Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.200
Peer-review started: September 2, 2019
First decision: November 13, 2019
Revised: November 18, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: January 6, 2020
Processing time: 126 Days and 15.9 Hours
Mortality due to septic shock is relatively high. The dynamic monitoring of plasma cell-free DNA (cfDNA) can guide the treatment of septic shock.
Herein, we present a typical case of septic shock syndrome caused by the bacilli Acinetobacter baumannii and Pantoea. The patient complained of abdominal pain, fever and chills upon admission to the Emergency Department. Marked decreases in white blood cells and procalcitonin (PCT) were observed after the patient received continuous renal replacement and extracorporeal membrane oxygenation. Plasma cfDNA levels were consistently high, peaking at 1366.40 ng/mL, as measured by a duplex real-time PCR assay with an internal control, which was developed as a novel method for the accurate quantification of cfDNA. The patient died of septic shock on HD 8, suggesting that cfDNA could be used to monitor disease progression more effectively than PCT and the other inflammatory factors measured in this case.
CfDNA may be a promising marker that complements other inflammatory factors to monitor disease progression in patients with septic shock.
Core tip: Mortality due to septic shock is relatively high. Several biomarkers have been evaluated for predicting mortality in patients with septic shock, but none have been shown to be entirely useful in clinical applications. Herein, we report a typical case and review the literature. This case might contribute to improving our understanding of cell-free DNA (cfDNA) in monitoring disease progression during septic shock. This report also suggests that cfDNA could be used to more effectively monitor disease progression than procalcitonin and other inflammatory factors.
- Citation: Liu JP, Zhang SC, Pan SY. Value of dynamic plasma cell-free DNA monitoring in septic shock syndrome: A case report. World J Clin Cases 2020; 8(1): 200-207
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.200
Septic shock is a subset of sepsis that often leads to metabolic disorders and multiorgan failure[1], resulting in hospital mortality rates greater than 40%[2]. The mechanism of septic shock is complex and poorly understood. Septic shock is associated with cell necrosis and apoptosis. Plasma cell-free DNA (cfDNA) is free, extracellular DNA, which is mainly derived from apoptosis in vivo or from the degradation of genomic DNA from necrotic cells. The dynamic monitoring of plasma cfDNA levels can guide the treatment of septic shock. The aim of this study was to determine the efficacy of plasma cfDNA for monitoring septic shock.
Abdominal pain, fever and chills.
A 29-year-old male patient presented at the Emergency Department of our hospital with complaints of abdominal pain, fever and chills on July 27, 2017. The onset of disease was one day before presentation with bilateral swelling and pain in the gums. On the morning of admission, he had a sudden chill and a high fever with a peak of 39.8 °C. He suffered from intermittent right upper quadrant pain, accompanied by nausea and retching.
He had a history of a left lateral malleolus fracture subluxation 6 years ago and a history of allergies to penicillin and amoxicillin. He denied a history of smoking, drinking, hyperlipemia, and chronic diseases, such as coronary heart disease, hypertension and diabetes.
In the emergency room, he had a blood pressure of 80/50 mmHg and a heart rate of 130 beats/min. The right upper abdomen was prominent and tender at palpation without guarding and rebound tenderness. In addition, he had a positive Murphy’s sign and liver percussion pain.
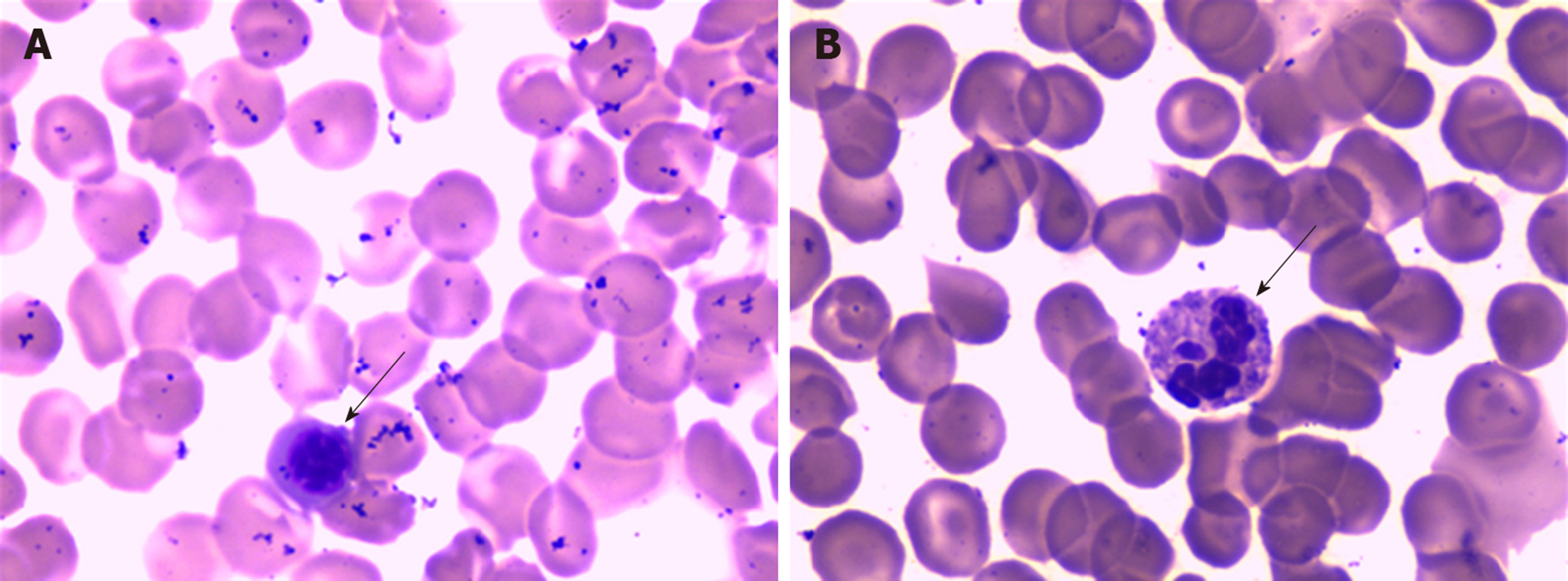
Laboratory tests showed leukocytopenia (2.38 × 109/L), a high percentage of neutrophils (83.2%), mild thrombocytopenia (68 × 109/L), abnormal blood coagulation function, a reduced international normalized ratio, hypokalemia, hyperbilirubinemia, elevated liver enzymes (both hepatocellular and cholestatic enzymes), an elevated procalcitonin (PCT) concentration (35.30 ng/mL), metabolic acidosis, and hyperlactatemia, suggesting progressive sepsis. On light microscopy, the peripheral blood smear showed a left shift of neutrophil nuclei, prominent toxic granulations and vacuolation in the neutrophil cytoplasm (Figure 1). Further laboratory results are shown in Table 1. Gram-negative bacilli were isolated from blood cultures.
| Parameter | Normal range | On admission 27 July | HD 2, 28 July | HD 3, 29 July | HD 4, 30 July | HD 5, 31 July | HD 6, 1 August | HD 7, 2 August | HD 8, 3 August |
| Hemoglobin (g/L) | 130-175 | 151 | 123 | 113 | 93 | 101 | 94 | 104 | 61 |
| RBC (× 1012/L) | 4.30-5.80 | 4.78 | 3.91 | 3.62 | 2.95 | 3.25 | 3.06 | 3.32 | 2.04 |
| WBC (× 109/L) | 3.50-9.50 | 2.38 | 14.57 | 29.40 | 12.11 | 10.21 | 8.54 | 1.00 | 0.43 |
| NEUT (%) | 40.0-75.0 | 83.2 | 96.6 | 97.6 | 86.3 | 73.7 | 11.5 | 61.0 | 2.4 |
| LYMPH (%) | 20.0-50.0 | 16.8 | 3.2 | 3.62 | 3.2 | 15.9 | 81.0 | 23.0 | 86.0 |
| Platelets (× 109/L) | 125-350 | 68 | 44 | 42 | 51 | 43 | 40 | 21 | 16 |
| Glucose (mmol/L) | 3.9-6.1 | / | 7.3 | 10.9 | / | 6.5 | 6.3 | 4.7 | / |
| Sodium (mmol/L) | 137-147 | 138.9 | 148.3 | 150.5 | 146.7 | 148.7 | 149.8 | 155.0 | 155.5 |
| Potassium (mmol/L) | 3.5-5.3 | 3.2 | 2.6 | 4.6 | 3.3 | 3.8 | 3.8 | 3.4 | 2.9 |
| Calcium (mmol/L) | 2.20-2.65 | 2.08 | 2.13 | 2.04 | 1.94 | 2.35 | 2.35 | 2.69 | 1.95 |
| Total bilirubin (μmol/L) | 6.0-22.0 | 42.0 | 67.9 | 65.6 | / | 69.1 | 98.3 | 93.9 | 51.3 |
| AST (IU/L) | 0-45 | 87.1 | 188.5 | 103.8 | 62 | 65.5 | 118.1 | 119.5 | 55.5 |
| ALT (IU/L) | 13-69 | 42.5 | 72.7 | 56.2 | 39.8 | 33.8 | 55.6 | 62.7 | 25.4 |
| GGT (IU/L) | 8-78 | 253 | 330.4 | 283.9 | / | 144.1 | 124 | 162.9 | 72.6 |
| Alkaline phosphatase (IU/L) | 38-126 | 88 | 62.8 | 88 | / | 107.4 | 99 | 182.6 | 57.2 |
| LDH (IU/L) | 140-271 | 411 | 626 | 559 | / | 402 | / | 417 | 295 |
| Albumin (g/L) | 40.0-55.0 | / | 39.5 | 43.1 | / | 47.5 | / | 47.6 | 44.0 |
| Globulin (g/L) | 20.0-40.0 | / | 16.1 | 22.9 | / | 20.8 | / | 28.0 | 15.5 |
| Urea (mmol/L) | 2.10-7.20 | 5.91 | 10.00 | 12.50 | / | 13.01 | 15.04 | 17.29 | 12.70 |
| Creatinine (μmol/L) | 44.0-132.0 | 110.8 | 143.3 | 147.3 | / | 80.6 | 84.4 | 74.8 | 110.8 |
| Lactate (mmol/L) | 0.5-1.6 | 9.5 | 3.5 | 2.9 | / | 3.9 | 5.3 | 4.1 | > 15 |
| Procalcito-nin (ng/mL) | 0-0.05 | 35.30 | 96.50 | 10.74 | 2.82 | 1.22 | 0.99 | 1.15 | 8.11 |
| C-reactive protein (mg/L) | 0-10 | / | > 90.00 | / | > 90.00 | / | / | / | > 90.00 |
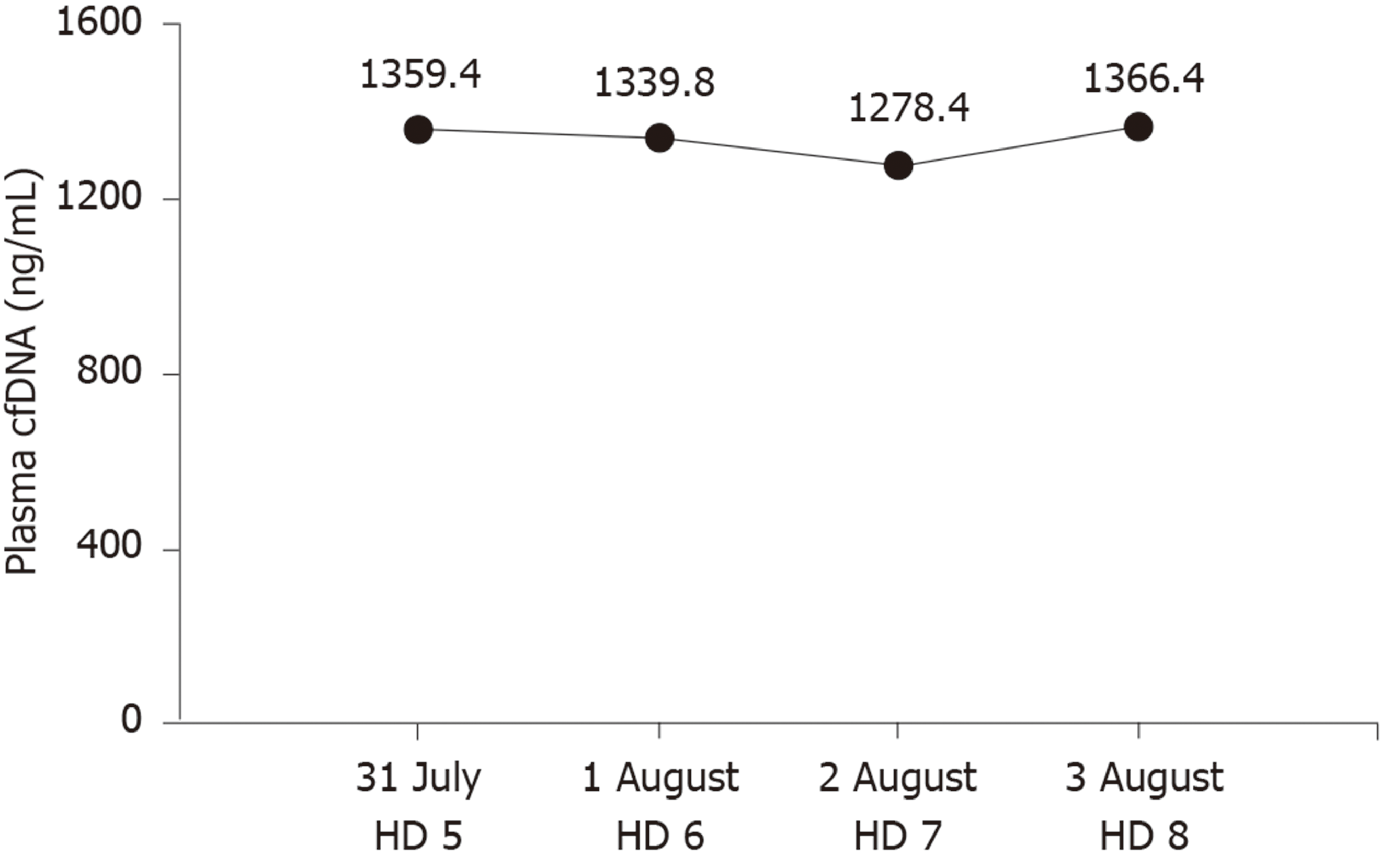
| Plasma cell-free DNA (ng/mL) | 0-52.5 | 1359.4 | 1339.8 | 1278.4 | 1366.4 |
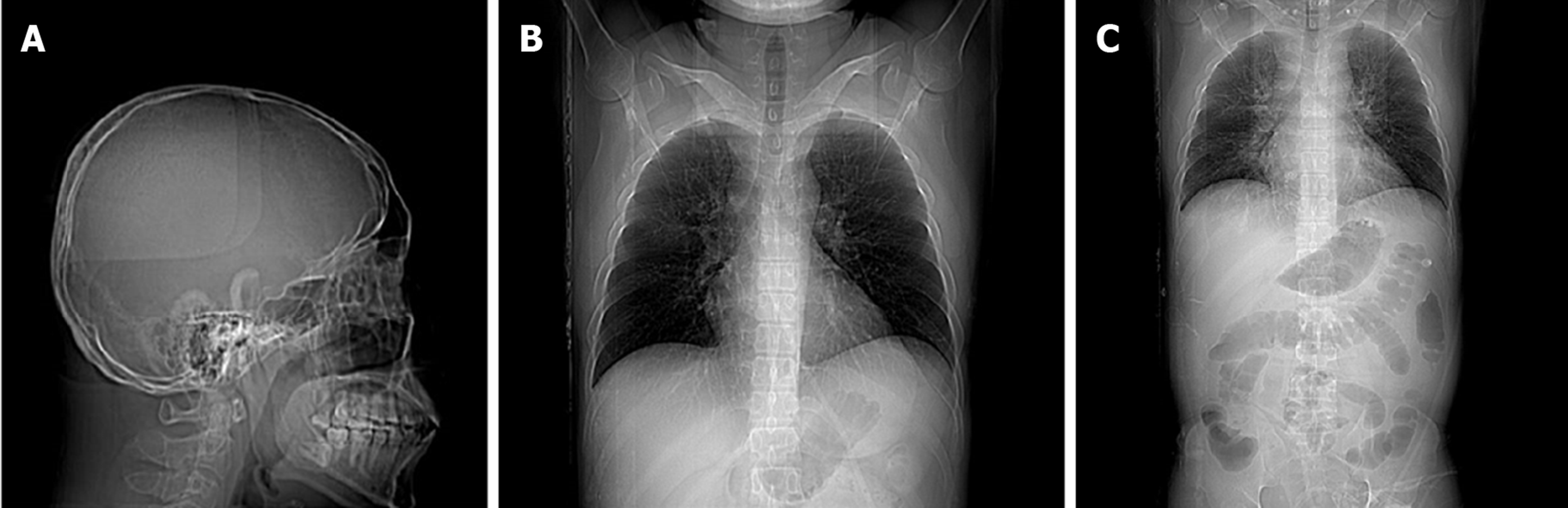
A routine examination involving electrocardiography and a head CT scan showed no significant abnormalities. A multislice chest CT scan performed in the Department of Radiology showed little interstitial change in the lower lung. Scanning of the abdomen showed gallbladder wall edema with increased gallbladder density, swelling of the kidneys, and an enlarged pancreas. There was interlobular septal thickening on both sides and bilateral pleural effusion with lower lung insufficiency of both longs, suggesting cardiac dysfunction with pulmonary edema (Figure 2).
Septic shock.
During the patient’s stay in the Department of Infectious Diseases, he received catecholaminergic therapy, fluid replacement, plasma transfusion and antibiotics (imipenem). The general condition of the patient rapidly worsened. Seven hours after presentation at the hospital, a decision was made to admit him to the intensive care unit (ICU). Treatment consisting of intravenous administration of broad-spectrum antibiotic therapy (imipenem, ganciclovir, moxifloxacin and phosphate oseltamivir), plasma and cryoprecipitate was initiated. Despite this therapy, acute respiratory distress syndrome occurred within the next few hours. Intensive treatment, which included mechanical ventilation, the continuous infusion of catecholamines and pharmacological therapy, was started. The typical clinical signs of septic shock, such as facial flushing, mild cyanosis of the lips, and conjunctival and pharynx congestion, were observed. Gram-negative bacilli were isolated from blood cultures. The patient then received tigecycline combined with imipenem/cilastatin to treat the infection. The inflammation markers remained high [C-reactive protein (CRP) and PCT].
On the 3rd day, due to unresponsiveness of the patient to conventional intensive therapy and the presence of a life-threatening condition, continuous renal replacement and extracorporeal membrane oxygenation (ECMO) were performed as rescue therapies. A significant improvement was observed in terms of the laboratory findings. Marked decreases in white blood cells (WBCs) (29.40 × 109/L to 8.54 × 109/L) and PCT (96.50 ng/mL to 0.99 ng/mL) and a reduction in the catecholamine dosage were achieved three days later.
However, plasma cfDNA levels were constantly high, peaking at 1366.40 ng/mL (Figure 3). cfDNA levels were measured by a duplex real-time PCR assay with an internal control, which was developed as a novel method for the accurate quantification of plasma cfDNA in our previous study. Within the 95% confidence interval, the normal reference intervals were 0-52.5 ng/mL for males and 0-45.8 ng/mL for females[3].
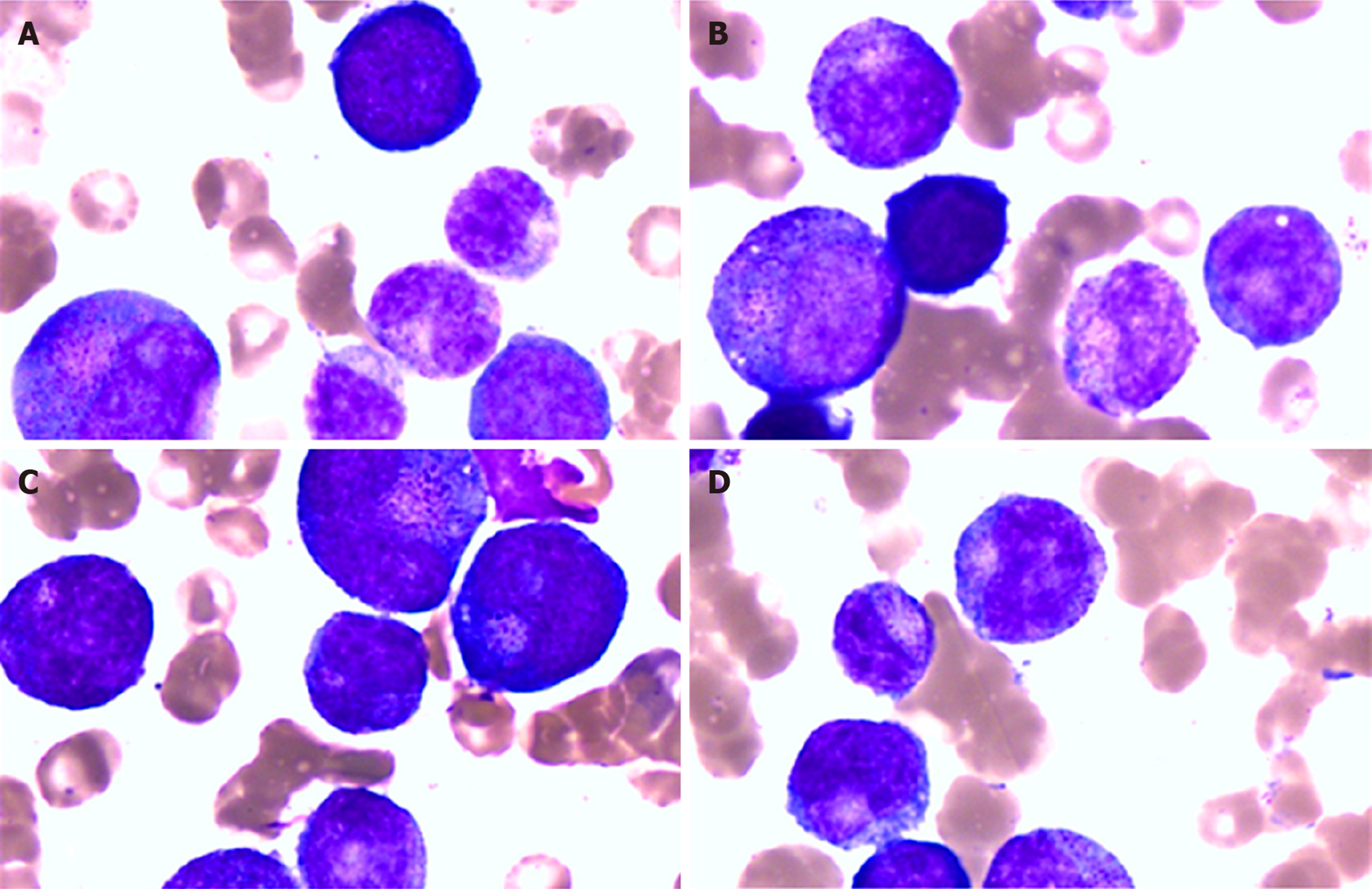
On the 7th day, the patient developed facial flushing, with increased body temperature (39.6 °C) and heart rate (140-150 beats/min), and decreased WBCs (1.00 × 109/L) and central venous pressure (9-10 mmHg). To maintain his blood pressure, he received a fluid infusion and catecholaminergic therapy. Peripheral blood and bone marrow examinations showed granulocyte maturation disorders, which may have been caused by infection (Figure 4). Therefore, he received treatment with leukocyte drugs, such as granulocyte-colony stimulating factor. At night, his urine volume began to decrease, the urine color was deeper and the lactate level gradually increased to greater than 15 mmol/L. Metabolic acidosis, hypernatremia and hypokalemia followed. Then, cyanosis, a deep coma, bilateral scattered pupils, and lungs full of coarse wet rales were observed. Treatment with rapid liquid infusion, high dose norepinephrine and pituitary vasopressin and ECMO were ineffective. His arterial blood pressure continued to decline.
The patient died of septic shock at 00:10 on August 4, 2017.
We report a case of septic shock syndrome caused by Acinetobacter baumannii and Pantoea, which was characterized by high fever, chills and abdominal pain. The patient had fever, diarrhea, toothache and a history of self-administered intravenous drugs. The onset of septic shock was acute and progressed rapidly into a state of shock. His symptoms were significantly more severe than those of the bacteremia caused by the hospital-wide panresistant Acinetobacter baumannii. This effect was possibly associated with the endotoxemia of sensitive bacteria, which were lysed by antibiotics.
Any type of bacteria can cause septic shock, but this condition is mainly caused by gram-negative bacilli[4]. Acinetobacter baumannii, a gram-negative coccobacillus, can act as an opportunistic pathogen in humans, affecting people with a range of different diseases. Acinetobacter baumannii is becoming an increasingly important hospital-derived infection that can cause septic shock. Bacterial septic shock is frequently caused by the overproduction of inflammatory mediators as a result of the immune recognition of bacteria or bacterial products[5]. The administration of effective antibacterial drugs as early as possible to control primary underlying diseases and improve shock by correcting metabolic acidosis and supplementing the blood volume are critical to treatment. In addition, a timely and effective evaluation of the patient’s therapeutic response is also critical. Conventional microbiological cultures for diagnosing septic shock are usually time consuming, do not reflect the systemic inflammatory response or the onset of organ dysfunction and may sometimes be misleading, generating false-positive or false-negative results.
Some traditional indicators, such as body temperature, WBCs, and blood lactate levels, can reflect the condition and efficacy of treatment, but these indicators are not very reliable. The clinical manifestations of septic shock are highly variable[4]. In this case, the WBC count was significantly reduced on admission and in the late stage of septic shock. This result may have been associated with the increased adhesion of leukocytes in severe infections. Thus, WBCs may have adhered to the vascular endothelium and may not have entered the circulation pool.
In septic shock, mitochondrial oxygen delivery is reduced due to hypoxia and hypoperfusion, and lactate synthesis increases, resulting in hyperlactatemia. Lactate plays an important role in assessing the severity of septic shock and objectively predicting the prognosis. In the 2017 Surviving Sepsis Campaign international guidelines and 2016 Sepsis-3 definitions, lactate levels were used to define patients with septic shock[2,6]. A high level of blood lactate is associated with the mortality and prognosis of patients with septic shock, but it is not specific. Trauma, seizures, cardiac arrest and other conditions can also lead to increased levels of lactate. Normal lactate levels are not always a marker for good prognosis in septic shock[7].
Acute-phase proteins, such as CRP or PCT, can also be used as biomarkers of the severity and prognosis of patients with septic shock. CRP, a liver-derived protein, is an attractive marker for diagnosing septic shock due to its easy access and low cost. CRP is well correlated with the severity of infection. The CRP level of the patient in our case remained high throughout the entire course. However, the specificity of CRP is limited[8]. A previous study demonstrated that CRP cannot reflect sepsis severity[9] because it fails to distinguish infectious from noninfectious inflammatory responses[10]. Therefore, CRP could only be considered an inflammatory biomarker.
PCT, the precursor of the hormone calcitonin, is significantly elevated in septic shock patients; PCT has high specificity and is positively correlated with disease severity and mortality[11]. Activation of the lymphatic system can produce large amounts of PCT during infections or when the body is otherwise stimulated. The half-life of PCT is 25-30 h[12], and PCT is increased earlier than CRP and other inflammatory factors in patients with bacterial infections. Septic shock patients can have a rapid decline in PCT in response to effective treatment with antibacterial agents, suggesting that PCT has important clinical implications for monitoring disease progression. In this case, PCT was significantly reduced starting on day 3, which made physicians mistakenly think that the patient was getting better. However, the patient’s condition worsened. We hypothesized that the decline in PCT may have been due to organ failure, which was caused by septic shock.
In addition to these classic biomarkers, cfDNA, which was first described in 1948[13], has the potential to be a useful marker in septic shock. CfDNA is released into the circulation through cell lysis, necrosis, apoptosis and active DNA release, resulting in higher concentrations of cfDNA in patients with microbial infections, trauma, cancer and other clinical conditions[14]. Although elevated levels of cfDNA are not specific to a single disease, elevated cfDNA has been shown to be an extremely sensitive and promising prognostic marker in septic shock[15]. This observation may be associated with the shorter half-life of cfDNA[16,17] than that of PCT and CRP. According to Ahmed, cfDNA is a good prognostic predictor for patients in the ICU and, to a lesser extent, is a good marker of septic shock[18]. However, the inevitable loss of cfDNA during extraction has become a considerable detriment, hindering its clinical applications. We previously developed a duplex real-time PCR assay with an internal control as a novel method for the accurate quantification of plasma cfDNA, which can eliminate preanalytical errors and increase the precision and accuracy[3]. Our previous studies showed the clinical value of plasma cfDNA levels, as measured by this novel method, in several conditions. CfDNA levels can be useful not only in evaluating chemotherapy effects and guiding treatment in advanced lung cancer patients, but also in assessing liver injury in hepatitis B patients[19-21]. In this case, cfDNA remained high until the patient died, suggesting that cfDNA could be used to monitor disease progression more effectively than PCT. Although the death of patients with septic shock in our hospital is rare, the conclusion of our study is based on only one case. In future, we will conduct large-scale clinical trials to confirm the value of cfDNA in septic shock.
Using a single biomarker for the diagnosis or prognosis of septic shock is not practical. Therefore, cfDNA could be a promising marker that complements PCT, CRP, lactate and other inflammatory factors to better manage patients with septic shock.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Das U, Stanciu C S-Editor: Ma YJ L-Editor: Webster JR E-Editor: Liu JH
| 1. | Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 998] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 3. | Chen D, Pan S, Xie E, Gao L, Xu H, Xia W, Xu T, Huang P. Development and Evaluation of a Duplex Real-Time PCR Assay With a Novel Internal Standard for Precise Quantification of Plasma DNA. Ann Lab Med. 2017;37:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2315] [Cited by in RCA: 2564] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 5. | House AA, Ronco C. Extracorporeal blood purification in sepsis and sepsis-related acute kidney injury. Blood Purif. 2008;26:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1986] [Article Influence: 248.3] [Reference Citation Analysis (1)] |
| 7. | Dugas AF, Mackenhauer J, Salciccioli JD, Cocchi MN, Gautam S, Donnino MW. Prevalence and characteristics of nonlactate and lactate expressors in septic shock. J Crit Care. 2012;27:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 1216] [Article Influence: 55.3] [Reference Citation Analysis (2)] |
| 9. | Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31:1737-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, Yan P, Jia Y, Feng D, Xie L. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;12:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Huang MY, Chen CY, Chien JH, Wu KH, Chang YJ, Wu KH, Wu HP. Serum Procalcitonin and Procalcitonin Clearance as a Prognostic Biomarker in Patients with Severe Sepsis and Septic Shock. Biomed Res Int. 2016;2016:1758501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49 Suppl 1:S57-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil. 1948;142:241-243. [PubMed] |
| 14. | Maruchi Y, Tsuda M, Mori H, Takenaka N, Gocho T, Huq MA, Takeyama N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care. 2018;22:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 15. | Rhodes A, Cecconi M. Cell-free DNA and outcome in sepsis. Crit Care. 2012;16:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2240] [Cited by in RCA: 2108] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 17. | Yao W, Mei C, Nan X, Hui L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene. 2016;590:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Ahmed AI, Soliman RA, Samir S. Cell Free DNA and Procalcitonin as Early Markers of Complications in ICU Patients with Multiple Trauma and Major Surgery. Clin Lab. 2016;62:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Pan S, Xia W, Ding Q, Shu Y, Xu T, Geng Y, Lu Y, Chen D, Xu J, Wang F, Zhao C, Huang P, Huang P, Shen H, Hu Z, Lu S. Can plasma DNA monitoring be employed in personalized chemotherapy for patients with advanced lung cancer? Biomed Pharmacother. 2012;66:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Wang H, Zhang B, Chen D, Xia W, Zhang J, Wang F, Xu J, Zhang Y, Zhang M, Zhang L, Lu Y, Geng Y, Huang P, Huang P, Wang H, Pan S. Real-time monitoring efficiency and toxicity of chemotherapy in patients with advanced lung cancer. Clin Epigenetics. 2015;7:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Xia WY, Gao L, Dai EH, Chen D, Xie EF, Yang L, Zhang SC, Zhang BF, Xu J, Pan SY. Liquid biopsy for non-invasive assessment of liver injury in hepatitis B patients. World J Gastroenterol. 2019;25:3985-3995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |