Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.234
Peer-review started: October 22, 2019
First decision: November 11, 2019
Revised: November 14, 2019
Accepted: November 27, 2019
Article in press: November 27, 2019
Published online: January 6, 2020
Processing time: 77 Days and 0.3 Hours
Primary intestinal extranodal natural killer/T-cell lymphoma, nasal type (PI-ENKTCL) is a rare non-Hodgkin’s lymphoma (NHL) subtype, and its prognosis is extremely poor. Clinical characteristics of the disease are not obvious and easily misdiagnosed. In this case report, we describe a patient with PI-ENKTCL who presented with intermittent hematochezia. The advantages of positron emission tomography/computed tomography (PET-CT) as a useful diagnostic tool and the role of surgery as an important therapy are highlighted.
A 45-year-old man, hospitalized due to intermittent hematochezia, underwent gastroscopy, colonoscopy, biopsy and CT, but no cause was found. Hence, we carried out a multidisciplinary team (MDT) discussion on the causes and treatment of this patient, and it was decided to perform PET-CT imaging with a MDT discussion of the results. PET-CT demonstrated a diagnosis of lymphoma and it was decided to surgically resect the lesion, and a R0 resection was successfully performed. Postoperative pathology showed negative resection margins, and examination of the lesion confirmed the diagnosis of PI-ENKTCL. After surgery, the patient underwent a follow-up period of 6 mo and received 6 cycles of gemcitabine, oxaliplatin and L-asparaginase. No recurrence or metastasis occurred.
PI-ENKTCL is rare, and MDT discussion is required during diagnosis. PET-CT can be performed for imaging diagnosis. Treatment is based on surgical resection, and the best treatment regimen is determined according to postoperative pathological results to improve prognosis and to extend survival in patients.
Core tip: Primary intestinal extranodal natural killer/T-cell lymphoma, nasal type (PI-ENKTCL) is a very rare disease and its prognosis is extremely poor. Due to its rarity, PI-ENKTCL has not been thoroughly investigated. Moreover, knowledge of its clinical characteristics is limited. Hence, we report a patient with PI-ENKTCL and review the corresponding literature to improve our knowledge of this disease and avoid misdiagnosis.
- Citation: Dong BL, Dong XH, Zhao HQ, Gao P, Yang XJ. Primary intestinal extranodal natural killer/T-cell lymphoma, nasal type: A case report. World J Clin Cases 2020; 8(1): 234-241
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/234.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.234
Primary intestinal extranodal natural killer/T-cell lymphoma, nasal type (PI-ENKTCL) is a rare non-Hodgkin’s lymphoma (NHL) subtype, accounting for 3.1% of cases, and its prognosis is extremely poor[1]. As only a few cases have been reported and the majority have been in the form of national and international case reports and case reviews, there is no unified treatment regimen; moreover, knowledge of the clinical characteristics of this disease is limited. This poses a challenge for early diagnosis, which may be one of the key reasons for delayed diagnosis and poor prognosis[2]. Herein, we report a patient with PI-ENKTCL who was treated at our hospital in January 2019. By combining this report with national and international literature, we analyzed the clinicopathological and immunological characteristics of PI-ENKTCL to improve awareness of this tumor type and to provide a reference for accurate early diagnosis and treatment.
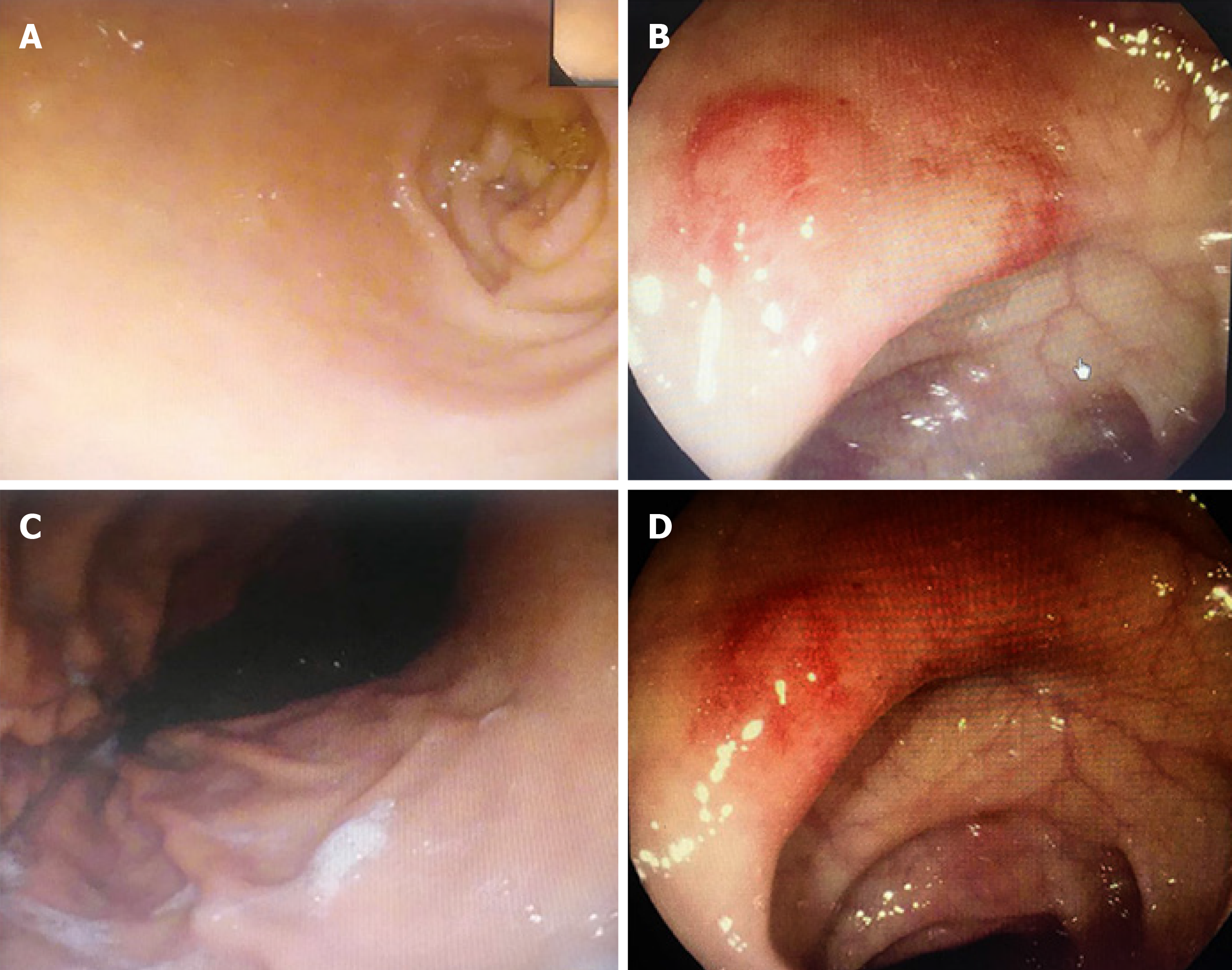
The patient was a 45-year-old man who was referred to our department in January 2019 due to intermittent hematochezia for 20 days and recurrence and exacerbation for 1 d. Twenty days prior to referral, he experienced intermittent hematochezia, with a high volume of blood, for no apparent reason, which spontaneously stopped without treatment. He sought medical attention at our gastroenterology department where gastroscopy, colonoscopy, biopsy and abdominal computed tomography (CT) were performed. Pathology showed the following results: “superficial gastritis (Figure 1A and B), (transverse colon) necrosis, inflammatory exudates, and granulomatous tissue” (Figure 1C and D). Abdominal CT showed local intestinal wall changes at the descending colon, and he was diagnosed with gastrointestinal bleeding (unknown cause). One day prior to referral, the patient experienced large volume hematochezia and was transferred to our department to determine the etiology of bleeding. Hemostasis, supplementation of blood volume, and other symptomatic treatments were performed. Relevant examinations were conducted to obtain a definitive diagnosis and for further treatment measures.
The patient’s vital signs were as follow: Temperature: 36.5°C, pulse rate: 78 bpm, respiratory rate: 19 breaths/min, and blood pressure: 108/56 mmHg. Physical examination showed the following: Anemic countenance; absence of superficial lymph node enlargement and heart abnormalities; abdomen flat; liver and spleen not palpated below the costal margin; hypogastric region tenderness present; shifting dullness absent; borborygmus of 5 times/min, with no increase or decrease; and gurgles and abdominal vascular murmurs not audible.
Laboratory tests showed the following results: White blood cell count: 7.1 × 109/L; red blood cell count: 2.91 × 1012/L; hemoglobin: 86 g/L; platelet count: 223 × 109/L; liver and kidney function normal; lactate dehydrogenase (LDH): 643 U/L; prothrombin time: 16.5 s; fibrinogen: 4.14 g/L; erythrocyte sedimentation rate: 84 mm/h; tumor marker CA-125: 96.90 U/mL (normal < 35 U/mL); remaining parameters, normal range; anti-Epstein-Barr virus (EBV) capsid antigen IgG, positive; tuberculosis antibodies and tubercle specific immune responses, negative; and immune function, normal.
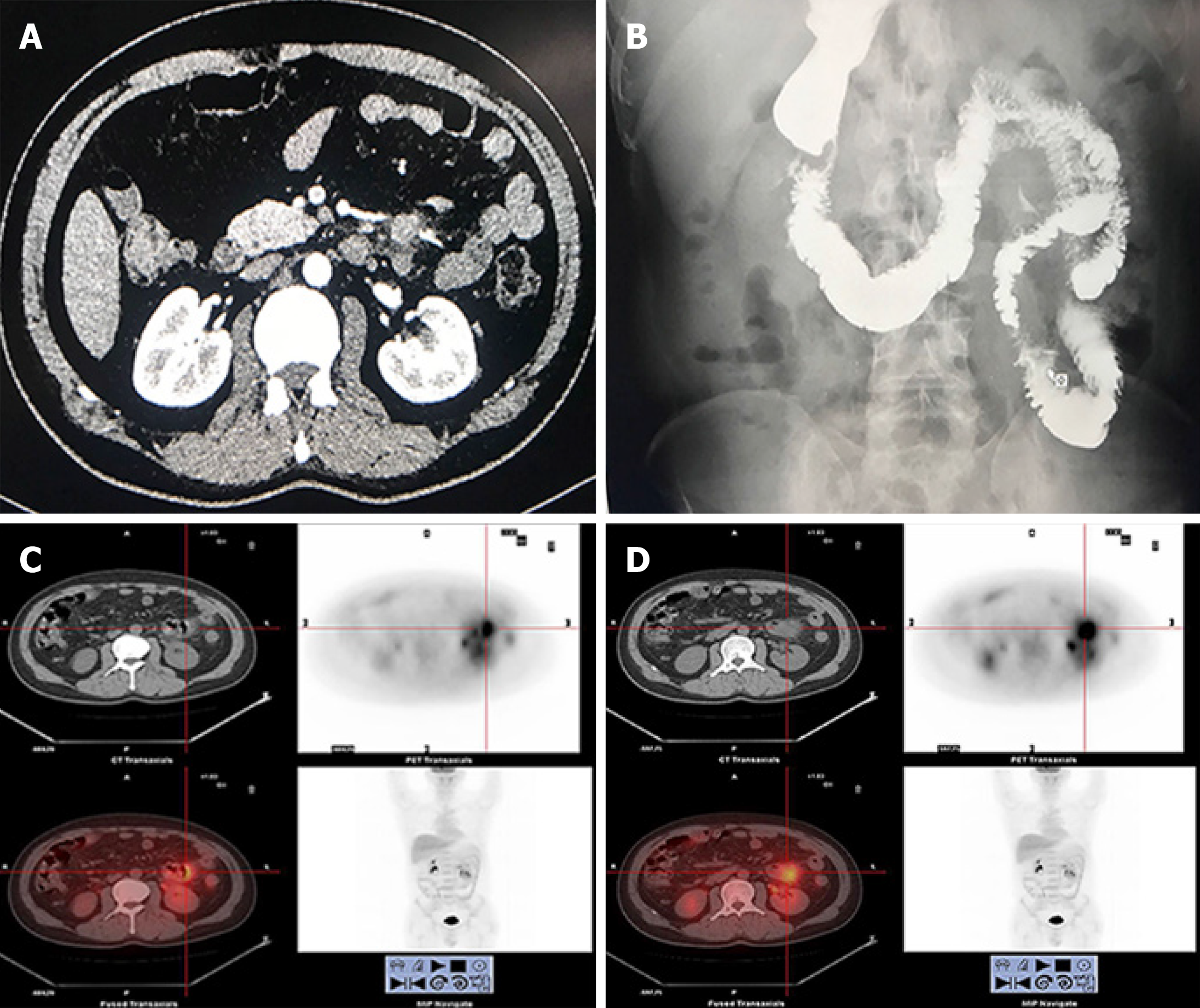
Abdominal CT showed local intestinal wall changes at the descending colon (Figure 2A). Digestive tract imaging showed local space-occupying lesion near the jejunum without obstruction (Figure 2B). Positron emission tomography (PET)-CT showed malignant small intestine lesions (suspected lymphoma), along with local lymph node metastases (Figure 2C, D).
Two days before surgery, the patient was given liquid food. He fasted for 8 h before surgery, and 2 g of cefotiam was administered intravenously 30 min before surgery to prevent infection. After surgery, a patient-controlled analgesic pump was provided; hemostasis, electrocardiography, and nutrient, water, and electrolyte supplementation were performed. Routine blood and biochemical markers were monitored.
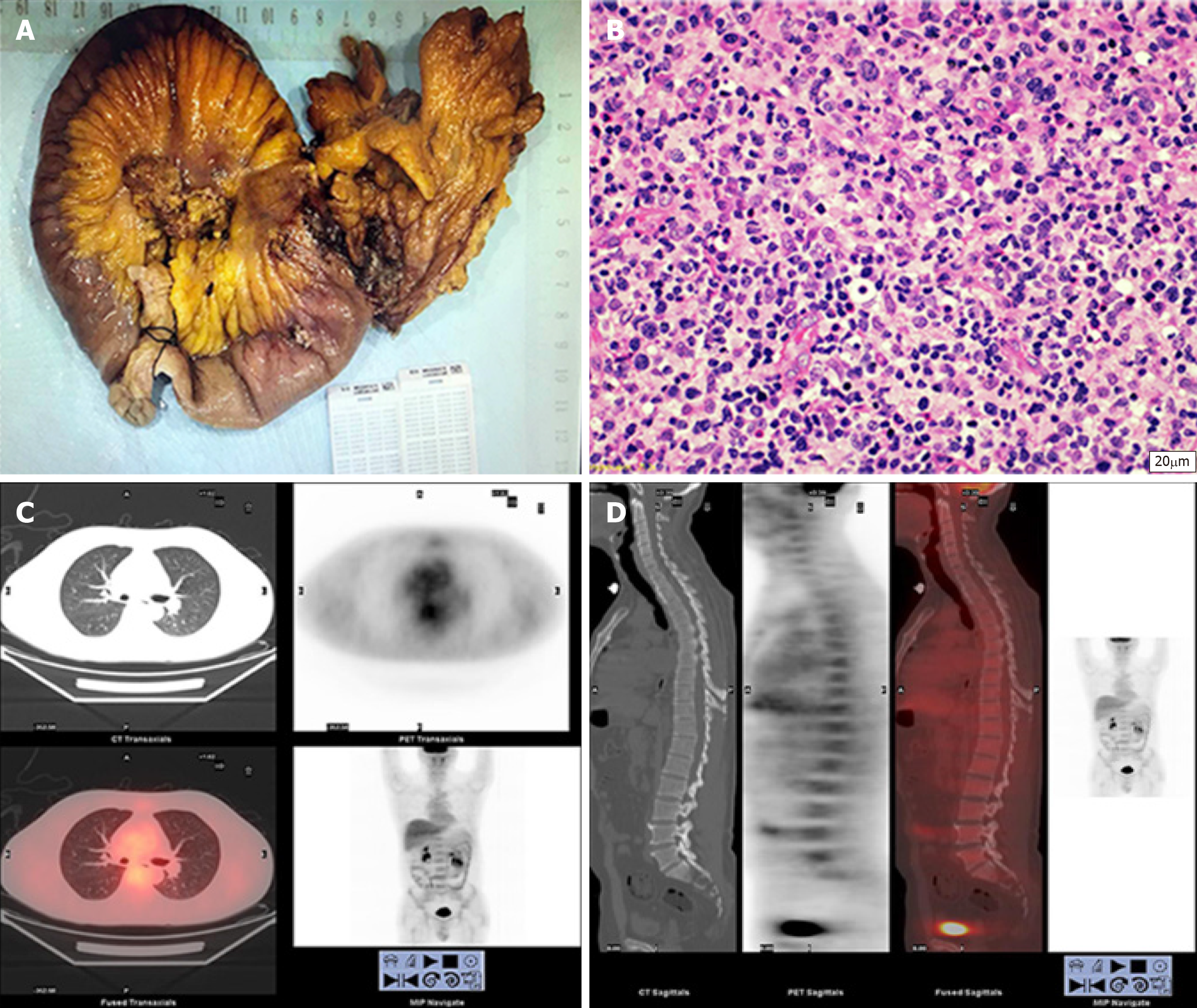
Following general anesthesia, the patient was placed in the supine position, followed by disinfection and trocar insertion. Examination found a 3 cm × 2 cm × 1 cm primary tumor in the jejunum 10 cm from the ligament of Treitz. The tumor had invaded the nearby descending colon to form a mass. The proximal jejunum 80 cm from the ligament of Treitz was dilated, and the distal small intestine was empty (Figure 3A). The surrounding organs were dissected, and the lesion-containing jejunum and descending colon were resected. A jejuno-jejunal anastomosis and an anastomosis between the residual ends of the descending colon were made. The surgery was successful, and the tumor was completely resected. The duration of surgery was 280 min, and intraoperative blood loss volume was 50 mL. Postoperative pathology tests showed that the resection margin was negative. No apparent surgical complications occurred after surgery and the patient was discharged 13 d after surgery.
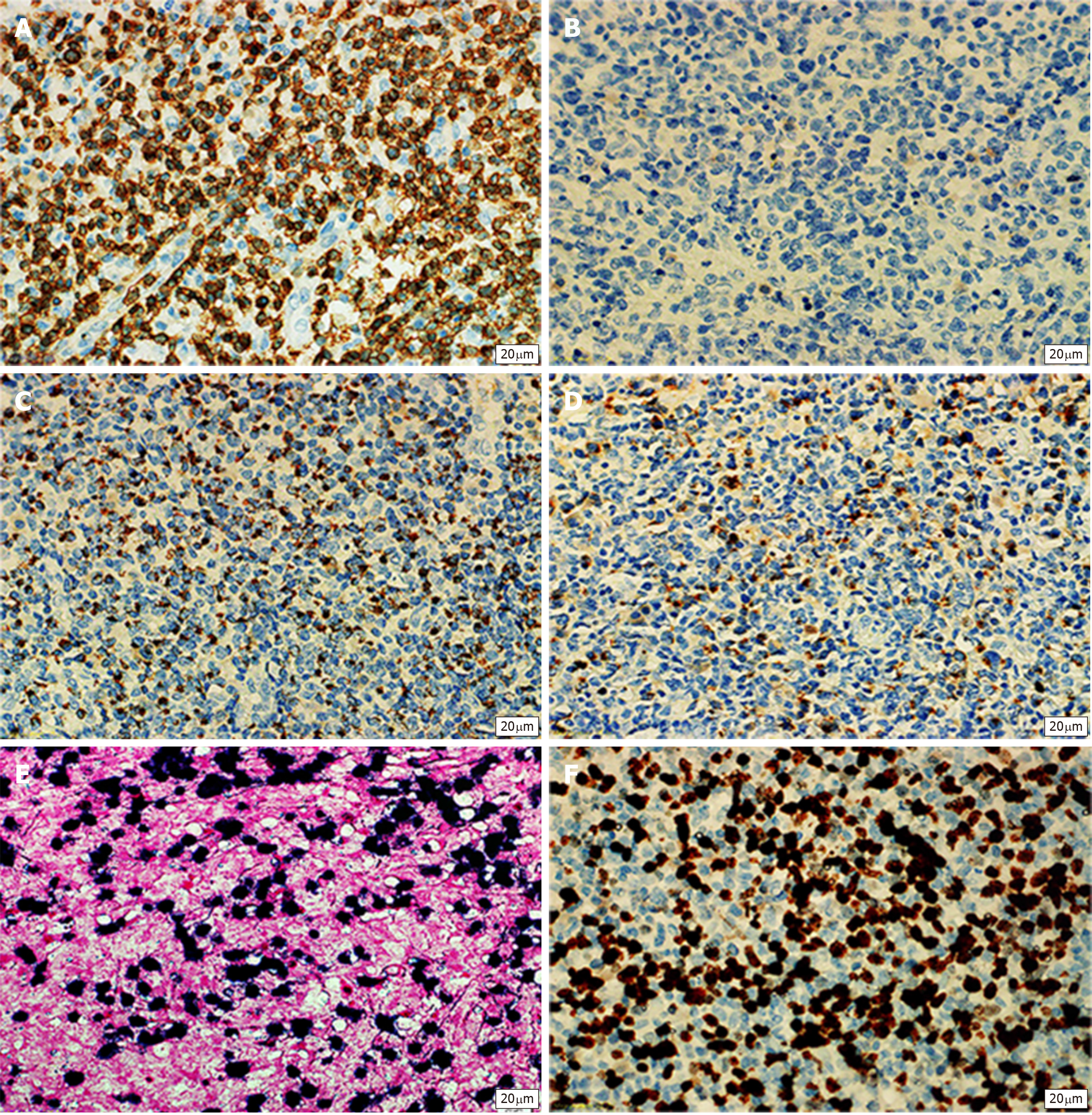
The mucous membrane at the ulceration site had disappeared and was covered with necrosis, inflammatory exudates, and granulomatous tissues. The entire layer of the intestinal wall showed diffuse invasion by lymphocyte-like atypical cells, with the involvement of extraserosal adipose tissue. The nuclei of these cells were irregular, and distortions, lobules, and notches could be seen. These cells were rich in chromatin and granules and possessed visible nucleoli; mitotic figures were widely seen, with pink or clear abundant cytoplasm. The muscle layer at the intestinal wall showed widespread degeneration and necrosis (Figure 3B). Combined immunohistochemical staining of the small intestine specimens showed the following results: ENKTCL: 1; CD3 (diffuse +): 2; CD20 (+/-): 3; TIA (+): 4; Gr-B (partial+): 5; CD56 (-): 6; EMA (-): 7; CD4 (diffuse +); 8; CD8 (diffuse +): 9; Perforin (focal +): 10; CD38 (diffuse +): 11; CD138 (diffuse +): 12; CD79a (diffuse +): 13; CD30 (-): 14; ALK-D5F3 (-): 15; CD45 (diffuse +): 16; Bcl-2 (diffuse +): 17; Ki-67 index of 80%: 18; and in situ hybridoma with EBV RNA test (+) (Figure 4A and B). The final diagnosis was ENKTCL (Figure 4).
After surgery, the patient underwent a follow-up period of 6 mo and received 6 cycles of gemcitabine, oxaliplatin and L-asparaginase (L-GMOEX regimen), which was successful, and no apparent abnormalities were observed on relevant tests. PET-CT was performed at the 6-mo follow-up, and no recurrence or metastasis was observed (Figure 3C and D). Further follow-up is required to determine long-term efficacy and prognosis.
PI-ENKTCL.
Surgery and systemic chemotherapy (L-GMOEX) was performed.
The patient underwent follow-up for 6 mo and received 6 cycles of gemcitabine, oxaliplatin and L-asparaginase. No recurrence or metastasis occurred.
Intestinal T-cell lymphoma and NK cell lymphoma are highly invasive and malignant tumors of the intestinal tract and account for 5.2% and 14.7% of primary lymphomas of the gastrointestinal tract, respectively[3]. PI-ENKTCL is rare and accounts for 3.1% of NHL in Europe and North America. However, it is more common in Asia and South America[4]. We performed a literature review and found that PI-ENKTCL tends to occur in middle-aged males around the age of 40 years and has a poorer prognosis than intestinal T-cell lymphoma or NK cell lymphoma[5].
Kim et al[6] reported that PI-ENKTCL mainly affects the small intestine, particularly the ileum and jejunum. This is different from B-cell lymphoma that usually affects the stomach, terminal ileum, and the cecum. Most PI-ENKTCL lesions do not have specific clinical presentations or endoscopic characteristics. The early symptoms of PI-ENKTCL are similar to gastrointestinal tuberculosis and Crohn’s disease, with highly similar endoscopic findings; the biopsy positivity rate is low[7-9]. Therefore, the misdiagnosis rate is high. PI-ENKTCL often results in bleeding, perforation, and other complications. Surgical resection of the primary tumor is mainly performed for diagnosis and treatment. There are slight differences in the factors that affect PI-ENKTCL prognosis, according to different studies. These factors generally include age, LDH levels, lymph node metastasis, clinical stage, and myelosuppression[10]. Currently, there are no unified prognostic factors.
PI-ENKTCL does not show a specific endoscopic presentation, with deep lymphoma lesions and a large amount of necrotic tissue at the surface. Therefore, it is usually difficult to diagnose PI-ENKTCL by biopsy[11]. Other diagnostic imaging methods do not show significant advantages in PI-ENKTCL. In addition, PI-ENKTCL laboratory tests are often accompanied by EBV infection and LDH elevation. Therefore, it is necessary to test for EBV and LDH when PI-ENKTCL is suspected. According to our literature review, when the diagnosis of PI-ENKTCL is suspected, PET-CT is needed for diagnosis and to exclude primary tumors of the nasal cavity. In addition, differences in intake values can be used for differential diagnosis and can have clinical significance in guiding clinical stage, treatment, and diagnosis[12,13].
PI-ENKTCL has histological characteristics similar to those of ENKTCL at other sites and often presents with invasion of blood vessel centers, expression of cytotoxic proteins (granzyme B and TIA-1), and significant necrosis[14]. Immunophenotypic characteristics include positivity for CD2, CD3, CD43, CD56, and cytotoxic factors (granzyme B and TIA-1). EBV and cytotoxic factor positivity are the key to diagnosis[15]. A unique case involving CD56 negativity was reported, but a definitive diagnosis can be achieved when at least one cytotoxic factor and EBV are positive[16].
As PI-ENKTCL is rare and there is a lack of clinical tissue heterogeneity, there is currently no confirmed, optimal first-line treatment regimen. In addition, it is difficult to diagnose PI-ENKTCL, particularly those that occur in the small intestine, and PI-ENKTCL tends to result in complications such as obstruction, perforation, and bleeding. Therefore, early surgical resection is beneficial[17]. Some studies also found that the occurrence of complications is one of the factors that directly affect prognosis[5]. In addition, combined chemotherapy is more beneficial than surgery alone for patients that fulfill the criteria for surgery[6,18]. Currently, there is no standard chemotherapy regimen for PI-ENKTCL. The chemotherapy regimen for PI-ENKTCL is similar to that for ENKTCL. Many previous clinical studies have used CHOP or SMILE as first-line chemotherapy regimens, and these regimens have fair efficacy considering drug resistance[1]. Recently, the chemotherapy regimen of L-asparaginase combined with GMOEX has shown good therapeutic efficacy[19]. Due to the popularity of immunotherapy, the use of the programmed death ligand 1 (PD-L1) inhibitor pembrolizumab has given new hope to ENKTCL patients. ENKTCL cells express PD-L1, which binds to PD-1 on the surface of T-cells infiltrating the tumor microenvironment to inhibit tumor-specific T-cell activity or inactivate T-cells to block immune responses and to enable immune evasion in tumor cells[20,21]. Therefore, overcoming this immune evasion mechanism and increasing T-cell activity will increase immunotherapy effects.
PI-ENKTCL has a low incidence, high invasiveness, and lacks specific clinical presentation, which makes it prone to misdiagnosis. This delays treatment and affects prognosis. Therefore, for suspected PI-ENKTCL patients, PET-CT and tests for EBV and LDH can provide a definitive diagnosis or diagnosis of exclusion, leading to multidisciplinary-assisted diagnosis and treatment. Surgery should be the mainstay of treatment for patients with no metastasis or surgical contraindications. Appropriate chemotherapy should be administered based on postoperative pathology results to improve the survival and prognosis of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Etreby SA S-Editor: Ma YJ L-Editor: Webster JR E-Editor: Qi LL
| 1. | Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410-4416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Yong W. Clinical study of l-asparaginase in the treatment of extranodal NK/T-cell lymphoma, nasal type. Hematol Oncol. 2016;34:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, Konoplev S, Bueso-Ramos CE, Vega F, Medeiros LJ, Yin CC. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013;37:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Fang JC, Xia ZX, Wang CN, Li Z. Clinicopathologic and Immunophenotypic Features of Primary Intestinal Extranodal NK/T-Cell Lymphoma, Nasal Type. Int J Surg Pathol. 2015;23:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Jiang M, Chen X, Yi Z, Zhang X, Zhang B, Luo F, Jiang Y, Zou L. Prognostic characteristics of gastrointestinal tract NK/T-cell lymphoma: an analysis of 47 patients in China. J Clin Gastroenterol. 2013;47:e74-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kim SJ, Jung HA, Chuang SS, Hong H, Guo CC, Cao J, Hong XN, Suzuki R, Kang HJ, Won JH, Chng WJ, Kwong YL, Suh C, Song YQ, Zhu J, Tay K, Lim ST, Suzumiya J, Lin TY, Kim WS; Asia Lymphoma Study Group. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma Study Group. J Hematol Oncol. 2013;6:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Sun J, Lu Z, Yang D, Chen J. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol. 2011;24:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, Liao YL, Lin SH, Hsieh YC, Lu CL, Sheu MJ, Liu H. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009;33:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Wan M, Chow J, Lei K, Chan W. Allelotyping of gastrointestinal nasal-type NK/T-cell lymphoma. Leuk Res. 2004;28:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Avilés A, Díaz NR, Neri N, Cleto S, Talavera A. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 2000;22:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Yang L, Tang X, Peng X, Qian D, Guo Q, Guo H. Clinical characteristics of primary intestinal NK/T cell lymphoma, nasal type: Case series and review of the literature. Pathol Res Pract. 2018;214:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Chan WK, Au WY, Wong CY, Liang R, Leung AY, Kwong YL, Khong PL. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010;35:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Jhuang JY, Chang ST, Weng SF, Pan ST, Chu PY, Hsieh PP, Wei CH, Chou SC, Koo CL, Chen CJ, Hsu JD, Chuang SS. Extranodal natural killer/T-cell lymphoma, nasal type in Taiwan: a relatively higher frequency of T-cell lineage and poor survival for extranasal tumors. Hum Pathol. 2015;46:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, Kayasut K, Mitarnun W, Khuhapinant A, Bunworasate U, Puavilai T, Bedavanija A, Garcia-Herrera A, Campo E, Cook JR, Choi J, Swerdlow SH. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Li X, Babayi A, Sang W, Abulajiang G, Li Q, Cui W, Zhang W. Clinicopathologic, immunophenotypic, and EBER in situ hybridization study of extranodal natural killer/T-cell lymphoma, nasal type in ãmulti-ethnic groups. Clin Lab. 2014;60:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao XY, Li JJ, Zhang R. Primary intestinal non-Hodgkin's lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol. 2011;17:4625-4631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, Shirakawa S, Fukumoto M. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017;10:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 20. | Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 685] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 21. | Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, Cha HJ. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |