Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.903
Peer-review started: December 19, 2018
First decision: January 6, 2019
Revised: January 24, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: April 6, 2019
Processing time: 108 Days and 1.8 Hours
Congenital extrahepatic portosystemic shunt, also known as Abernethy deformation, is a rare malformation caused by dysplasia in the portal vein system. There are few reports of liver transplantation as a treatment for Abernethy deformation, and our report is the first case in China. This is the second reported case with congenital extrahepatic portosystemic shunt combined with focal nodular hyperplasia and hepatopulmonary treated with liver transplantation.
The patient was a 14-year-old girl, diagnosed preoperatively as type Ib Abernethy deformation, intrahepatic multiple space-occupying lesion, and hepatopulmonary syndrome. The patient recovered well after undergoing classic orthotopic liver transplantation. Liver function, pulmonary function, and portal vein computed tomography angiography imaging were reexamined 20 mo postoperatively, and no abnormality was observed.
Liver transplantation is an effective treatment for type I Abernethy deformation combined with focal nodular hyperplasia and hepatopulmonary syndrome.
Core tip: Congenital extrahepatic portosystemic shunt is a rare congenital abnormal vascular anastomosis between the portal vein and vena cava. This report discusses clinical, angiographic diagnostic and histopathological features in one patient with congenital extrahepatic portosystemic shunt, as well as its surgical strategy. Liver transplantation appears to be a feasible treatment option for patients with congenital extrahepatic portosystemic shunt because it achieves optimal treatment effectiveness.
- Citation: Xiang W, Wang H, Si ZZ, Chen GS, Wang GW, Li T. Type I congenital extrahepatic portosystemic shunt treated by orthotopic liver transplantation: A case report. World J Clin Cases 2019; 7(7): 903-907
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/903.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.903
The first case of congenital absence of the portal vein was found during an autopsy on a girl aged 1 yr and 10 mo by John Abernethy in 1793[1]. With a gradual understanding of the disease and development of vascular ultrasound, abdominal computed tomography (CT), nuclear magnetic resonance, abdominal vascular ultrasound, more clinical cases have been published[2]. It has been reported that a patient with Abernethy deformation can be diagnosed as early as the prenatal stage. The oldest age at diagnosis has been 64 yr. Approximately 80% of patients are diagnosed in childhood. Clinical manifestations of Abernethy deformation are primarily associated with hepatic vascular shunt. Liver transplantation is the only treatment that can reverse Abernethy deformation symptoms. However, there is still no unified regimen for the selection of the surgical procedure. There are a few reports on liver transplant as a treatment for Abernethy deformation. This article is the first reported case of Abernethy deformation treated with orthotopic liver transplantation in China and is the second reported case of congenital extrahepatic portosystemic shunt combined with focal nodular hyperplasia and hepatopulmonary syndrome treated with liver transplantation.
A 14-year-old girl had pain in the left epigastrium for more than 10 d, with no tarry stool, hematemesis, or coma history. She normally liked squatting, and her activity tolerance was low. She was normally developed without positive signs.
The results of laboratory examinations were as follows: routine blood examination: red blood cell 4.64 × 109/L, white blood cell 4.83 × 109/L, blood platelet 218 × 109/L; blood biochemistry: alanine aminotransferase 36.9 μ/L, aspartate aminotransferase 30.6 μ/L, serum albumin 41.9 g/L, total bilirubin 21.7 μmol/L, direct bilirubin 8.0 μmol/L, PT 12.8 s, international normalized ratio 1.13. Blood ammonia 22.4 μmol/L, lactic acid 3.28 mmol/L. No abnormality was observed in arterial blood gas parameters at resting state.
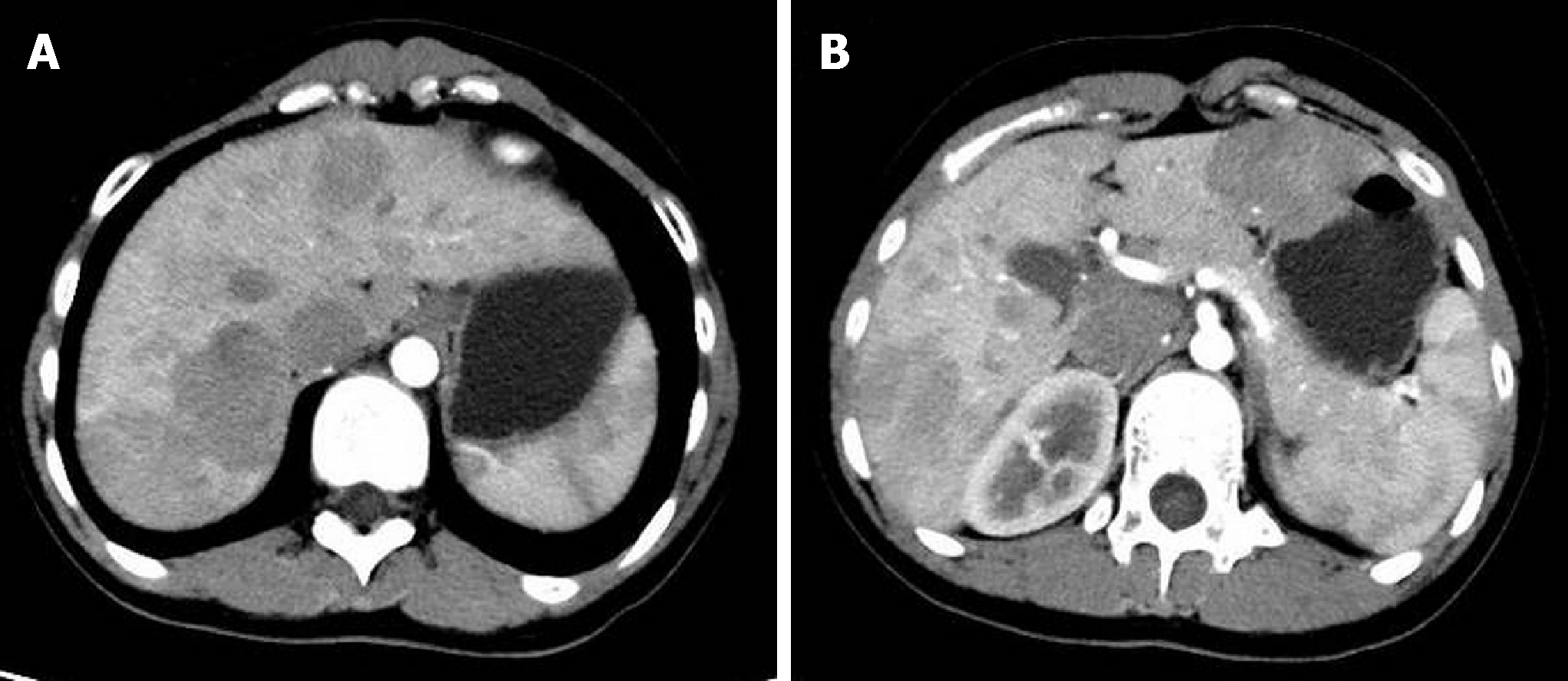
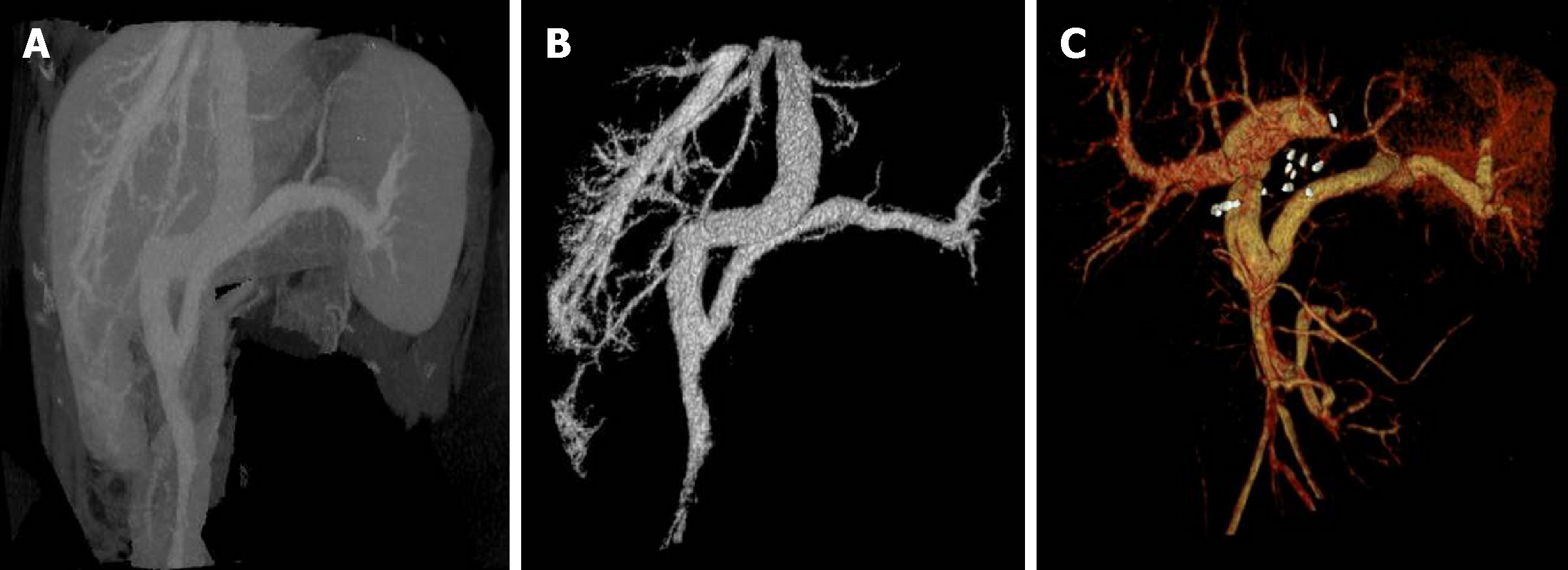
CT suggested congenital extrahepatic portacaval diversion (Type Ib Abernethy deformation). The superior mesenteric vein and splenic vein joined to form a short extra-hepatic portal vein that drained into a postcava with left hepatic vein, associated with double pulmonary congestion and pulmonary arterial hypertension. There were multiple space-occupying lesions in the liver, and the size of the relatively large tumor was approximately 50 mm. To exclude malignant tumor transformation, positron emission tomography-CT examination was performed (Figure 1 and Figure 2). The results indicated nodules and mass imaging with multiple increased glycometabolism in the liver parenchyma and increased 11C-choline metabolism in some lesions. Multiple focal nodular hyperplasia combined with partial malignant transformation was highly possible. Echocardiography suggested the mean pressure of pulmonary artery at 36 mmHg and light pulmonary arterial hypertension. Pulmonary function examination indicated severe diffusion impairment.
(1) Type 1b Abernethy deformation; (2) Focal nodular hyperplasia; and (3) Hepa-topulmonary disease.
Liver transplantation from donation after cardiac death of the donor liver was performed. The patient recovered well after being given anti-infective therapy, baliximab immune induction, and FK-506 + mycophenolate mofetil antirejection therapy postoperatively.
After discharge, the patient was followed up for 20 mo. Reexamination results showed normal liver function, with no abnormalities observed during the pulmonary respiratory function examination.
Congenital extrahepatic portosystemic shunt, also known as Abernethy deformation, is a rare malformation in congenital extrahepatic portosystemic shunt caused by dysplasia in the portal vein system. It is characterized by congenital abnormal vascular anastomosis between the portal vein and vena cava. Blood flow in the portal vein does not or only partially drains into the liver, mostly via the lateral branch of the extrahepatic portal vein.
Based on the characteristics of abnormal vascular anastomosis between the portal vein and vena cava, the congenital portal-systemic shunt can be divided into two types. Type I: complete loss of portal vein perfusion in the liver or type II: partial portal vein blood flow draining to the liver. Type I is further divided into type Ia and type Ib. Type Ia: separate drainage of the superior mesenteric vein and splenic vein; type Ib: superior mesenteric vein and splenic vein join and drain into the inferior vena cava[3]. Congenital portal vein absence belongs to type I Abernethy deformation. The occurrence may be related to abnormal development of the ovarian vein and azygos vein during embryogenesis. Type I deformation mostly occurs in females. The most common abnormality is the communication between the superior mesenteric vein and infrahepatic vena cava or left renal vein, often associated with congenital mal-formation of other organs (such as heart malformation, biliary atresia, and polysplenia), hepatic nodular hyperplasia, and tumors. Generally, type II is the deformation of the portal vein alone, which often occurs in men. Venous blood in the gastrointestinal tract shunts into the vena cava via abnormal side-to-side anastomosis.
The clinical manifestations of congenital hydrodynamic shunt patients are primarily related to liver vascular shunt. The main manifestations include hepatic encephalopathy, pulmonary arterial hypertension, and hepatopulmonary syndrome. Some patients may also have with spontaneous hypoglycemia, followed by normal manifestations of liver function damage, such as hyperammonemia, jaundice, weakness, and right upper quadrant pain.
The patient in this study had clinical hepatopulmonary characteristics: liver disease, right-left shunt caused by hypoxia and pulmonary vasodilation, and pulmonary arterial hypertension. It could be related to the influence of portal-systemic shunt on the metabolism of hepatic vasoactive substances[4]. Cirrhosis combined with portal-systemic shunt is the most common hepatopulmonary pathogenesis[5]. Patients with Abernethy deformation often have no obvious or delayed portal hypertension, due to abnormal blood flow between the portal vein and vena cava[6]. Young patients often develop hyperammonemia, and most older patients show hepatic encephalopathy, which may be related to increased sensitivity to blood ammonia and other toxicants and portal-systemic shunt in the aging brain. Patients with 60% portal-systemic shunt or above have a high possibility of developing hepatic encephalopathy[7,8]. In this case, the patient had normal blood ammonia without symptoms of hepatic encephalopathy. A few patients may have hepatic space-occupying lesions, such as liver focal nodular hyperplasia, hepatic adenoma, and liver cancer[9,10]. It may be related to nutrient substance transfer in the liver and increased blood flow in the hepatic artery, leading to changes in liver function and regeneration function.
Because all patients have the possibility of malignant transformation, we should regularly follow up with serology and imaging, whether combined with space-occupying liver or hyperplastic lesion. Some patients may display deformations in other sites, such as heart, vessel, biliary tract, pancreas, and intestinal tract[4]. Treatment for Abernethy deformation, typing and symptom complications are related to the accompanying diseases. For type I Abernethy deformation patients, given the development into hepatic nodular regeneration, hepatic benign tumor, or the possibility of malignant transformation, liver transplantation has been considered a good treatment. Transplantation not only hinders the development of hepatic pathological changes, but also rebuilds a normal portal vein system, with recovery to a normal anatomical structure[11,12]. Liver transplantation is also considered an ideal therapeutic method for Abernethy deformation combined with hepatopulmonary defects[13,14]. Liver transplantation is the only treatment that can reverse Abernethy deformation symptoms.
However, the selection of surgical procedure is still controversial[7,8,10]. Residual donor liver cannot be guaranteed to meet the requirements of a normal body. More importantly, the characteristics of hepatic focal nodule of Abernethy deformation are not clear, and the possibility of malignant transformation of lesions or recurrence after transplantation is very high. Thus, assisted partial orthotopic liver transplantation is not a recommended surgical procedure[8,15]. Therefore, we chose orthotopic liver transplantation from a corpse donor. The patient has recovered well without appearance of hepatic encephalopathy symptoms or regenerative nodules in the transplanted liver.
Liver transplantation is an effective treatment for Abernethy deformation combined with focal nodular hyperplasia and hepatopulmonary syndrome.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Boin IF, Stanciu C, Takama Y S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Abernethy J. Account of Two Instances of Uncommon Formation in the Viscera of the Human Body: From the Philosophical Transactions of the Royal Society of London. Med Facts Obs. 1797;7:100-108. [PubMed] |
| 2. | Brasoveanu V, Ionescu MI, Grigorie R, Mihaila M, Bacalbasa N, Dumitru R, Herlea V, Iorgescu A, Tomescu D, Popescu I. Living Donor Liver Transplantation for Unresectable Liver Adenomatosis Associated with Congenital Absence of Portal Vein: A Case Report and Literature Review. Am J Case Rep. 2015;16:637-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg. 1994;29:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 296] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 5. | Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Witters P, Maleux G, George C, Delcroix M, Hoffman I, Gewillig M, Verslype C, Monbaliu D, Aerts R, Pirenne J, Van Steenbergen W, Nevens F, Fevery J, Cassiman D. Congenital veno-venous malformations of the liver: widely variable clinical presentations. J Gastroenterol Hepatol. 2008;23:e390-e394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Crespin J, Nemcek A, Rehkemper G, Blei AT. Intrahepatic portal-hepatic venous anastomosis: a portal-systemic shunt with neurological repercussions. Am J Gastroenterol. 2000;95:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Singhal A, Srivastava A, Goyal N, Vij V, Wadhawan M, Bera M, Gupta S. Successful living donor liver transplant in a child with Abernethy malformation with biliary atresia, ventricular septal defect and intrapulmonary shunting. Pediatr Transplant. 2009;13:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Chevallier P, Oddo F, Souci J, Diaine B, Padovani B. [Macroscopic intrahepatic portosystemic venous shunt: review of the literature and reclassification]. J Radiol. 2000;81:597-604. [PubMed] |
| 10. | Murray CP, Yoo SJ, Babyn PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Hobeika J, Houssin D, Bernard O, Devictor D, Grimon G, Chapuis Y. Orthotopic liver transplantation in children with chronic liver disease and severe hypoxemia. Transplantation. 1994;57:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 12. | Howard ER, Davenport M. Congenital extrahepatic portocaval shunts--the Abernethy malformation. J Pediatr Surg. 1997;32:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 169] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Guérin F, Blanc T, Gauthier F, Abella SF, Branchereau S. Congenital portosystemic vascular malformations. Semin Pediatr Surg. 2012;21:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Kanamori Y, Hashizume K, Kitano Y, Sugiyama M, Motoi T, Tange T. Congenital extrahepatic portocaval shunt (Abernethy type 2), huge liver mass, and patent ductus arteriosus--a case report of its rare clinical presentation in a young girl. J Pediatr Surg. 2003;38:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Elias N, Scirica CV, Hertl M. Liver transplantation for the Abernathy malformation. N Engl J Med. 2008;358:858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |