and cerebral ipsilateral hemispheres.
- Citation: Beyhan M, Acu B, Gökçe E, Fırat MM. Evaluation of ischemic lesions after carotid artery stenting with diffusion-weighted imaging. World J Clin Cases 2020; 8(10): 1859-1870
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1859.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1859
Atherosclerosis of the carotid artery is a major cause of ischemic cerebrovascular disease[1]. Stenosis in the proximal carotid artery secondary to atherosclerosis is among the leading causes of transient ischemic strokes and cerebral infarction. Carotid endarterectomy (CE) or carotid artery stenting (CAS) are the methods proposed to prevent ischemic strokes[2]. CE is still the best treatment modality for symptomatic patients and patients with carotid stenosis of over 70% (North American Symptomatic Carotid Endarterectomy Trial, NASCET)[3]. On the other hand, CAS should be used in patients with contralateral occlusion that poses high risk in surgery, patients with anatomical variations that cause technical difficulties in surgical accessing [such as high-placement internal carotid artery (ICA) bulb, history of prior neck dissection and presence of tracheostomy and radiation injury] or patients with serious comorbidities[4]. Current medical guidelines suggest the use of embolic protection devices (EPDs) during CAS to prevent periprocedural ischemic events[5].
In diffusion-weighted imaging (DWI), Brownian movement of water in tissues and random movement of water molecules due to their kinetic (thermal) energies are visualized[6]. Higher signal intensity in DWI shows lower water diffusion, which in turn is an early and sensitive indication of brain ischemia that develops as a result of irreversible cellular damage[7]. Therefore, DWI has been proposed as a highly sensitive and robust method for the identification of new ischemic events during CAS[8]. One advantage of using DWI during the interventions is the ability to provide pre- and post-treatment measurements of the lesion for comparison[9]. Most ischemic lesions do not manifest themselves in clinical neurological events and are potentially reversible, as indicated by follow-up examinations using magnetic resonance imaging (MRI)[10]. In the present study, number, volume and cerebral parenchymal and vascular distributions of newly detected ischemic lesions in cases with carotid artery stenosis revealed by DWI after CAS were evaluated retrospectively.
The study included 64 cases that underwent CAS procedures between October 2006 and July 2012 in the Interventional Radiology Unit of the Radiology Department at Tokat Gaziosmanpasa University, Faculty of Medicine. Forty-seven of the cases (73.4%) were male, and seventeen (26.6%) were female. The average age was 67.96 ± 8.03 years (range: 42-84). Twelve of the cases (18.8%) had asymptomatic and fifty-two (81.2%) had symptomatic carotid stenosis. Demographic data and average stenosis rates of the cases with carotid artery stenosis are given in Table 1. The diagnoses of carotid stenosis in asymptomatic cases were made incidentally during examinations for other reasons. The most common comorbid diseases and risk factors in the cases were a history of hypertension (HT), diabetes mellitus (DM) and cerebrovascular disease. Accompanying comorbid diseases and their frequencies in carotid artery stenosis patients are given in Table 2.
| Characteristic | n | % | |
| Gender | Female | 17 | 26.6 |
| Male | 47 | 73.4 | |
| Clinical appearance | Asymptomatic | 12 | 18.8 |
| Symptomatic | 52 | 81.2 | |
| Average stenosis rate | Asymptomatic | - | 68.33 |
| Symptomatic | - | 74.69 | |
| Comorbid diseases and risk factors | n | % |
| Hypertension | 38 | 30.2 |
| Diabetes mellitus | 22 | 17.5 |
| Cerebrovascular disease | 20 | 15.9 |
| Coronary artery disease | 15 | 11.9 |
| Hyperlipidemia | 9 | 7.1 |
| Smoking | 8 | 6.3 |
| Goiter | 5 | 4.0 |
| Chronic obstructive pulmonary disease | 4 | 3.2 |
| Congestive heart failure | 2 | 1.6 |
| Asthma | 1 | 0.8 |
| Glaucoma | 1 | 0.8 |
| Familial mediterranean fever | 1 | 0.8 |
In 5 cases, stenting was performed in different sessions due to bilateral ICA stenosis, and these cases were evaluated as two separate procedures. Upon detection of dissection in the ICA bulb, stenting was performed for one case.
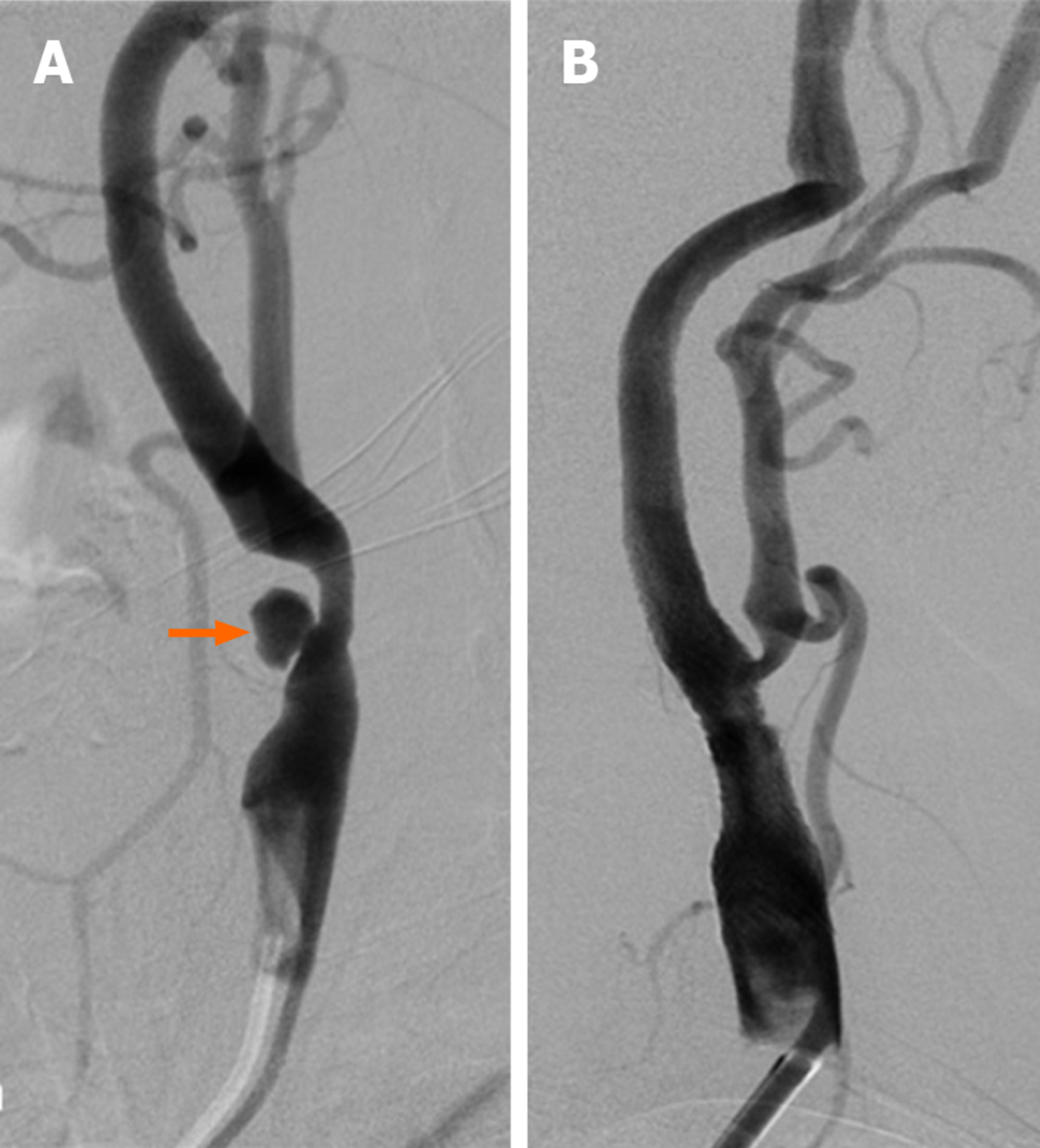
The atherosclerotic plaques in the carotid arteries less than 1 cm were defined as short segments, while those greater than 1 cm were classified as long segments. The surface properties of plaques causing stenosis were also considered using digital subtraction angiography (DSA). These plaques were classified as regular surfaced, irregular surfaced and ulcerated plaques. Ulcerated plaques were defined as extension of contrast media beyond the vascular lumen within the plaque (Figure 1). Data regarding the side and location of carotid artery stenosis and the distribution of atherosclerotic plaque morphology are given in Table 3.
| Parameters | n | % | |
| Side of stenosis | Right | 33 | 51.6 |
| Left | 31 | 48.4 | |
| Location of stenosis | CCA distal and ICA bulb | 6 | 9.3 |
| ICA bulb | 42 | 65.6 | |
| Dissection of ICA bulb | 1 | 1.6 | |
| Distal of ICA bulb | 4 | 6.3 | |
| ICA bulb origin | 3 | 4.6 | |
| Proximal of ICA bulb | 2 | 3.1 | |
| Post-bulbar ICA and bulb | 1 | 1.6 | |
| Petrous ICA | 1 | 1.6 | |
| Post-bulbar ICA | 4 | 6.3 | |
| Plaque morphology | Short segment regular surfaced | 4 | 6.3 |
| Short segment irregular surfaced | 2 | 3.2 | |
| Short segment ulcerated | 2 | 3.2 | |
| Long segment regular surfaced | 19 | 30.1 | |
| Long segment irregular surfaced | 12 | 19.1 | |
| Long segment ulcerated | 24 | 38.1 | |
The study was approved by the Ethics Committee of the Tokat Gaziosmanpasa University Faculty of Medicine (No: B.30.2.GOU.0.01.00.00/219).
CAS procedure was employed because of ICA occlusion in 10 cases and because of serious comorbid diseases and risk factors in other cases. All procedures were performed under the supervision of an anesthesia team. In all cases but one (case No: 4), “Angioguard RX” (Cordis Endovascular, Santa Clara, CA, United States) distal protection devices were used. Dissection, vasospasm or unfavorable anatomy, such as the type and length of aortic arches and excessive tortuosity of the carotid arteries, were the factors limiting the use of EPDs. Therefore, EPD was not used in one case (case No: 4) who underwent CAS because of stenosis due to dissection in ICA bulb. In all other cases, “Angioguard RX” EPDs were used. Stenting was performed to assure that 1-2 cm normal segments in the proximal and distal segments of the plaque were covered. When the targeted stent opening could not be achieved by the radial force of the stent, balloon angioplasty was performed in appropriate cases with post-dilatation (all cases except for No: 4, 11 and 89). Surgical procedure in our patients was completed in about 30 min.
All patients were examined using DSA before CAS. DSA examination was conducted using a GE Innova 3100 (Milwaukee, WI, United States) angiography device. Images were obtained using 1000 × 1000 and 750 × 750 matrices. Stenosis rate was determined by NASCET on DSA. In the NASCET method, stenosis rate was calculated as a percentage using the diameters of ICA in the maximum stenosis area and in a more distal normal area. Stenosis measurements were performed at the segments with the highest stenosis among the images taken from lateral and anterior-posterior projections in “GE Advantage Windows Workstation 4.3.” All cases had two routine DWI examinations, 1 h before and 1 h after the procedure using a 1.5 T MR machine (Signa Excite HD 12.0 M5B software; GE Healthcare, Milwaukee, WI, United States, 2005). A neurovascular head-neck coil (General Electric, 1.5 T, 8 Ch) was used in the imaging. The images were evaluated using DWI and FLAIR sequences (repetition time: 8800, echo time: 155.8, number of extractions: 1.5, slice thickness: 5 mm, gap distance: 1.5 mm, field of view: 24 cm × 24 cm, matrix: 288 × 288). Diffusion gradients were activated in all three orthogonal planes using b = 0 and b = 1000 s/mm² values (in DWI, repetition time: 8000, echo time: 81.5, number of extractions: 1.0, slice thickness: 5 mm, gap distance: 1.5 mm, field of view: 28 cm × 28 cm, matrix: 160 × 160). DWIs obtained before and after CAS were evaluated retrospectively by two radiologists experienced in neuroradiology work in “GE Advantage Windows Workstation 4.2.” Ischemic lesions detected in the DWI taken in the first hour after the operation were considered new lesions. Also, ischemic lesions detected in DWI carried out for symptomatic cases manifesting neurological finding during the follow-ups within 5-24 h were assumed to emerge due to CAS, and they were considered new ischemia. No new lesions were observed in the patients due to CAS after the first 24 h. Ischemic lesions that appeared after the procedure were seen in TRACE images as hyperintense areas, but they were observed as hypointense areas representing diffusion limitations in apparent diffusion coefficient mapping. Using the TRACE images, the lesion volume was calculated as cm3 using “Volume Viewer” software and producing reformat images. Although the size of the ischemic areas was determined using the largest diameters taken in three dimensions, it was considered that the affected area could be determined more accurately through measuring the volume rather than the size since the lesions of cortical-subcortical location have irregular shapes.
Descriptive data were given as mean ± standard deviation, while categorical data were expressed as n (%). χ2 test was used to compare categorical variables between the study groups. The variables with continuous distribution based on expressing normal or non-normal distribution were analyzed using paired samples t test, independent two samples t test or Mann-Whitney U test among two groups and one-way analysis of variance test among three groups. Spearman correlation analysis was used to evaluate the relationships between variables. P values below 0.05 were considered statistically significant. Analyses were performed using SPSS software (IBM SPSS Statistics 19, SPSS Inc., an IBM Co., Armonk, NY, United States).
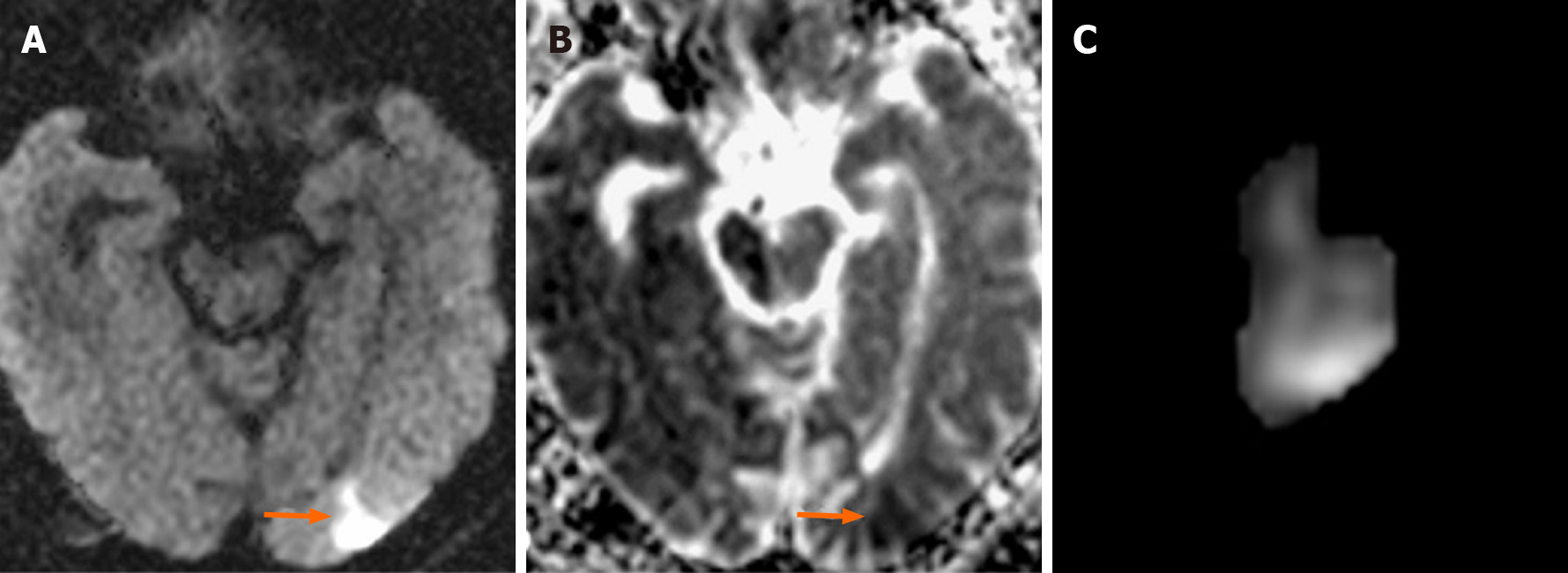
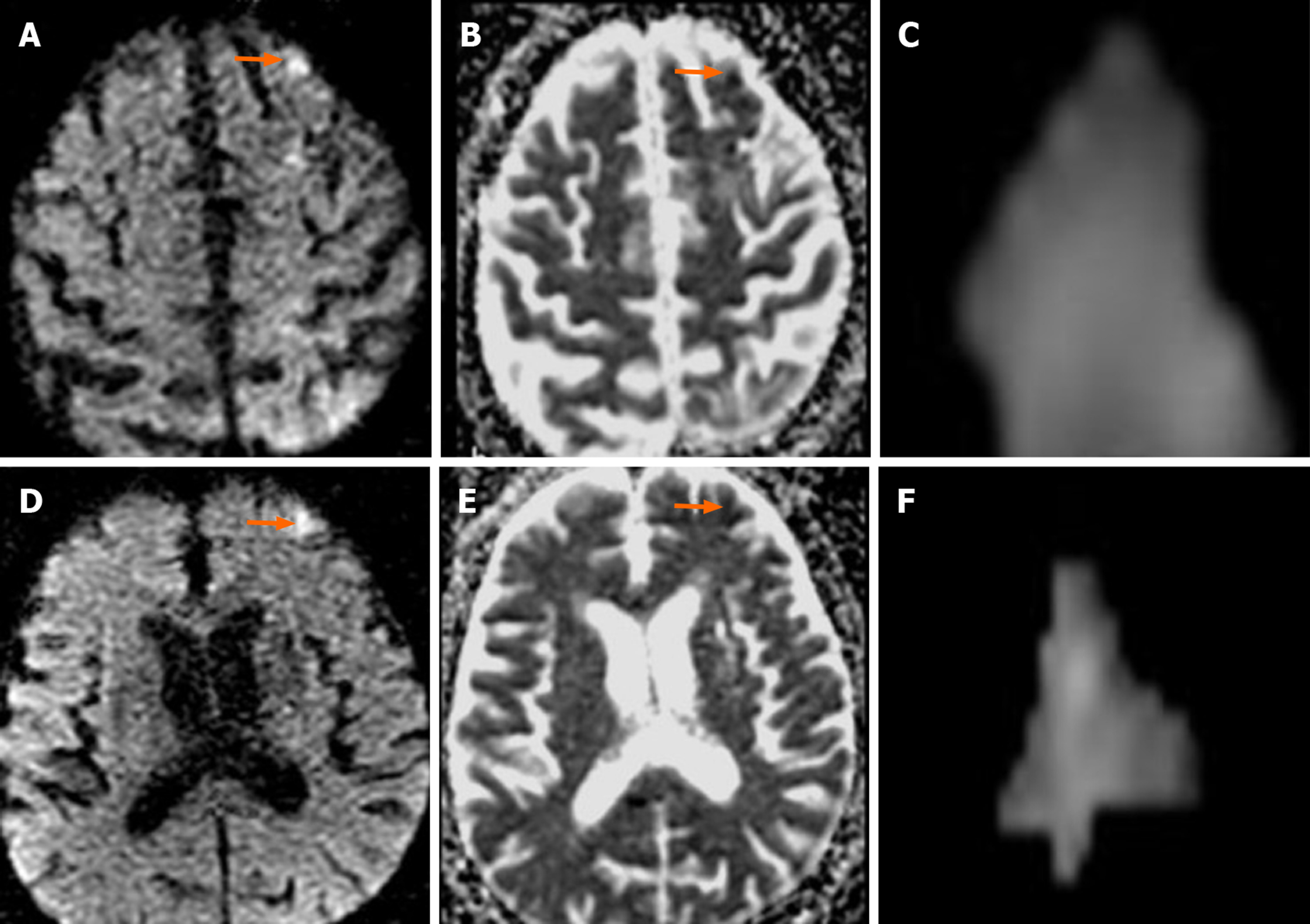
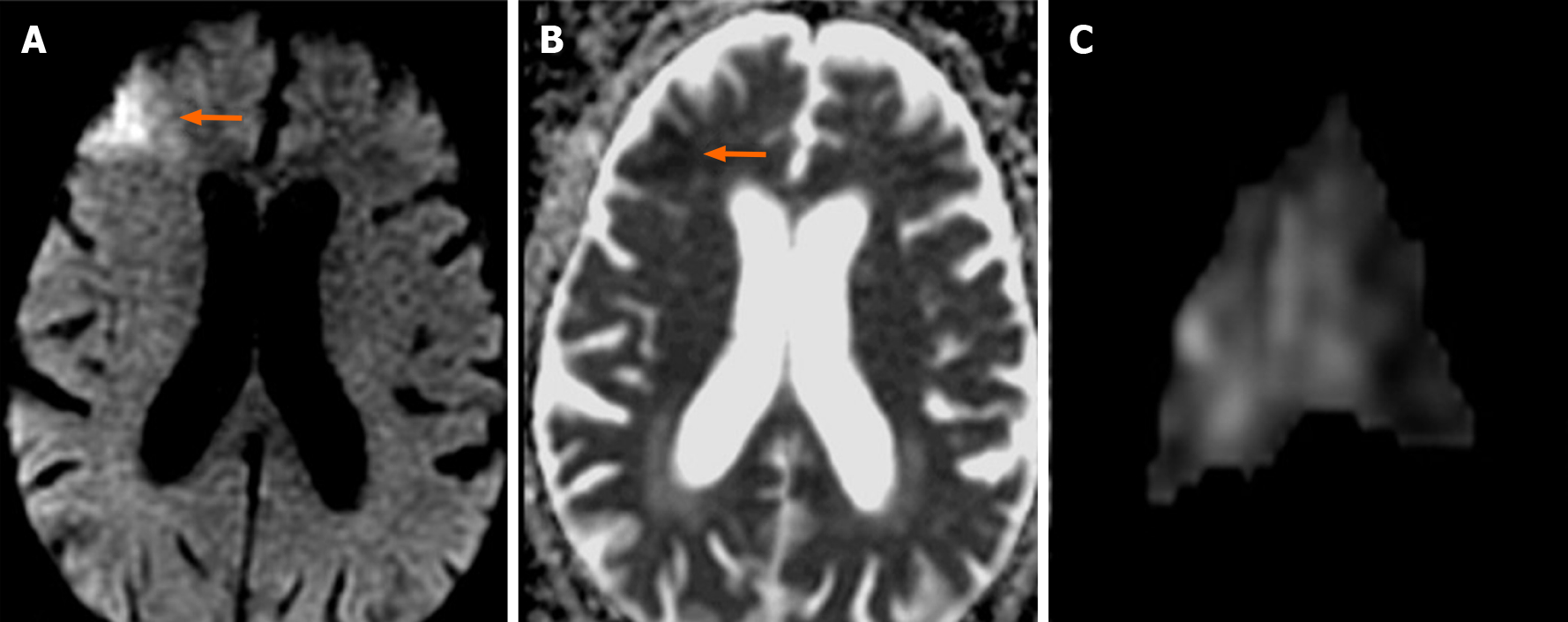
In 20 (31.2%) of the 64 cases on which CAS was performed, 39 new ischemic lesions were observed. When all cases undergoing CAS were evaluated, the mean number of new lesions was 0.62. New ipsilateral lesions (Figures 2 and 3) were found in 17 of 64 cases (26.5%) after CAS, while 3 cases (4.6%) had new contralateral lesions (Figure 4) and one case (1.5%) had bilateral lesions. The average volume of new ischemic lesions calculated by the two radiologists was 1.10 cm³. Lesion numbers, locations and volumes reported by the two observers were not statistically different (P = 0.602; paired samples t test). New ischemic lesions were of occipital and frontal lobe locations and were most common in middle cerebral artery (MCA) and posterior cerebral artery (PCA) territories. Distributions of new ischemic lesions in DWI after CAS by location and vascular distribution areas are given in Table 4. After the CAS procedure, 15 cases (23.4%) were found to have new ischemic lesions without any neurological symptoms and were considered silent ischemias. On the other hand, 5 cases (7.8%) were found to have symptomatic indications after the procedure. Technical details of CAS procedures, ischemic lesion numbers and complications are listed in Table 5. Three of the symptomatic cases healed without sequelae in their follow-ups while sequelae (minimal muscle loss) was observed in 2 patients. In symptomatic patients, time to symptom development, neurological symptoms and prognosis are given in Table 6.
| Parameters | n | % |
| Location of cerebral parenchymal lesion | ||
| Occipital lobe | 11 | 28.2 |
| Frontal lobe | 10 | 25.6 |
| Parietal lobe | 8 | 20.5 |
| Basal ganglions | 3 | 7.7 |
| Cerebellum | 2 | 5.1 |
| Frontoparietal | 1 | 2.6 |
| Parietooccipital | 1 | 2.6 |
| Frontoparietooccipital | 1 | 2.6 |
| Occipitotemporoparietal | 1 | 2.6 |
| Corpus callosum | 1 | 2.6 |
| Cerebral vascular territory | ||
| MCA | 15 | 38.5 |
| PCA | 8 | 20.5 |
| MCA-PCA “watershed” | 7 | 17.9 |
| ACA | 3 | 7.7 |
| ACA-MCA “watershed” | 2 | 5.1 |
| ACA-MCA and MCA-PCA “watershed” | 2 | 5.1 |
| PICA | 1 | 2.6 |
| SCA | 1 | 2.6 |
| Case No. | Age | Gender | Stenosis localization | Balloon angioplasty | Embolic protection device | Ipsilateral lesion | Contralateral lesion | Complication |
| 3 | 59 | M | L ICA bulb | + | + | 5 | - | Silent ischemia |
| 6 | 71 | M | L ICA bulb | + | + | 1 | - | Silent ischemia |
| 8 | 72 | M | L ICA bulb | + | + | 3 | - | Silent ischemia |
| 9 | 71 | F | L ICA bulb | + | + | 2 | - | Silent ischemia |
| 11 | 78 | M | L ICA bulb | - | + | 3 | - | Silent ischemia |
| 13 | 72 | M | L ICA distal to bulb | + | + | 1 | - | Symptomatic ischemia |
| 15 | 71 | M | R ICA bulb | + | + | 1 | - | Silent ischemia |
| 16 | 62 | F | L ICA bulb | + | + | 4 | 3 | Silent ischemia |
| 17 | 75 | F | R ICA bulb | + | + | - | 1 | Silent ischemia |
| 19 | 68 | M | L post-bulbar ICA | + | + | 2 | - | Silent ischemia |
| 23 | 67 | M | R ICA bulb | + | + | 1 | - | Silent ischemia |
| 26 | 84 | M | L ICA bulb | + | + | - | 1 | Symptomatic ischemia |
| 29 | 76 | M | L post-bulbar ICA | + | + | 1 | - | Symptomatic ischemia |
| 36 | 70 | F | R ICA bulb | + | + | - | 1 | Silent ischemia |
| 38 | 76 | M | R ICA bulb | + | + | 1 | - | Silent ischemia |
| 46 | 74 | M | L ICA bulb | + | + | 3 | - | Silent ischemia |
| 52 | 68 | F | R ICA bulb | + | + | 1 | - | Symptomatic ischemia |
| 54 | 72 | F | R ICA bulb | + | + | 2 | - | Silent ischemia |
| 58 | 67 | M | R ICA bulb | + | + | 1 | - | Silent ischemia |
| 59 | 67 | M | L ICA bulb | + | + | 1 | - | Symptomatic ischemia |
| Case No. | Time to symptom development | Neurological symptoms | Prognosis |
| 13 | 1st hour | Loss of upper limb strength, right lower limb hemiparesis | Right upper limb muscle strength 4/5 |
| 26 | 5th hour | Restlessness, agitation | Recovery without sequelae |
| 29 | 19th hour | Motor aphasia, right upper limb muscle strength 2/5, plegia of right leg | Right upper limb muscle strength 4/5, dysdiadochokinesia |
| 52 | 22nd hour | Hemiparesis | Recovery without sequelae |
| 59 | 24th hour | Cloudy vision, vertigo, ataxia | Recovery without sequelae |
Thirty-three plaques causing carotid artery stenosis (51.6%) were in the right carotid artery, while 31 of them (48.4%) were in the left. Most of the plaques were observed in the ICA bulb. In terms of plaque morphologies, it was determined that the majority of plaques causing stenosis were long segmented and ulcerated. In addition, the stenosis rate was higher in the symptomatic group (74.69%) than in the asymptomatic group (68.33%).
Regarding gender distribution, 14 of the new lesions were in men and six in women. The volumes of the lesions were significantly higher in men than in women (P = 0.039; Mann Whitney U test). The number of ischemic lesions observed in DWI was significantly associated with plaque sizes over 1 cm and with stenting in the left carotid artery (P = 0.042; Mann Whitney U test and P = 0.032; independent samples t test, respectively). The average stenosis rate in the carotid artery was 78.87 ± 10.67 in regular-surfaced plaques, 81.43 ± 10.27 in irregular-surfaced plaques and 64.07 ± 16.23 in ulcerated plaques. Carotid stenosis rate was significantly lower in cases with ulcerated plaques (P < 0.001; one-way analysis of variance).
It is recognized that CAS can cause new ischemic lesions detected by DWI in the range of 22%-54%[11]. DWI lesions associated with CAS also occur in the cerebral cortex, mostly in watershed areas[12]. In our study, the prevalence of post-CAS DWI lesions was 31.2%, which was close to the lower limits in the literature. Instead of watershed areas, new ischemic lesions were observed in cerebral cortex, MCA and PCA territories in more than half the cases in the present study.
Despite the advent of stent technology, thromboembolic events remain obstacles to be overcome in endovascular therapy. Although stenotic carotid lesions have fragile thrombotic and atherosclerotic components that are vulnerable to fragmentation, these particulates are responsible for most of the neurological events that can develop during the stenting process[13]. Cerebral embolism may be observed in most diagnostic, invasive and surgical procedures performed in supra-aortic vessels. Most of these embolisms are clinically silent and in only a few cases do clinically persistent strokes develop. For this reason, DWI is used as a marker for clinical strokes after neurovascular interventions. In a study by Hauth et al[14], 22 of 105 cases (21%) developed 64 new ischemic lesions in DWI after CAS procedures. Fifty-five of these lesions were reported as ipsilateral, and nine were contralateral. Lövblad et al[9] found new ipsilateral lesions in 4 of 19 patients, and neurological symptoms were observed in 2 of these 4 patients. Broussalis et al[11] performed CAS for 65 out of 110 cases due to symptomatic carotid stenosis. In their studies, new ischemic lesions were detected in 8 cases after CAS, and all lesions were clinically silent ischemia. Jaeger et al[15] reported new ipsilateral lesions in 20 of 70 cases (29%) after CAS, and new contralateral lesions in 6 cases (9%). They found that the mean number of ipsilateral lesions after the intervention was 2.6, and the number of contralateral lesions was 1.2. Forty-seven of the lesions detected after intervention (80%) were in frontal and parietal lobes. Fifty-two of the 59 newly diagnosed lesions (88%) were found in ACA and MCA territories. In our study, 17 of 64 cases after CAS (26.5%) had new ipsilateral lesions, while 3 cases (4.6%) had new contralateral lesions and one (1.5%) had bilateral lesions. The mean number of ipsilateral lesions after the intervention was 1.94, while that of contralateral ones was 1.5. Twenty-nine of them (74.3%) were in occipital, frontal and parietal lobes. Twenty-three of the 39 new lesions (59%) were in MCA and PCA territories. The higher number of new ischemias observed by Jaeger suggests that process-related microembolisms develop more frequently[15]. Since ischemic lesions were observed in watershed areas in 28.1% of our cases, hemodynamic factors along with the microembolisms were also established as factors for the development of new ischemias.
Jaeger et al[15] showed that wire or catheter manipulations could lead to new lesions. In the present study, the number of new ischemic lesions was higher in cases where stenting was applied to the left carotid artery. Difficulty in accessing the left common carotid artery, longer access time and an increase in the number and length of manipulations may explain the higher number of ischemic lesions on the left.
Mathur et al[16] showed that longer or multiple stenosis events are independent determinants for stroke. Broussalis et al[11] detected plaque ulceration in the carotid artery in 17 out of 107 cases (15.9%). Similarly, the number of new lesions detected by DWI in our study was significantly higher in long plaque segments (> 1 cm). In addition, plaque ulceration was observed in 27 cases (42.2%), and the carotid stenosis rate was lower in these cases. In the group with asymptomatic carotid stenosis, the rate of stenosis was lower, and ulceration was evident in two-thirds of the cases.
It is recognized that the tendency for atherosclerosis is high in men, while in women the tendency increases after menopause and reaches levels close to men in advanced age[17]. Since men constituted three quarters of the cases in the present study, such a finding lends support to the idea that atherosclerosis risk is higher in men.
Using DWI, Taha et al[18] determined 117 new ischemic lesions in 42 of 95 patients after CAS and found that 3 patients had strokes (two minor and one major ischemia) on the ipsilateral side. Clinically silent ischemias were observed in the remaining patients. The formation of new embolic lesions was lower in proximal flow reverse protection compared to double balloon protection (33% vs 48.4%, respectively). However, the respective volumes of the new ipsilateral ischemic lesions were 0.13 cm³ and 0.08 cm³. Ruffino et al[19] found 15 new ipsilateral ischemic lesions in seven of 23 patients after CAS (30.4%) with an average lesion volume of 0.076 cm³. In the present study, however, the average lesion volumes measured by the two observers were 1.10 cm³. The average lesion volumes determined by the two observers were not significantly different.
Yan et al[20] reported that the most common risk factors for the development of ischemia were HT followed by smoking and coronary artery disease. Fukumitsu et al[21], on the other hand, found that the most common risk factors for ischemia development, in decreasing order, were HT, hyperlipidemia and DM. In accordance with the literature, HT was the most common risk factor in the present study for ischemia development followed by DM and cerebrovascular disease history.
The present study has some limitations. First, the failure of the DWI series taken before and after CAS to pass through the same planes might have caused errors in the number, localization and dimensions of the lesions. Second, the patient population was relatively small. Third, although the size was taken as the ischemic area with the largest diameter in three planes, volume measurement was performed rather than size measurement since the lesion shape was irregular especially in cortically-subcortically located lesions. Fourth, the study was retrospective.
In conclusion, new ischemic lesions may appear in the main arterial territories due to CAS. They may also occur in watershed areas. Difficulty in accessing the left common carotid artery, the increased number of manipulations and the long intervention time could explain the high number of ischemic lesions on the left. In addition, more ischemic lesions could be seen in long segment plaques.
Carotid artery stenting (CAS) is a relatively less-invasive method in high grade carotid artery stenosis. New symptomatic or asymptomatic cerebral ischemia lesions can develop due to CAS. Diffusion-weighted imaging (DWI) can be used to determine the number and volume of new ischemic lesions. In the present study, cerebral locations, vascular distribution areas and volumes of ischemic lesions that developed due to CAS were evaluated using DWI.
The frequency and size of new ischemias that can develop due to CAS in cases with carotid artery stenosis can be determined using DWI, and thus precautions can be taken against complications that might arise during the follow-up period.
Cerebral locations, vascular distributions and volumes of new ischemic lesions that developed due to CAS were investigated. In addition, associations between stenosis rate, morphology of the plaque causing the stenosis and new lesion development were studied.
The study included 64 cases that underwent CAS procedures at Tokat Gaziosmanpasa University, Faculty of Medicine, Department of Radiology, between October 2006 and July 2012. Demographic data, average stenosis rate, accompanying comorbid diseases and their frequencies and distribution of atherosclerotic plaque morphology of patients who had DSA due to CAS were evaluated. DWI images after CAS were evaluated retrospectively by two experienced neuroradiologists in a comparative manner. The number of new lesions and their volumes were determined. Associations between the number of new lesions and the side of the stenosis and those between gender and plaque morphologies were studied.
Thirty-nine new lesions were observed in 20 of 64 cases (31.2%) with CAS. The lesions were ipsilateral in 17 cases (26.5%) and contralateral in 3 cases (4.6%). The average volume of the new ischemic lesions was determined as 1.10 cm³ by two observers. The most common locations for the new ischemic lesions were occipital and frontal lobes. In terms of watershed areas, they were common in middle cerebral artery and posterior cerebral artery territories. Silent ischemias were found in 15 cases (23.4%) after CAS procedures, while 5 cases (7.8%) had symptomatic ischemias.
New CAS-originated ischemic lesions are predominantly observed in main arterial territories, but they may also be seen in watershed areas. The higher number of ischemic lesions located on the left side could be explained by the difficulty of accessing the left common carotid artery, the higher number of attempts and longer intervention time. Despite lower stenosis rates, ischemic lesions can be more frequent in ulcerated plaques.
The number, size and localizations of new lesions that can develop due to CAS can be determined through DWI. Thus, arising symptoms can be determined and managed effectively.
We thank Osman Demir for the help with the statistical analyses.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvadori M, Xie Q, Yang L S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Ji A, Lv P, Dai Y, Bai X, Tang X, Fu C, Lin J. Associations between carotid intraplaque hemorrhage and new ipsilateral ischemic lesions after carotid artery stenting: a quantitative study with conventional multi-contrast MRI. Int J Cardiovasc Imaging. 2019;35:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Ichinose N, Hama S, Tsuji T, Soh Z, Hayashi H, Kiura Y, Sakamoto S, Okazaki T, Ishii D, Shinagawa K, Kurisu K. Predicting ischemic stroke after carotid artery stenting based on proximal calcification and the jellyfish sign. J Neurosurg. 2018;128:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Eckstein HH. European Society for Vascular Surgery Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2018;55:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Wabnitz AM, Turan TN. Symptomatic Carotid Artery Stenosis: Surgery, Stenting, or Medical Therapy? Curr Treat Options Cardiovasc Med. 2017;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Stabile E, Sannino A, Schiattarella GG, Gargiulo G, Toscano E, Brevetti L, Scudiero F, Giugliano G, Perrino C, Trimarco B, Esposito G. Cerebral embolic lesions detected with diffusion-weighted magnetic resonance imaging following carotid artery stenting: a meta-analysis of 8 studies comparing filter cerebral protection and proximal balloon occlusion. JACC Cardiovasc Interv. 2014;7:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Battal B, Akgün V, Kocaoğlu M. Diffusion-weighted MRI beyond the central nervous system in children. Diagn Interv Radiol. 2012;18:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Oster J, Doherty C, Grant PE, Simon M, Cole AJ. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44:852-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Aytac E, Gürkaş E, Akpinar CK, Saleem MA, Qureshi AI. Subclinical ischemic events in patients undergoing carotid artery stent placement: comparison of proximal and distal protection techniques. J Neurointerv Surg. 2017;9:933-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lövblad KO, Baird AE, Schlaug G, Benfield A, Siewert B, Voetsch B, Connor A, Burzynski C, Edelman RR, Warach S. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol. 1997;42:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 294] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Huang KL, Ho MY, Chang CH, Ryu SJ, Wong HF, Hsieh IC, Chang TY, Wu TC, Lee TH, Chang YJ. Impact of silent ischemic lesions on cognition following carotid artery stenting. Eur Neurol. 2011;66:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Broussalis E, Griessenauer C, Mutzenbach S, Pikija S, Jansen H, Stevanovic V, Killer-Oberpfalzer M. Reduction of cerebral DWI lesion burden after carotid artery stenting using the CASPER stent system. J Neurointerv Surg. 2019;11:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Park KY, Chung PW, Kim YB, Moon HS, Suh BC, Yoon WT. Post-interventional microembolism: cortical border zone is a preferential site for ischemia. Cerebrovasc Dis. 2011;32:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Macdonald S, Venables GS, Cleveland TJ, Gaines PA. Protected carotid stenting: safety and efficacy of the MedNova NeuroShield filter. J Vasc Surg. 2002;35:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hauth EA, Jansen C, Drescher R, Schwartz M, Forsting M, Jaeger HJ, Mathias KD. MR and clinical follow-up of diffusion-weighted cerebral lesions after carotid artery stenting. AJNR Am J Neuroradiol. 2005;26:2336-2341. [PubMed] |
| 15. | Jaeger HJ, Mathias KD, Hauth E, Drescher R, Gissler HM, Hennigs S, Christmann A. Cerebral ischemia detected with diffusion-weighted MR imaging after stent implantation in the carotid artery. AJNR Am J Neuroradiol. 2002;23:200-207. [PubMed] |
| 16. | Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW, Gomez CR, Yadav JS, Chastain HD, Fox LM, Dean LS, Vitek JJ. Predictors of stroke complicating carotid artery stenting. Circulation. 1998;97:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol. 2014;8:49-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Taha MM, Maeda M, Sakaida H, Kawaguchi K, Toma N, Yamamoto A, Hirose T, Miura Y, Fujimoto M, Matsushima S, Taki W. Cerebral ischemic lesions detected with diffusion-weighted magnetic resonance imaging after carotid artery stenting: Comparison of several anti-embolic protection devices. Neurol Med Chir (Tokyo). 2009;49:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Ruffino MA, Faletti R, Bergamasco L, Fonio P, Righi D. Incidence of New Ischaemic Brain Lesions After Carotid Artery Stenting with the Micromesh Roadsaver Carotid Artery Stent: A Prospective Single-Centre Study. Cardiovasc Intervent Radiol. 2016;39:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Yan D, Tang X, Shi Z, Wang L, Lin C, Guo D, Fu W. Perioperative and Follow-up Results of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Carotid Near-Occlusion. Ann Vasc Surg. 2019;59:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fukumitsu R, Yoshida K, Kurosaki Y, Torihashi K, Sadamasa N, Koyanagi M, Narumi O, Sato T, Chin M, Handa A, Yamagata S, Miyamoto S. Short-Term Results of Carotid Endarterectomy and Stenting After the Introduction of Carotid Magnetic Resonance Imaging: A Single-Institution Retrospective Study. World Neurosurg. 2017;101:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |