Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.120
Peer-review started: October 31, 2019
First decision: November 13, 2019
Revised: November 26, 2019
Accepted: December 13, 2019
Article in press: December 13, 2019
Published online: January 6, 2020
Processing time: 67 Days and 2.8 Hours
Gastrointestinal stromal tumors (GISTs) originate from interstitial cells of Cajal. GISTs can occur anywhere along the gastrointestinal tract. Large lesions have traditionally been removed surgically. However, with recent innovations in advanced endoscopy, GISTs located within the stomach are now removed endoscopically. We describe a new innovative endoscopic technique to close large and hard to access defects after endoscopic full-thickness resection of gastric GISTs.
We present a series of three patients who were diagnosed with a gastric GIST. All patients underwent full-thickness endoscopic resection. In all cases, for closure of the surgical bed, conventional endoscopic techniques including hemoclips, endoloop and suturing were unsuccessful. We performed a new technique in which we pulled omental fat into the gastric lumen and completely closed the defect using endoscopic devices. All patients performed well post-procedure and computed tomography was carried out one day after the procedures which showed no extravasation of contrast.
The omental plug technique may be used as an alternative to surgery in selected cases of gastric perforation.
Core tip: We present three patients who were diagnosed with gastric gastrointestinal stromal tumors. All patients underwent endoscopic full-thickness resection. We describe a new technique in which the omental fat is pulled into the gastric lumen and clipped to the edges of the defect. Then, continuous endosutures or an endoloop were placed in the surrounding gastric mucosa and cinched leading to complete closure of the defect. This novel closure technique may be an alternative to surgery in selected cases of gastric perforation.
- Citation: Sachdev AH, Iqbal S, Ribeiro IB, de Moura DTH. Use of omental patch and endoscopic closure technique as an alternative to surgery after endoscopic full thickness resection of gastric intestinal stromal tumors: A series of cases. World J Clin Cases 2020; 8(1): 120-125
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/120.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.120
Gastrointestinal stromal tumors (GISTs) originate from interstitial cells of Cajal. GISTs can occur anywhere along the GI tract but are most commonly found in the stomach (60%-70% of cases), occurring less frequently in the small intestine (20%-30%), colon and rectum (5%), and esophagus (4%)[1]. Large lesions have traditionally been removed surgically. However, with recent innovations in advanced endoscopy, GISTs located in the stomach are now removed endoscopically[2]. If the lesion is located deep in the muscularis propria, the en-bloc removal entails endoscopic full-thickness resection (EFTR). EFTR is limited by the size of the full-thickness defect created. The currently available methods for closing defects endoscopically include metallic clipping (+/- endoloop placement), closure using over-the-scope metal clips, and suturing. However, these closure methods may not be successful for large and hard to access defects especially when operating in the retroflexed position. Hence, such defects will need emergent surgical closure. Here we describe a new innovative endoscopic technique to close large and hard to access defects after EFTR of gastric GISTs.
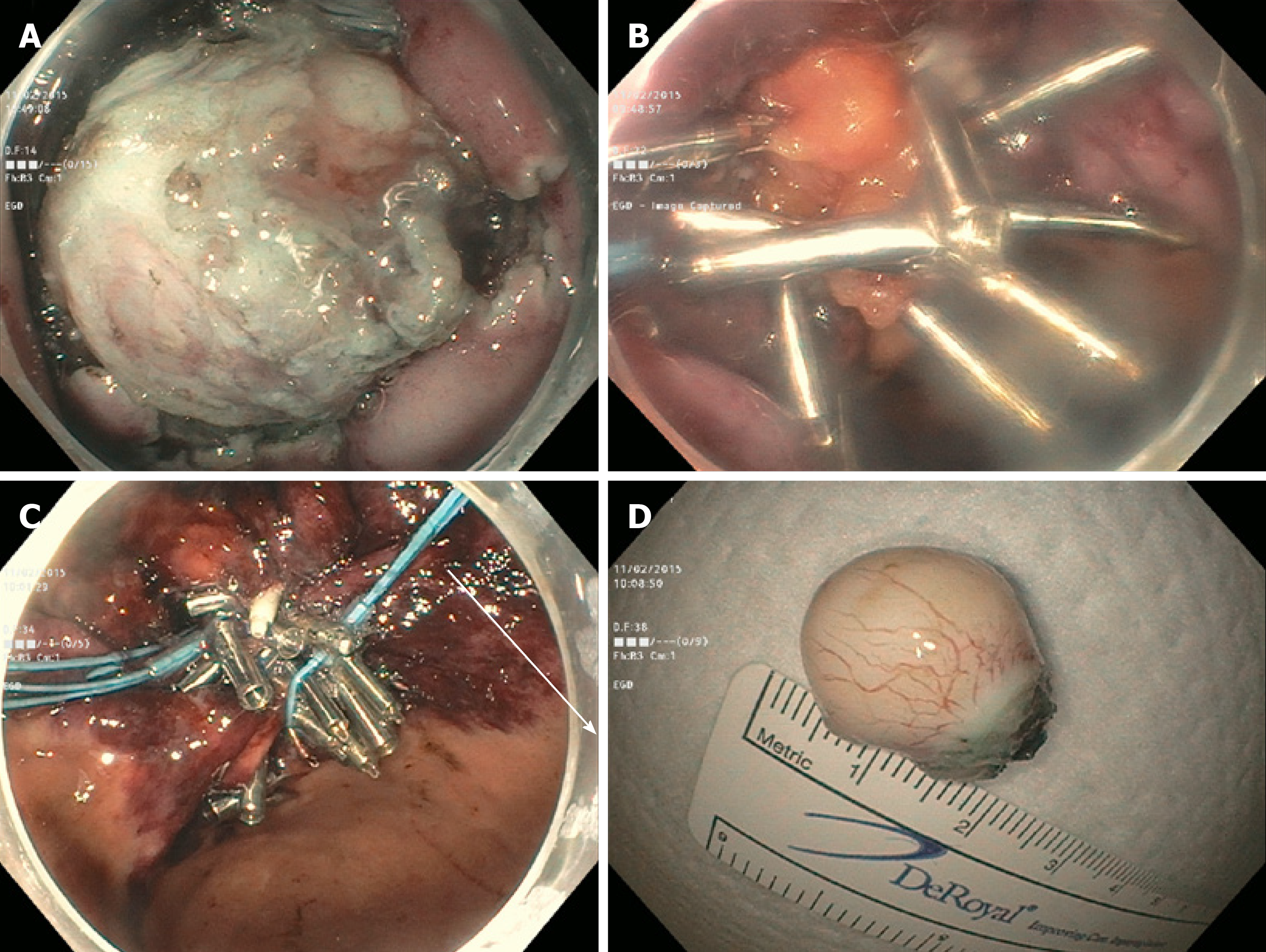
An 81-year-old female was found to have a 22 mm gastric fundus muscularis propria (layer IV) subepithelial lesion on endoscopic ultrasonography (EUS). EUS-fine needle aspiration was performed and pathology confirmed a GIST. The lesion was initially marked circumferentially by the tip of a Dual Knife electrocautery system (Olympus, Tokyo, Japan). Submucosal solution was injected. The overlying mucosa was incised. The lesion was identified, and removed by a combination of Dual and IT-2 knife electrocautery (Olympus, Tokyo, Japan). A large full-thickness defect was noted. Despite the use of CO2, capnoperitoneum was diagnosed and decompressed. Initial attempts at closure using endosuturing failed due to the slippage of sutures and T-tags. Attempts to close the defect using hemoclips and an endoloop were also unsuccessful. It was decided to pull omental fat into the gastric lumen and clip to the edges of the defect. Then, hemoclips along with endoloop placement (“tulip-bundle” technique) were used to close the defect (Figure 1). The patient did well post-procedure. Abdominal computed tomography (CT) was performed the next day, and extravasation of contrast was not observed.
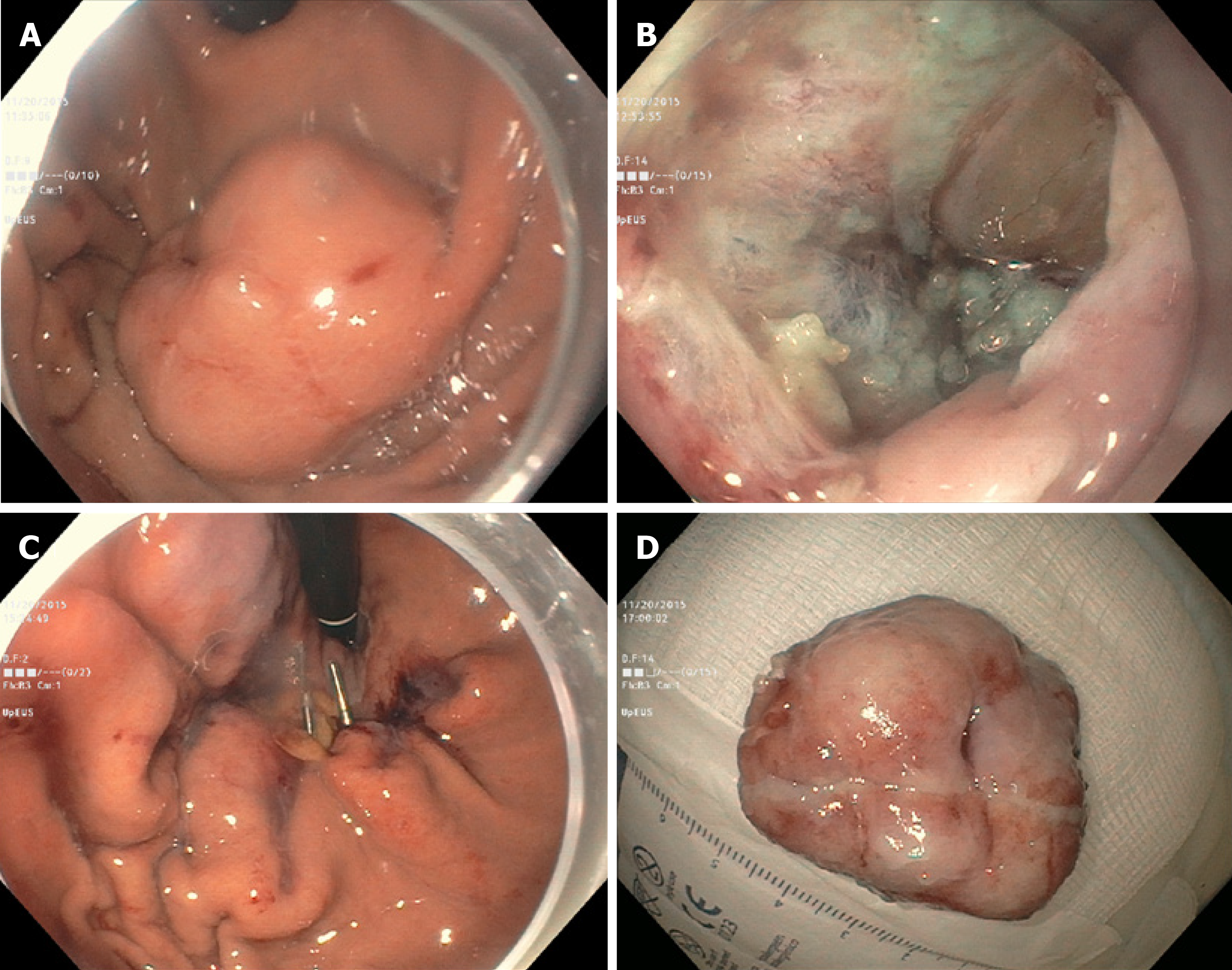
The second case was a 79-year-old gentleman with a 28 mm gastric cardia muscularis propria GIST. The lesion was removed by EFTR. The resulting capnoperitoneum was decompressed. However, initial attempts at closing the large defect using an endosuture was only partially successful. Only the distal margin was closed. The proximal margin was difficult to visualize. Attempts at closure using hemoclips and an endoloop were unsuccessful. The omental fat was then pulled into the gastric lumen and clipped to the edges of the defect. Continuous endosutures were placed at four different areas in a purse-string fashion in the surrounding mucosa, and cinched leading to successful closure (Figure 2). A nasogastric tube was inserted, and attached to intermittent suction. No contrast extravasation was noted on abdominal CT the following day. The nasogastric tube was subsequently removed, and the patient was placed on a PO diet.
The third case was a 54-year-old gentlemen with a 35 mm gastric cardia muscularis propria GIST. The lesion was exophytic in location, and difficult to identify after incision of overlying mucosa. After identification with repeat EUS, the lesion was removed by a combination of IT-2 knife and snare polypectomy. The resulting capnoperitoneum was decompressed. The lesion was closed by the endoscopic omental patch technique as described in case two.
The three patients were diagnosed with muscularis propria GIST of the stomach.
“Omental patching” was initially introduced in 1937 by Roscoe Reid Graham of Toronto to successfully close perforated duodenal ulcers. Generally, three or four full-thickness or seromuscular (as introduced by Lambert) sutures are placed perpendicularly between the edges of the perforation and are laid out on each side of the duodenum. A patch of omentum is then brought upward and the sutures are tied across[3]. Here we describe omental patching via endoscopic techniques to close large and hard to access defects after EFTR of gastric GISTs.
In our practice, we have successfully used either hemoclipping along with endoloop placement (“tulip-bundle” technique) or endosuturing to close iatrogenic defects. However, when lesions are located in either the gastric fundus or cardia these closure methods do not always succeed. In the three cases presented, the proximal edges (especially) were difficult to access due to the large size of the defects. The omentum was noted nearby. It was pulled inside the gastric lumen, and clipped to the edges of the defect. In two cases, continuous endosutures in a purse-string fashion were placed at four different sites around the defect and cinched. In one case, when endosuturing was not available, we used hemoclips along with endoloop placement (“tulip-bundle” technique). The resulting capnoperitoneum was decompressed in all cases by passing a 10 mL syringe half-filled with sterile saline into the right upper quadrant of the abdomen under aseptic conditions. The plunger was removed to let the CO2 evacuate. Abdominal CT was performed one day post-procedure to rule out any contrast extravasation. Prophylactic antibiotics were given for 3-5 d. All procedures were performed under general anesthesia.
All lesions were successfully removed and iatrogenic defects were closed endoscopically. No post-procedure complications were noted. Post-procedure hospital length of stay ranged from 3-4 d. Histopathology examination showed GIST with complete R0 resection in all three cases. Pathology showed low-grade dysplasia, with the exception of case two which showed moderate grade dysplasia (with 6 per 50 HPF). No further surgical or oncological treatment was required. Patients were followed clinically in an outpatient setting with surveillance abdominal CT performed at 6 month intervals. The follow-up period ranged from 8-17 mo. No recurrence was noted (Table 1).
| Patient | Age (yr) | Sex | Lesion location | Indication for EFTR | Size of resected specimen | Length of hospital stay |
| One | 81 | F | Gastric fundus, proximal body | GIST | 22 mm | 3 |
| Two | 79 | M | Gastric cardia | GIST | 28 mm | 4 |
| Three | 54 | M | Gastric cardia | GIST | 35 mm | 4 |
Traditionally, the majority of localized GISTs larger than 1 cm and involving the muscularis propria are managed via surgical resection. The consensus guidelines dictate that all GISTs greater than or equal to 2 cm in size should be resected[1]. Although a 2 cm cutoff is somewhat arbitrary, the most recent data indicates that this is appropriate[4]. The goal of surgery is complete resection of the tumor, leaving the pseudocapsule intact and obtaining negative margins. However, with recent innovations in advanced endoscopy, it is feasible to offer patients a less invasive approach for removal of their tumors[5].
Endoscopic resection of GISTs has been reported; however, as the procedure is technically challenging and numerous obstacles may arise, the role of endoscopic resection is controversial. First, tumors may be difficult to access by endoscopy. Second, if a tumor is located deep in the muscularis propria, removal involves EFTR, which is technically challenging and is limited by the size of the defect created. Thus, it presents the inherent risk of positive margins. Furthermore, full-thickness resection imparts a risk of tumor spillage. Finally, the EFTR results in an iatrogenic perforation. Historically, iatrogenic GI perforations were referred for surgery. However, developments in advanced endoscopy have paved the way for novel endoscopic closure techniques[6-8].
To date, different techniques have been designed for the closure of GI defects. Certain therapeutic methods have been more thoroughly explored in iatrogenic colonic perforations[9-11]. With the development of endoscopic mucosal resection and endoscopic submucosal dissection, iatrogenic perforations have been seen more frequently over the last 20-30 years; this has allowed endoscopists additional opportunities to perform endoscopic closure.
A retrospective study from Japan by Minami et al[12] revealed that endoclips are an effective conservative closure method for perforation caused by endoscopic mucosal resection. Multiple studies have reported on the use of the over-the-scope clip technique to close perforations[13-16]. All studies concluded that this technique is an effective endoluminal closure method; however, the studies in question only assessed method efficacy on relatively small defects. For closure of full-thickness defects, Stavropoulos et al[17] described the effectiveness of endoscopic suturing in clinical practice using the OverStitch. One limitation of this procedure is that it must be performed with a double channel gastroscope, which limits flexibility. Thus, suturing in a location such as the gastric fundus is often challenging.
The previously-noted closure methods may not be successful for large or difficult-to-access defects. Full-thickness resections will inherently leave a large defect. Hence, a novel technique for the closure of large and difficult-to-access defects caused by EFTR of GISTs located in the stomach was assessed in our series. Similar methods have been reported as early as 2001 in porcine models[18]. Comparable techniques have also been attempted and described in case reports[19,20]. While our report includes three cases, a larger sample size is needed to accurately assess the efficacy of this technique. If the closure of iatrogenic perforations with an omental patch using endoscopic techniques can be shown to be a reliable and reproducible procedure, it may become an attractive alternative to surgery, with the hope of reducing morbidity and mortality.
The omental plug closure technique may be used as an alternative to surgery in selected cases of gastric perforation. However, the technique requires the endoscopist to be experienced and proficient in endoscopic closure devices.
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ribeiro IB, Kwon KA, Aydin M S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Liu MY
| 1. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 822] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc. 2016;8:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 484] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 5. | Mori H, Kobara H, Nishiyama N, Masaki T. Current status and future perspectives of endoscopic full-thickness resection. Dig Endosc. 2018;30 Suppl 1:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Al Ghossaini N, Lucidarme D, Bulois P. Endoscopic treatment of iatrogenic gastrointestinal perforations: an overview. Dig Liver Dis. 2014;46:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Barrichello Junior SA, Ribeiro IB, Fittipaldi-Fernandez RJ, Hoff AC, de Moura DTH, Minata MK, de Souza TF, Galvão Neto MDP, de Moura EGH. Exclusively endoscopic approach to treating gastric perforation caused by an intragastric balloon: case series and literature review. Endosc Int Open. 2018;6:E1322-E1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | de Moura DTH, Ribeiro IB, Funari MP, Baptista A, Thompson CC, de Moura EGH. Novel use of a cardiac septal occluder to treat a chronic recalcitrant bariatric fistula after Roux-en-Y gastric bypass. Endoscopy. 2019;51:E111-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J Gastrointest Endosc. 2019;11:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 11. | Inayat F, Aslam A, Grunwald MD, Hussain Q, Hurairah A, Iqbal S. Omental Patching and Purse-String Endosuture Closure after Endoscopic Full-Thickness Resection in Patients with Gastric Gastrointestinal Stromal Tumors. Clin Endosc. 2019;52:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, Fockens P; CLIPPER Study Group. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion. 2012;85:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Yılmaz B, Unlu O, Roach EC, Can G, Efe C, Korkmaz U, Kurt M. Endoscopic clips for the closure of acute iatrogenic perforations: Where do we stand? Dig Endosc. 2015;27:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Stavropoulos SN, Modayil R, Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc. 2015;7:777-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Hashiba K, Carvalho AM, Diniz G, Barbosa de Aridrade N, Guedes CA, Siqueira Filho L, Lima CA, Coehlo HE, de Oliveira RA. Experimental endoscopic repair of gastric perforations with an omental patch and clips. Gastrointest Endosc. 2001;54:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Abidi WM, Thompson CC. Endoscopic closure of an iatrogenic perforation with an omental patch. Gastrointest Endosc. 2016;83:652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Mönkemüller K, Sarker S, Baig KR. Endoscopic creation of an omental patch with an over-the-scope clip system after endoscopic excavation and resection of a large gastrointestinal stromal tumor of the stomach. Endoscopy. 2014;46 Suppl 1 UCTN:E451-E452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |