Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.97
Peer-review started: September 11, 2019
First decision: October 24, 2019
Revised: November 5, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: January 6, 2020
Processing time: 117 Days and 22.2 Hours
Few studies have addressed the efficacy of pembrolizumab in pulmonary sarcomatoid carcinoma (PSC), a rare, previously rapidly fatal subtype of non-small-cell lung cancer.
We report the case of a 69-year-old man presented with respiratory distress caused by a large left upper lung lobe mass diagnosed as PSC with programmed death-ligand 1 expressed on more than 50 percent of tumor cells. The patient was started on pembrolizumab and, after 5 cycles, there was a more than 80 percent decrease in the size of the tumor mass. Further decrease was seen at the end of 10 cycles. The patient has been tolerating pembrolizumab well, with no limiting side-effects. Fourteen months after first coming into the hospital, he remains asymptomatic.
Pembrolizumab appears as a viable emerging treatment for PSC.
Core tip: Pulmonary sarcomatoid carcinoma (PSC) is classified as a rare, aggressive subtype of non-small-cell lung cancer. In recent years, pembrolizumab, a humanized monoclonal IgG-kappa isotype antibody against the programmed death-1 receptor, has become the first-line treatment for NSCLE with programmed death-ligand-1 (PD-L1) expression on at least 50% of tumor cells. We report the case of an elderly man diagnosed with invasive PSC with PD-L1 greater than 50%. The patient experienced a highly positive response to pembrolizumab. The recommendation to treat PSC with pembrolizumab is supported by the small number of published papers available in the English literature.
- Citation: Cimpeanu E, Ahmed J, Zafar W, DeMarinis A, Bardarov SS, Salman S, Bloomfield D. Pembrolizumab - emerging treatment of pulmonary sarcomatoid carcinoma: A case report. World J Clin Cases 2020; 8(1): 97-102
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/97.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.97
Pulmonary sarcomatoid carcinoma (PSC) represents a high-grade histologic subtype of non-small-cell lung cancer (NSCLC), accounting for only about 1% of NSCLC and 0.4% of all lung cancers in the United States[1,2]. Most are diagnosed at advanced stages and have an aggressive clinical course and lower overall survival than other histologic subtypes, even on the rare occasions when discovered incipiently[2]. Management of metastatic disease has been challenging, owing to high rates of resistance to conventional platinum-based chemotherapy, which, up to recently, was the preferred treatment option for all metastatic types[2-4]. While response to pembrolizumab in the more common subtypes of NSCLC has been reported by several studies, very few have addressed the efficacy of pembrolizumab in PSC. We report a case of excellent response to pembrolizumab in a patient with PSC characterized by programmed death-ligand 1 (PD-L1) expression greater than 50%.
A 69-year-old man presented with one week’s duration of respiratory distress and diffuse, intermittent and non-radiating left upper chest pain.
The patient began experiencing occasional dry cough two months prior to presentation but denied having any other symptoms.
There was a past medical history of 60 pack-year smoking, hypertension, diabetes mellitus type II, hypothyroidism, Parkinson’s disease and depression.
The patient had labored and irregular breathing but did not use the accessory muscles of respiration. There was tenderness to palpation of the left chest wall and dullness to percussion in the left upper lung field, with decreased breath sounds, rhonchi and slight wheezing. There was mild diffuse abdominal tenderness, without guarding.
Laboratory workup revealed white blood cell count 22.9 K/uL, hemoglobin 10.8 g/dL, hematocrit 37.0%, platelet count 943 K/uL, erythrocyte sedimentation rate 130, serum potassium 5.4 mmol/L and serum calcium 11.6 mg/dL.
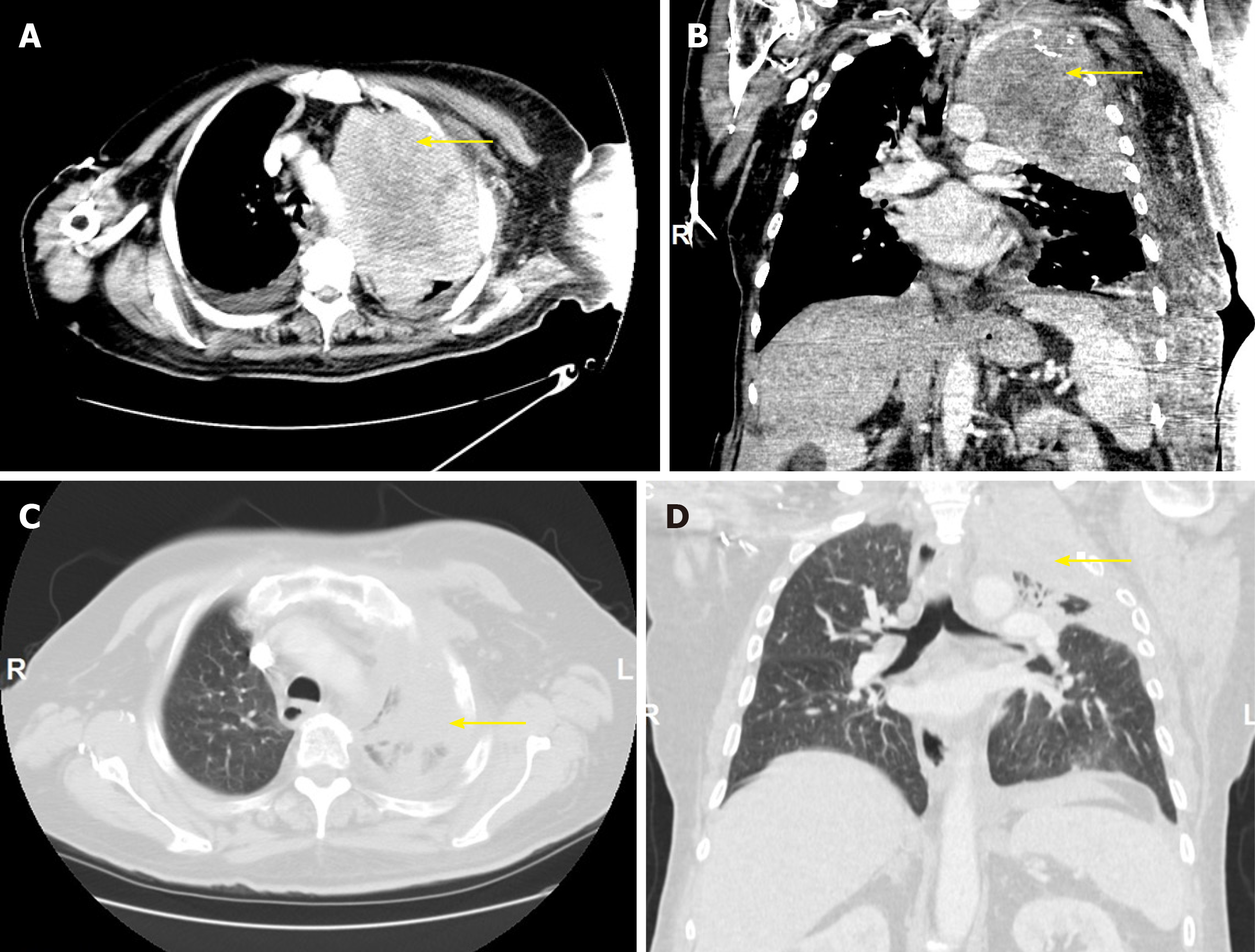
Chest computed tomography (CT) demonstrated a solid, heterogeneous, partially necrotic mass occupying the left upper lobe, encasing the left subclavian artery and extending into the left mediastinum (Figure 1A, 1B). Abdominal and pelvic CT showed hepatomegaly. There was extensive destruction of the left first rib, with less severe involvement of the left second rib, but no evidence of mediastinal, hilar or axillary adenopathy.
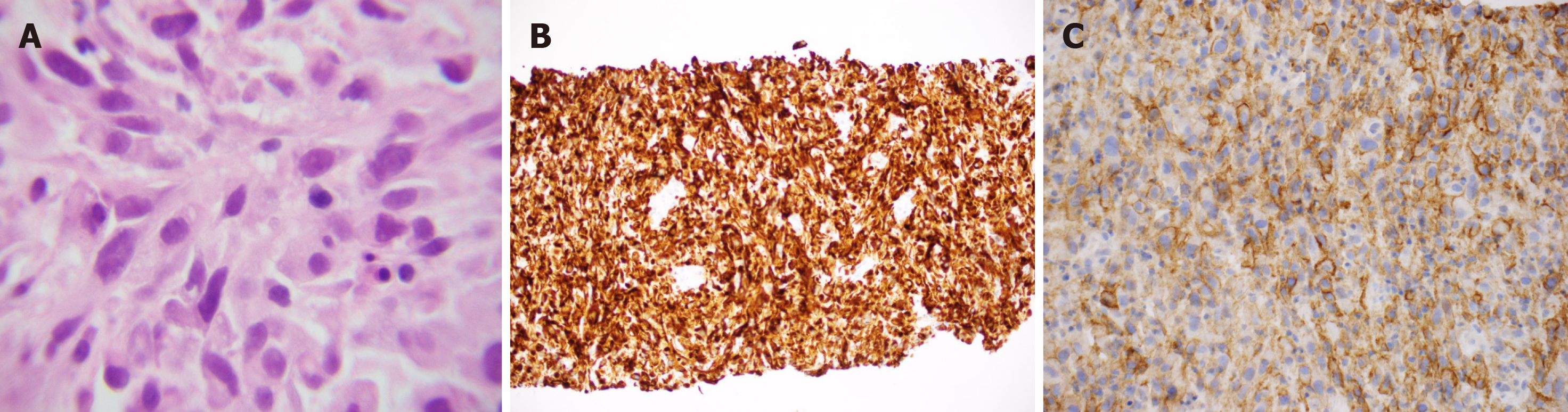
Biopsy of the mass revealed a poorly differentiated neoplasm composed predominantly of spindle cells, with rare epithelioid cells and large bizarre nuclei (Figure 2A). The immunohistochemical analysis of the lesion demonstrated that the neoplastic cells were positive for cytokeratin-7 (CK-7) (Figure 2B) but negative for thyroid transcription factor-1, p40, CK20, prostate-specific antigen, and MelanA. In addition, immunohistochemical stains for mesothelial origin, specifically Calretinin, CK5/6 and podoplanin (D2-40) were negative. The negative p40 and CK5/6 also ruled out sarcomatoid squamous cell carcinoma. The neoplastic cells tested positive for PD-L1, with a tumor proportion score greater than 50% (Figure 2C). No mutations in epidermal growth factor receptor (EGFR) exons 18, 19 or 21 and KRAS codons 12, 13 or 61 were present. An exon 20 insertion was identified but the mass was EGFR T790M-negative. Anaplastic lymphoma kinase (ALK) and receptor tyrosine kinase (ROS) translocations were not performed since EGFR and KRAS mutations are mutually exclusive with these translocations. The tumor was classified as stage IIIa (T4N1M0) PSC.
IV fluids, Pamidronate and antibiotics were administered, and the patient’s condition stabilized. Given multiple medical comorbidities, surgical debulking was not feasible. As the tumor was causing airway compromise, palliative radiation therapy was initiated. The patient was also started on pembrolizumab (200 mg) every 21 d.
A repeat CT scan of the chest after 5 cycles of pembrolizumab showed a decrease of more than 80 percent in the size of the tumor mass (Figure 1C, 1D).
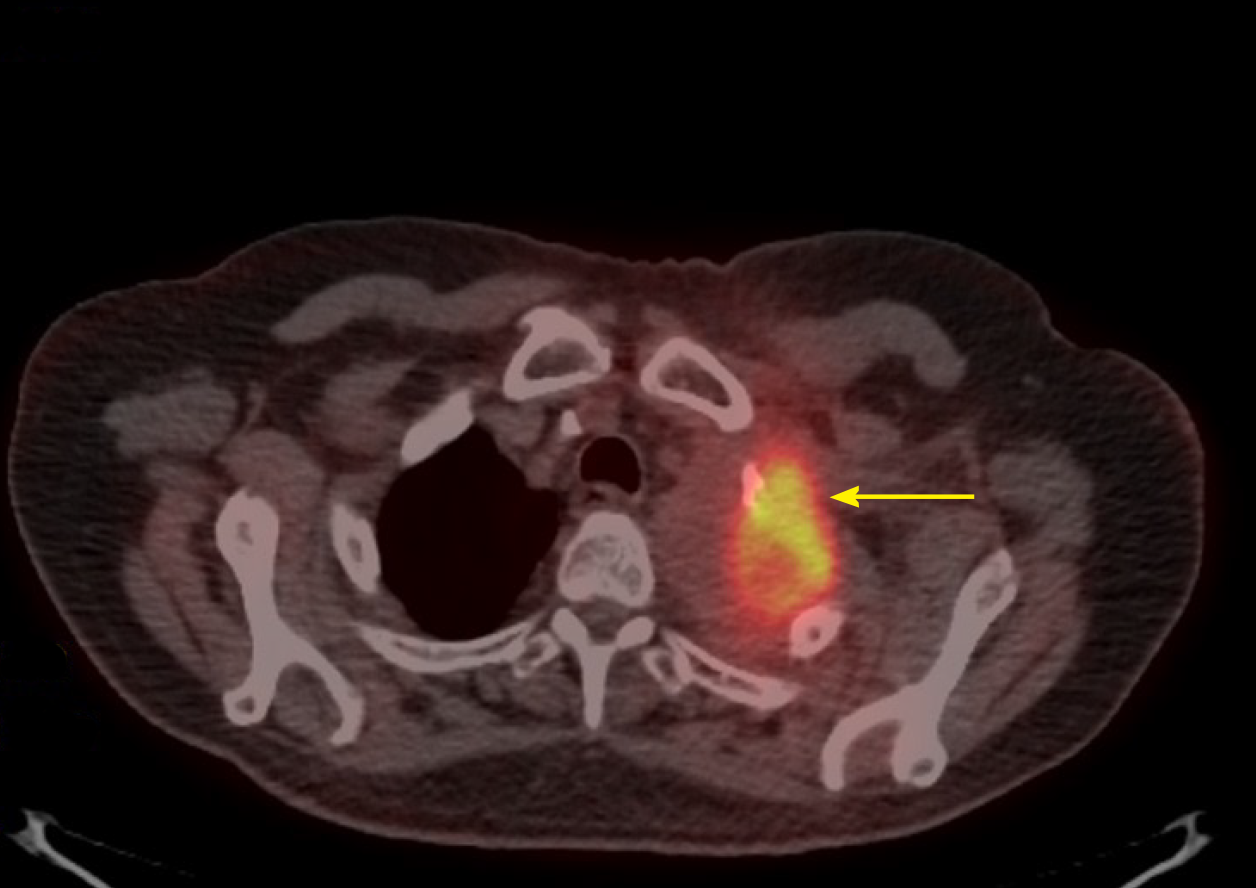
Positron emission tomography-CT (PET-CT) scan at the end of 10 cycles showed an even further decrease (Figure 3). The patient has been tolerating pembrolizumab well, with no limiting side-effects and a plan was made to continue the same treatment. At present, 14 mo after first coming into the hospital, he remains asymptomatic.
When diagnosed, PSCs are frequently bulky, peripherally located and already metastatic, with poor prognosis[1]. For a patient like ours, with stage III tumor, overall survival is estimated at 5.8 mo, whereas for stages I-II it is 16.9 mo and for stage IV 5.4 mo[5]. The typical patient has a history of heavy smoking[1]. PSCs are more widespread in Caucasians (89%) and males (59%)[5]. The mean age at diagnosis is 70 years[5]. Our patient fits these exact demographics - male, Caucasian, heavy smoker, in his late 60 s and with an advanced malignancy. Improved survival in PSC is seen when tumors are localized, amenable to complete surgical resection, 4 cm or less in size, and when patients are not underweight or anemic[6]. Our patient was not underweight but lacked other positive prognostic factors. He was, in fact, anemic and had a large, locally-invasive tumor, which put him at increased risk for a less favorable outcome.
Platinum-based chemotherapy has proven disappointing in PSC, with most patients (69%) experiencing disease progression and overall survival being only slightly increased compared to the non-platinum group (7.0 vs 5.3 mo)[3]. Compared to patients not receiving any treatment, platinum-based chemotherapy resulted in a median overall survival of only 51 d longer[7]. Decreased survival in PSC has been largely attributed to its aggressive nature as well as chemoresistance[1]. The marginal performance of available treatment options warranted a need for new therapeutic strategies.
The introduction of pembrolizumab, a monoclonal IgG4 kappa isotype antibody against the Programmed Death 1 pathway, for NSCLC lacking targetable EGFR or ALK mutations has resulted in improved overall survival and progression-free survival for NSCLC with PD-L1 on at least 50% of tumor cells[4,8]. Pembrolizumab has become the first-line treatment for such tumor[4]. KEYNOTE studies (021, 024 and 189) all showed improved treatment response when pembrolizumab was added to platinum-based chemotherapy[4,9,10]. In addition, patients on pembrolizumab benefited from increased overall survival, greater response rate, longer duration of response and fewer adverse effects secondary to treatment[10]. However, the application of pembrolizumab for PSC has been minimally reported. On a Pubmed search, there are three other individual cases published supporting our contention that pembrolizumab is effective in this previously rapidly fatal tumor[11-13]. There are six other cases in which a form of immunotherapy has been used, however, the outcome is unclear[14,15].
For PSCs with mutated EGFR, EGFR tyrosine kinase inhibitors (TKIs) can be a more suitable treatment option[16]. Third generation EGFR-TKIs have proven efficacious in tumors with EGFR mutations in exons 19 and 21 as well as exon 20 T790M mutations[17]. Osimertinib, a third-generation EGFR-TKI, is particularly indicated for EGFR-mutant NSCLC with an acquired T790M resistance mutation, progressing during or following treatment with EGFR-TKIs[17]. Our patient lacked EGFR targetable mutations. The tumor was in fact positive for an EGFR exon 20 insertion, which is seen in about 9% of all EGFR-mutated tumors and has been linked to de-novo resistance to EGFR-TKI[18]. For these reasons, EGFR-TKIs were not an appropriate choice.
The efficacy of pembrolizumab in the treatment of PSC has not been adequately studied. In our patient, it was proven a highly beneficial form of treatment. He continued to be asymptomatic, more than 14 mo after presentation. To date, this is one of the most sustained responses of PSC to an immune checkpoint inhibitor reported in the English literature. For PSC patients with PD-L1 expression on 50% or more of tumor cells, pembrolizumab is a viable option.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vanoli A S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Antoine M, Vieira T, Fallet V, Hamard C, Duruisseaux M, Cadranel J, Wislez M. [Pulmonary sarcomatoid carcinoma]. Ann Pathol. 2016;36:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, Demmy TL, Groman A, Reid M. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Vieira T, Girard N, Ung M, Monnet I, Cazes A, Bonnette P, Duruisseaux M, Mazieres J, Antoine M, Cadranel J, Wislez M. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8:1574-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4811] [Article Influence: 687.3] [Reference Citation Analysis (0)] |
| 5. | Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, Shin DM, Pillai RN, Pakkala S, Owonikoko TK, Khuri FR, Ramalingam SS. Pulmonary Sarcomatoid Carcinoma: An Analysis of the National Cancer Data Base. Clin Lung Cancer. 2017;18:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Lin Y, Yang H, Cai Q, Wang D, Rao H, Lin S, Long H, Fu J, Zhang L, Lin P, Xu G, Rong T, Xiong X, Ma G, Liang Y. Characteristics and Prognostic Analysis of 69 Patients With Pulmonary Sarcomatoid Carcinoma. Am J Clin Oncol. 2016;39:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Karim NA, Schuster J, Eldessouki I, Gaber O, Namad T, Wang J, Xie C, Morris JC. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget. 2018;9:4102-4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7247] [Article Influence: 724.7] [Reference Citation Analysis (0)] |
| 9. | Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L; KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 1157] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 10. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7532] [Article Influence: 836.9] [Reference Citation Analysis (0)] |
| 11. | Matsumoto Y, Miura T, Horiuchi H, Usui K. The Successful Treatment of Pulmonary Pleomorphic Carcinoma with Pembrolizumab: A Case Report. Case Rep Oncol. 2017;10:752-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ikematsu Y, Yoneshima Y, Ijichi K, Tanaka K, Harada T, Oda Y, Nakanishi Y, Okamoto I. Marked response to pembrolizumab in a patient with pulmonary pleomorphic carcinoma highly positive for PD-L1. Lung Cancer. 2017;112:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Tokuyasu H, Ishikawa S, Sakai H, Ikeuchi T, Miura H. Single pembrolizumab treatment causing profound durable response in a patient with pulmonary pleomorphic carcinoma. Respir Med Case Rep. 2019;28:100879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kotlowska MP, Rueda AG, Olmedo ME, Benito A, Roldán AS, Fernandez Méndez MA, Gorospe L, Palacios J, Garrido López P. Efficacy of immunotherapy in sarcomatoid lung cancer, a case report and literature review. Respir Med Case Rep. 2019;26:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Sukrithan V, Sandler J, Gucalp RA, Gralla RJ, Halmos B. Responses to immune checkpoint therapy in pulmonary sarcomatoid carcinoma: A retrospective review. J Clin Oncol. 2019;37:115-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Tiefenbacher A, Pirker R. EGFR tyrosine kinase inhibitors as first-line therapy in advanced EGFR mutation-positive non-small cell lung cancer: strategies to improve clinical outcome. J Thorac Dis. 2017;9:4208-4211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29:i20-i27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, Zakowski MF, Kris MG, Ladanyi M. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |