Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.872
Peer-review started: December 11, 2018
First decision: January 26, 2019
Revised: February 20, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 6, 2019
Processing time: 116 Days and 8.2 Hours
Hepatoblastoma (HB) is the most common hepatic malignant tumour in children, accounting for approximately 50%-60% of primary hepatic malignant tumours in children, mostly in children under 3 years old. In Western countries, the incidence of hepatoblastoma is approximately 1-2/100000. Da Vinci surgical system is fast becoming a key instrument in microinvasive surgery. The past decade has seen the rapid development of robot-assisted laparoscopy, which expends many fields including the liver surgery. This paper discusses the significance and feasibility of robot-assisted gallbladder-preserving hepatectomy for treating S5 hepatoblastoma in children. The aim of this essay is to compare the safety and effectiveness of robotic surgery with conventional laparoscopic surgery, and explore the meaning of preservation of the gallbladder by sharing this case.
A 3-year-old child with a liver mass in the 5th segment was treated using the Da Vinci surgical system, and the gallbladder was retained. The child was admitted to the hospital for 20 d for the discovery of the right hepatic lobe mass. Ultrasonography revealed a low echo mass, 46 mm × 26 mm × 58 mm in size, indicating hepatoblastoma in the right lobe, and enhanced computed tomography showed continuous enhancement of iso-low-density lesions with different sizes and nodules and unclear boundaries, without the dilation of the intrahepatic bile duct, no enlargement of the gallbladder, and uniform thickness of the wall. The diagnosis was “liver mass, hepatoblastoma”. It was decided to perform S5 liver tumour resection. During surgery, the tumour and gallbladder were isolated first, and the gallbladder could be completely separated from the tumour surface without obvious infiltration; therefore, the gallbladder was preserved. The cutting line was marked with an electric hook. The hepatic duodenal ligament was blocked with a urethral catheter using the Pringle method, and the tumour and part of the normal liver tissue were completely resected with an ultrasound knife along the incision. The hepatic portal interdiction time was approximately 25 min. An abdominal drainage tube was inserted. The auxiliary hole was connected to the lens, and the specimen was removed. The patient’s status was uneventful, and the operation time was 166 min. The robotic time was 115 min, and the bleeding amount was approximately 200 mL. In total, 300 mL of red blood cell suspension and 200 mL of plasma were injected. No serious complications occurred. Pathological findings confirmed fetal hepatoblastoma and R0 resection. A gallbladder contraction test was performed two weeks after surgery.
Robot-assisted S5 hepatectomy with gallbladder preservation is safe and feasible for specific patients.
Core tip: Our paper describes the key surgical points of gallbladder-preserving hepatectomy for treating S5 hepatoblastoma performed completely with the Da Vinci robotic system. A gallbladder-preserving hepatectomy was carried out for a boy at our hospital, and then systematic literature review was performed to discuss the significance and feasibility of preserving gallbladder during hepatectomy and the surgical safety and advantages, and compare the safety and effectiveness of robot-assisted surgery and traditional laparoscopic surgery.
- Citation: Chen DX, Wang SJ, Jiang YN, Yu MC, Fan JZ, Wang XQ. Robot-assisted gallbladder-preserving hepatectomy for treating S5 hepatoblastoma in a child: A case report and review of the literature. World J Clin Cases 2019; 7(7): 872-880
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/872.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.872
Hepatoblastoma (HB) is the most common hepatic malignant tumour in children, accounting for approximately 50%-60% of primary hepatic malignant tumours in children, mostly in children under 3 years old[1]. In Western countries, the incidence of hepatoblastoma is approximately 1-2/100000[2-4]. In Asian children, the incidence of hepatoblastoma is higher relative to that in other countries and regions[4,5]. Hepa-toblastoma has a high degree of malignancy and a short postoperative survival.
The Da Vinci system has been the first robotic surgery system approved by the Food and Drug Administration in the United States, and it is also the most widely used robot system both in China and other countries. In recent years, robotic surgery system has been gradually applied in paediatric surgery and now involves various types of operations in paediatric urology, cardiovascular surgery, and hepatobiliary surgery[6-11].
The development of paediatric surgical robotic surgery in China is in its infancy, and the therapeutic effects of paediatric surgery are influenced by various factors, such as the child’s age, disease status, anatomical positions of the lesions, posture, and surgical complexity, which also enable robotic surgery to give full play to its advantages in the field of paediatric surgical treatment[12-16].
Herein, we report on the application of the Da Vinci surgical system for the treatment of a child’s 5th segment liver tumour and the preservation of the gallbladder, with the aim to explore the feasibility of using the full robotic surgery to treat a child’s liver tumour and to summarize the application points of the technology.
A 3-year-old boy was admitted to the hospital for a liver mass for 20 d.
The abdomen was soft, without tender, rebound pain, or obvious mass. The liver and spleen were untouchable, Murphy's sign was negative, and the bowel sounds were normal.
Alpha fetoprotein (AFP) was 339 μg/L, alanine aminotransferase was 11.9 U/L, and aspartate aminotransferase was 22.4 U/L. International normalized ratio was 0.97, and total bilirubin was 5.6 μmol/L.
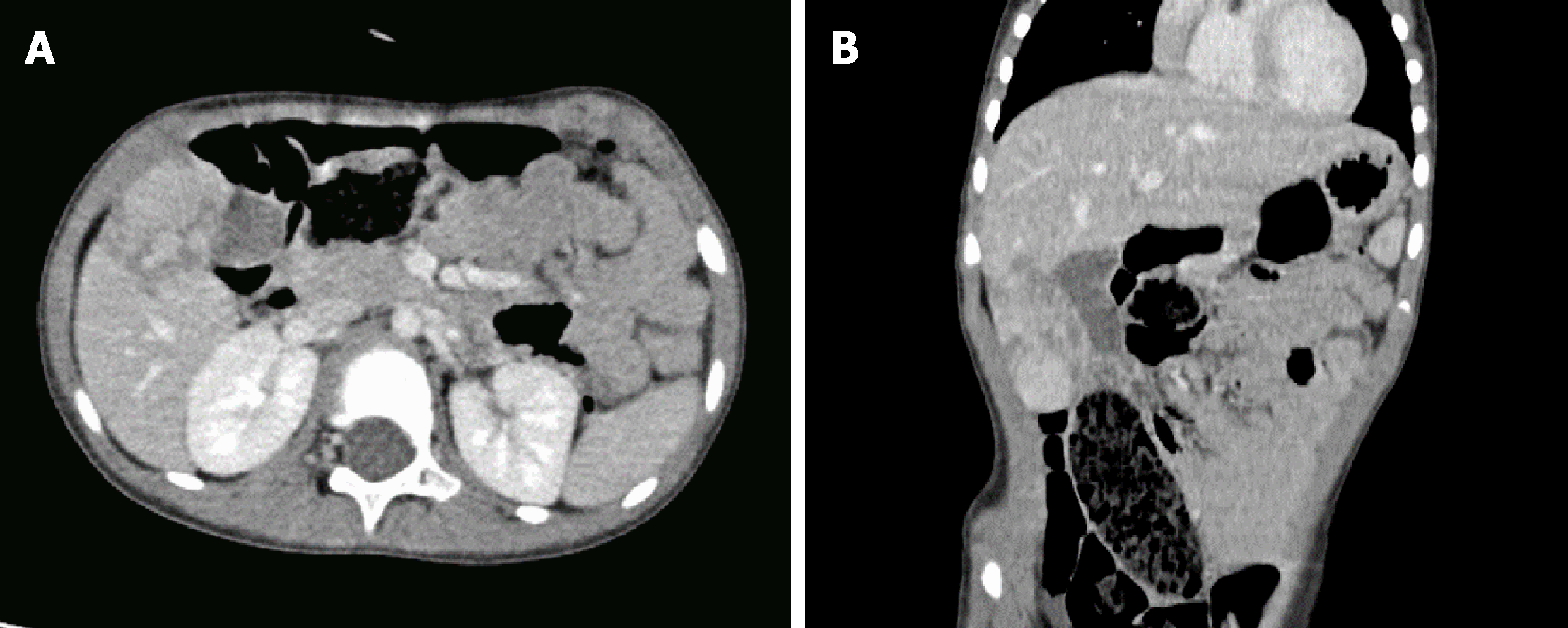
Ultrasonography revealed a low-echo mass measuring 46 mm × 26 mm × 58 mm in the liver right lobe. Enhanced computed tomography showed continuous en-hancement of iso-low-density lesions of different sizes and nodules, unclear boundaries, no dilation of the intrahepatic bile duct, no enlargement of the gallbladder, and uniform thickness of the wall (Figure 1).
The preoperative diagnosis was hepatoblastoma.
The preoperative examination was completed, and the robotic S5 liver tumour resection was performed.
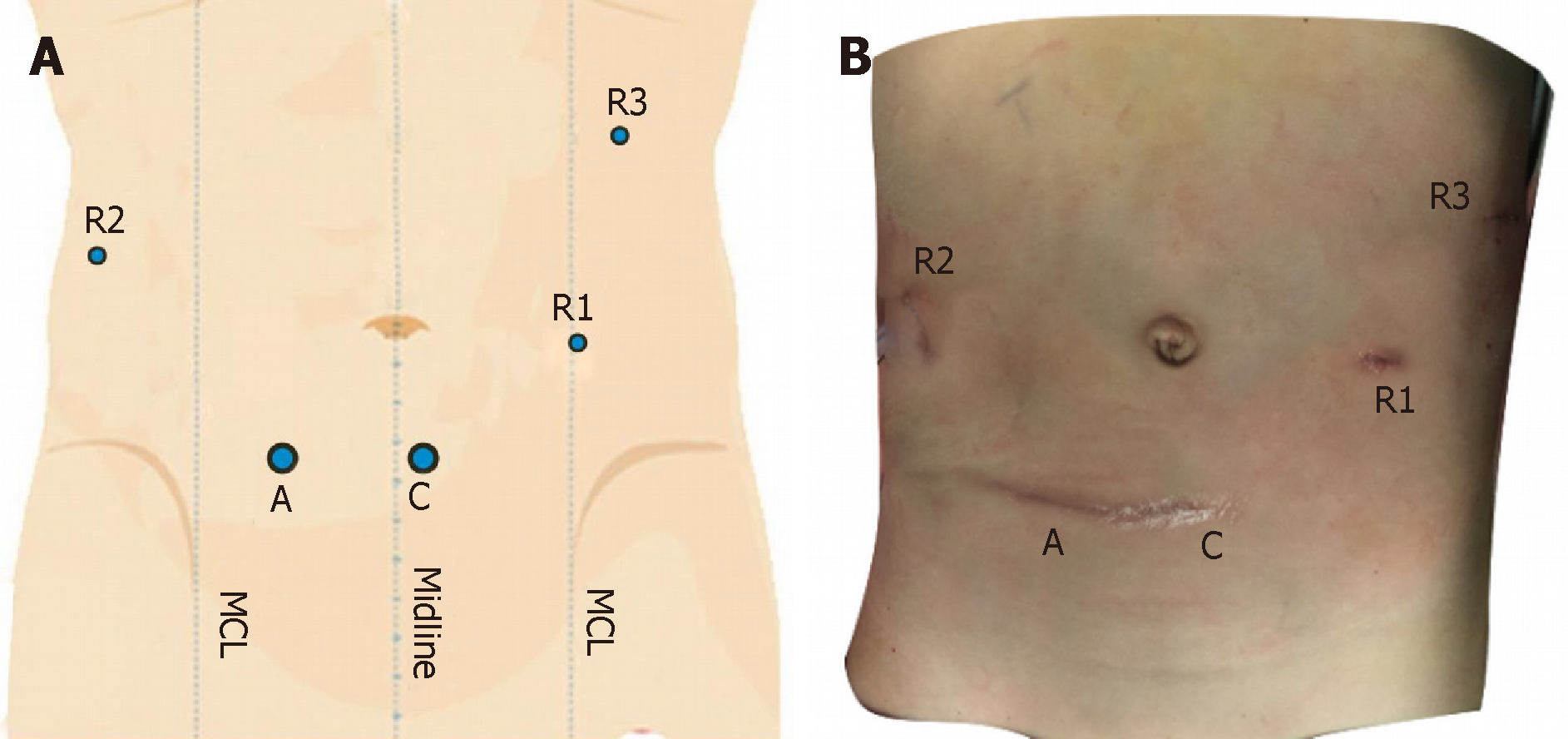
After successful anaesthesia, urethral catheterization was performed. The anaesthesiologist placed an internal jugular vein catheter, and the Trendelenburg position was adopted, after which the lower chest was raised with a mattress. The Direct Trocar Entry method was used to puncture suprapubic area to establish the pneumoperitoneum (Figures 2 and 3). The pressure was 10 cm H2O, and a 12-mm trocar and the robot lens were inserted. On the left and right sides of the lens, the arm 1 and arm 2 trocars with a diameter of 8 mm were inserted. Arm 3 was placed in the midline of the left upper quadrant axilla. A 12-mm auxiliary hole was placed between arm 2 and the lens. The robot arm was placed at the head end. After the mechanical arm was connected and pulled, the abdominal pressure was reduced to 8 mmHg.
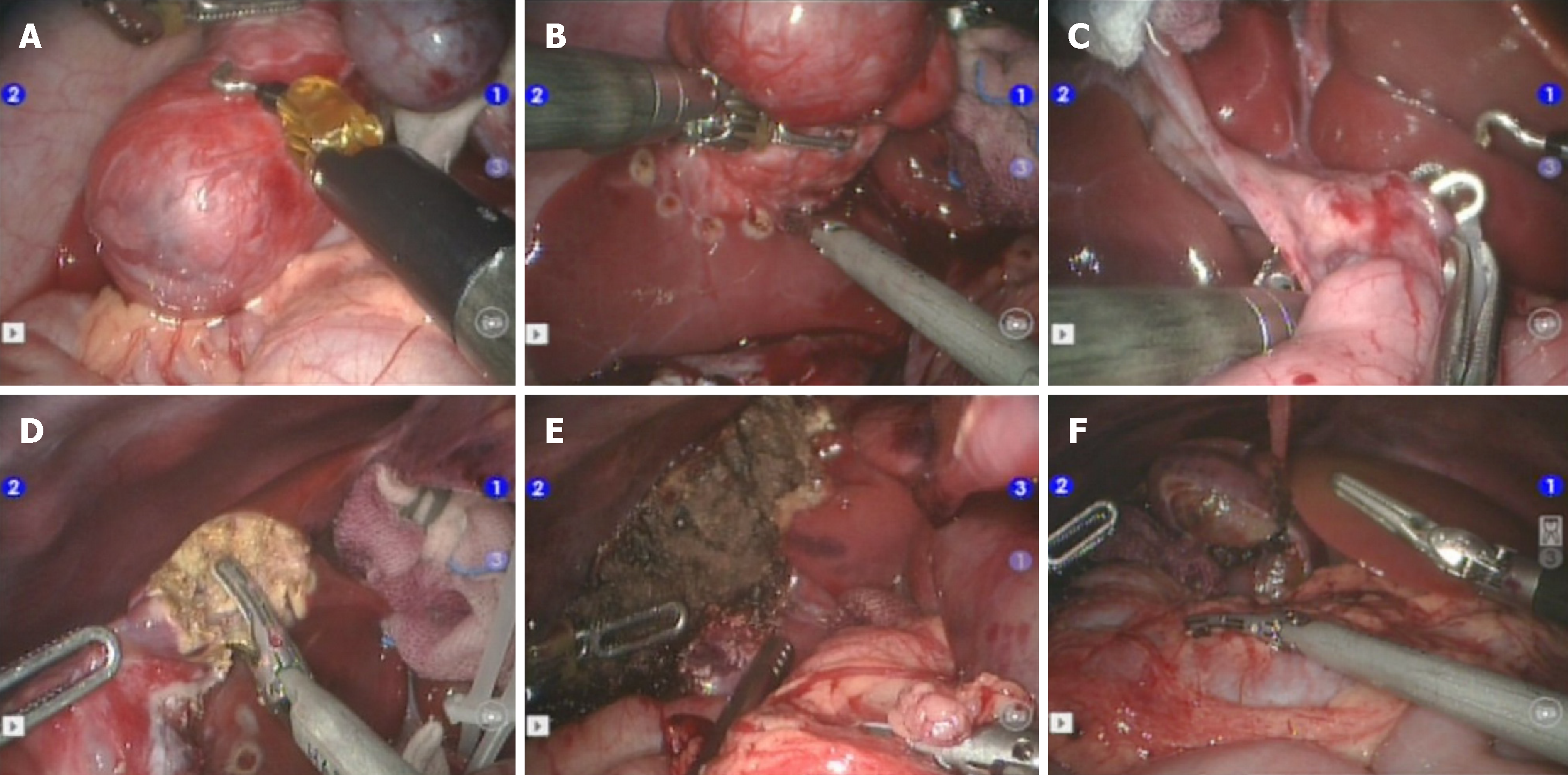
A 5th segment right hepatic tumour could be seen through exploration. The tumour and gallbladder were isolated first, and the gallbladder could be completely separated from the tumour surface without obvious infiltration; therefore, the gallbladder was preserved (Figure 4).
The incisal edge was marked with an electrical hook at the junction between the tumour and normal liver tissue at approximately 1 cm, and the hepatic duodenal ligament was blocked with a urethral catheter using the Pringle method. The tumour and part of the normal liver tissue were completely resected with an ultrasonic knife along the incision, and the blood vessel was ligated with a 5-0 prolene line for haemostasis. The first hepatic portal block time was approximately 25 min.
The liver was cogulated with an argon knife to stop bleeding. A liver needle was used to suture the broken edge. An abdominal drainage tube was inserted from the right lower abdomen via arm 2. The auxiliary hole was connected to the lens hole, and the specimen was removed (Figure 5).
The abdominal wall muscle at the trocar was sutured intermittently with 3-0 absorbable suture, the subcutaneous tissue was sutured discontinuously with 4-0 absorbable suture, and the skin was glued with bioprotein. The membranes of all incised skin were pulled tight and covered.
The operation was smooth, with 200 mL of bleeding, and the patient was returned to the ward after surgery.
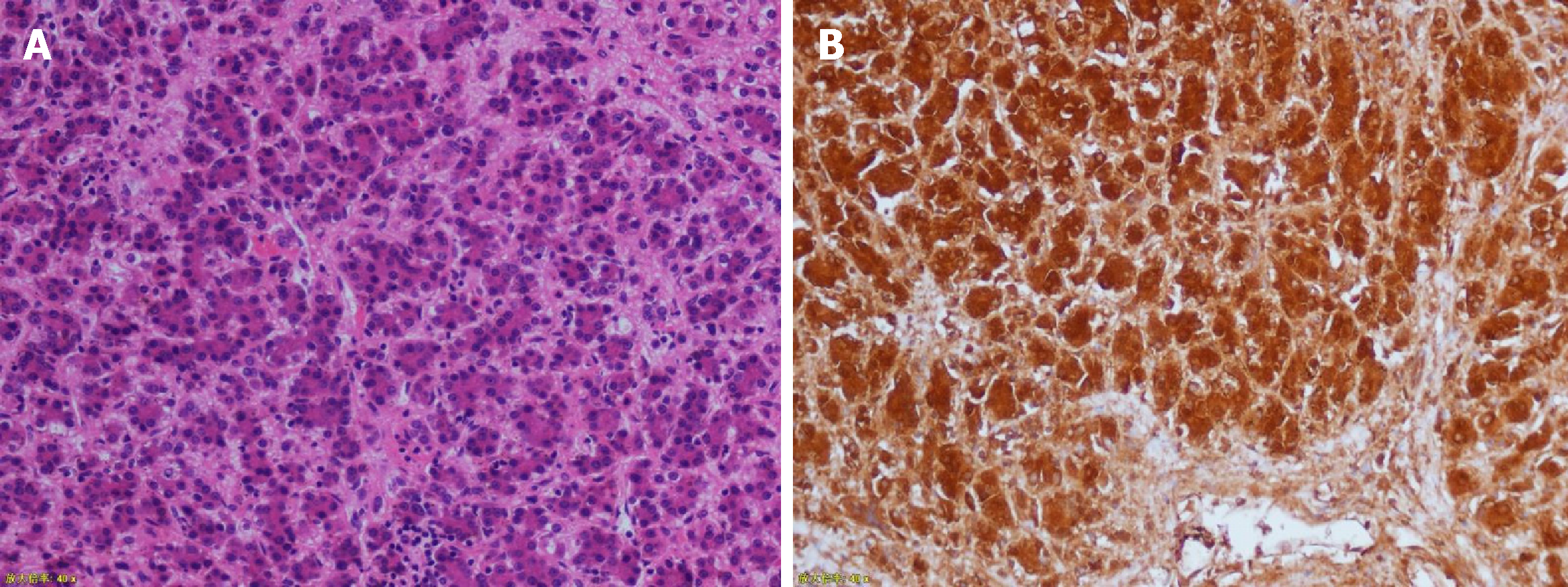
The operation went well. The total time was 166 min, of which the robot operation time was 115 min, and there was a blood loss of approximately 200 mL, with the provision of 300 mL of red blood cell suspension and 200 mL of plasma. The gastric tube was pulled out two days after the operation, and the patient was gradually given liquid food and semi-liquid food. Two combination antibiotics were administered for 5 d. The abdominal drainage tube was removed 6 d after the operation, and the patient was discharged 12 d after the operation. No serious complications occurred. The immunohistochemical results were as follows: hepatocytes (+), GPC-3 (+), AFP (focal +), ki-67 (+20%), Arg-1 (+), and CD99 (local) (Figure 6). The gallbladder contraction test conducted two weeks after the operation showed that the gallbladder size was approximately 5.3 cm × 2.0 cm, and the wall was not thick and smooth. Forty minutes after a meal, the gallbladder size was 5.4 cm × 1.8 cm. Ninety minutes after a meal, the gallbladder size was 2.9 cm × 1.2 cm.
Hepatoblastoma is the most common type of liver tumour for children in recent years. A number of scholars have studied the aetiology, pathogenesis, and possible course of hepatoblastoma in children from different perspectives, suggesting that it may be related to chromosomal abnormality, genetic factors, low birth weight, and various external adverse factors involving maternal exposure during pregnancy[17]. According to the American paediatric oncology group, higher preoperative clinical stage is associated with worse prognosis for hepatoblastoma in children. Multiple domestic and foreign studies have shown that the survival rate of hepatoblastoma can be significantly improved by combining surgery with chemotherapy, and the complete resection of the tumour is a prerequisite for the treatment of hepatoblastoma[18,19].
The Da Vinci surgery system (Intuitive Surgical, Inc., Mountain View, CA, USA) is the most widely used robotic system. The Da Vinci surgery robot system has a unique 3-dimensional HD magnification (10×) imaging system, and the separation process is more accurate, effectively avoiding secondary damage[20-22]. The robot’s artificial wrist arm with flutter filtration function has better dexterity and a larger range than traditional laparoscopic instruments, making the anastomosis process easier and capable of more delicacy.
Over the last decade, robot-assisted laparoscopy has been included in the surgical armamentarium to manage complex abdominal scenarios, including those encountered in liver surgery[23-25].
From a technical point of view, the use of a robotic surgical system can improve certain steps of minimally invasive right liver resection. Magnified 3-dimensional vision allows for better definition of the vascular anatomy and improved recognition of the fine branches that originate from the right portal trunk and are directed toward segment 1. Furthermore, by taking advantage of the wristed instruments, it is possible to use simple ligatures to control major vessels instead of the stapler, which can be cumbersome in small spaces[26].
Characterized by its precision and minimally invasive nature, hepatobiliary and pancreatic surgery is further developed on the basis of traditional laparoscopic surgery, which expands the advantages of laparoscopic surgery[27] and the application scope of minimally invasive technology represented by laparoscopy in some fields and overcomes some deficiencies of traditional laparoscopy. It also represents the developmental direction of surgery.
The first report of robotic liver resection dates back to 2003, when an initial study of robotic anatomic liver resection was published by Giulianotti et al[28], 10 years after the report of the first laparoscopic liver resection (LLR). Giulianotti et al[26] have proved through research that partial hepatectomy by robot offered the patient decreased abdominal wall morbidity, shorter postoperative hospital length of stay, and minimal need for blood transfusion.
Several studies have reported the efficacy and safety of robotic surgeries for various diseases, which have outcomes comparable to those of conventional or laparoscopic surgeries[22,29]. Lai et al[30] compared the perioperative indicators and long-term tumour outcomes of partial hepatectomy by robot (n = 100) and laparoscopic (n = 35) methods. Compared to the laparoscopic group, the robotic group had significantly higher mass liver resection rate (27% vs 2.9%) and upper posterior segment location rate (29% vs 0%). The mean operative time of the robot group was longer, and there were no statistically significant differences between the two groups in blood loss, complications and mortality, R0 resection rate, 5-year total survival, or 5-year disease-free survival.
Compared with laparoscopic hepatectomy, robot-assisted surgery has shown better safety and effectiveness in the treatment of liver tumours, and the system has greatly expanded the surgical indications for hepatectomy. Regarding safety considerations, compared with LLR, robotic-assisted liver resection (RALR) has the advantages of reducing total postoperative complications, postoperative bleeding, and postoperative biliary fistula, with no significant difference in the amount of intraoperative blood loss between the two surgical methods. Regarding effectiveness, RALR improved the rate of R1 excision compared with LLR, and there were no significant differences between the two groups in terms of postoperative hospitalization time, or rate of conversion to open surgery[30-34]. With improvements and upgrades of equipment and technology, the advantages of robot-assisted laparoscopic hepatectomy will become increasingly more obvious[35-37].
There are still some defects in robotic surgery. For example, the high cost of surgical robots limits the popularity of robotic surgery. Although special instruments for surgical robots have been developed, they are still not perfect, and some surgical instruments are urgently needed. The lack of a tactile feedback system with the surgical robot cannot fully play the characteristics of surgical robot. At present, training for robot operators can only be carried out in a few centres, which limits its further promotion. In addition, as robotic surgery is relatively new in the field of open surgery, its temporary high cost makes it less popular than laparoscopy, resulting in a lack of high quality, large prospective studies of robotic surgery. However, these shortcomings are improving over time.
In a study by Dong et al[38], the gallbladders were retained in 28 patients undergoing living donor liver transplantation (15 cases of right lobe liver and 13 cases of left lobe liver). The postoperative gallbladder function was good, and it was safe and feasible to retain the gallbladder when liver transplantation was performed. It has been proved that retaining the gallbladder can avoid post-cholecystectomy syndrome, reduce the incidence of biliary diseases, such as gallstones and Oddi sphincter dysfunction, reduce the occurrence of postoperative adipose diarrhoea, improve the quality of life of children, and reduce the incidence of possible colon cancer.
The main gallbladder changes after surgery included gallbladder enlargement and gallbladder wall thickening, and the main complications included biliary sludge, gallstone formation, and polypoid lesion of the gallbladder[39]. Su et al[39]’s results demonstrated that the rate of postoperative complications of the gallbladder in donors was relative low, thus preserving the gallbladder in liver transplantation donors during liver graft procurement is feasible and safe.
For patients who are children, the complete preservation of the gallbladder and its normal function on the basis of complete tumour resection is of great significance for improving quality of life and long-term prognosis.
In summary, robot-assisted hepatectomy with gallbladder preservation is safe and feasible. Preliminary experience showed that the surgical method is clearer and more flexible than conventional endoscopy, with more accurate surgery and less resulting damage. With continued technological development and the accumulation of surgeons’ experience, robotic surgery may become a new trend in surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Otto G, Marion R S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, Aronson DC, Malogolowkin MH, Perilongo G, von Schweinitz D, Ansari M, Lopez-Terrada D, Tanaka Y, Alaggio R, Leuschner I, Hishiki T, Schmid I, Watanabe K, Yoshimura K, Feng Y, Rinaldi E, Saraceno D, Derosa M, Czauderna P. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol. 2017;18:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 3. | Sumazin P, Chen Y, Treviño LR, Sarabia SF, Hampton OA, Patel K, Mistretta TA, Zorman B, Thompson P, Heczey A, Comerford S, Wheeler DA, Chintagumpala M, Meyers R, Rakheja D, Finegold MJ, Tomlinson G, Parsons DW, López-Terrada D. Genomic analysis of hepatoblastoma identifies distinct molecular and prognostic subgroups. Hepatology. 2017;65:104-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford). 2013;15:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Buendia MA, Armengol C, Cairo S. Molecular classification of hepatoblastoma and prognostic value of the HB 16-gene signature. Hepatology. 2017;66:1351-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Passerotti C, Peters CA. Pediatric robotic-assisted laparoscopy: a description of the principle procedures. ScientificWorldJournal. 2006;6:2581-2588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Casale P. Robotic pediatric urology. Expert Rev Med Devices. 2008;5:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Reinhardt S, Ifaoui IB, Thorup J. Robotic surgery start-up with a fellow as the console surgeon. Scand J Urol. 2017;51:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Berlinger NT. Robotic surgery--squeezing into tight places. N Engl J Med. 2006;354:2099-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Vasilyev NV, Dupont PE, del Nido PJ. Robotics and imaging in congenital heart surgery. Future Cardiol. 2012;8:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kant AJ, Klein MD, Langenburg SE. Robotics in pediatric surgery: perspectives for imaging. Pediatr Radiol. 2004;34:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hu MG, Xiao YH, Song DD, Zhao GD, Liu YZ, Wang Z, Li HY, Liu R. First experience of robotic spleen-preserving distal pancreatectomy in a child with insulinoma. World J Surg Oncol. 2017;15:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Li B, Liu DB, Gong EM. Robot-assisted laparoscopic transplant-to-native ureteroureterostomy of an intraperitoneal renal allograft. J Pediatr Urol. 2018;14:356-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sang H, Yun J, Monfaredi R, Wilson E, Fooladi H, Cleary K. External force estimation and implementation in robotically assisted minimally invasive surgery. Int J Med Robot. 2017;13:e1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wang XQ, Xu SJ, Wang Z, Xiao YH, Xu J, Wang ZD, Chen DX. Robotic-assisted surgery for pediatric choledochal cyst: Case report and literature review. World J Clin Cases. 2018;6:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wu EL, Garstka ME, Kang SW, Kandil E. Robotic Neck Surgery in the Pediatric Population. JSLS. 2018;22:e2018.00012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Kalish JM, Doros L, Helman LJ, Hennekam RC, Kuiper RP, Maas SM, Maher ER, Nichols KE, Plon SE, Porter CC, Rednam S, Schultz KAP, States LJ, Tomlinson GE, Zelley K, Druley TE. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res. 2017;23:e115-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | O'Neill AF, Towbin AJ, Krailo MD, Xia C, Gao Y, McCarville MB, Meyers RL, McGahren ED, Tiao GM, Dunn SP, Langham MR, Weldon CB, Finegold MJ, Ranganathan S, Furman WL, Malogolowkin M, Rodriguez-Galindo C, Katzenstein HM. Characterization of Pulmonary Metastases in Children With Hepatoblastoma Treated on Children's Oncology Group Protocol AHEP0731 (The Treatment of Children With All Stages of Hepatoblastoma): A Report From the Children's Oncology Group. J Clin Oncol. 2017;35:3465-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Iacob D, Serban A, Fufezan O, Badea R, Iancu C, Mitre C, Neamţu S. Mixed hepatoblastoma in child. Case report. Med Ultrason. 2010;12:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Kitisin K, Packiam V, Bartlett DL, Tsung A. A current update on the evolution of robotic liver surgery. Minerva Chir. 2011;66:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Warren H, Dasgupta P. The future of robotics. Investig Clin Urol. 2017;58:297-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Koh DH, Jang WS, Park JW, Ham WS, Han WK, Rha KH, Choi YD. Efficacy and Safety of Robotic Procedures Performed Using the da Vinci Robotic Surgical System at a Single Institute in Korea: Experience with 10000 Cases. Yonsei Med J. 2018;59:975-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Desai PH, Lin JF, Slomovitz BM. Milestones to optimal adoption of robotic technology in gynecology. Obstet Gynecol. 2014;123:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Robot-assisted laparoscopic surgery of the colon and rectum. Surg Endosc. 2012;26:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Autorino R, Zargar H, Kaouk JH. Robotic-assisted laparoscopic surgery: recent advances in urology. Fertil Steril. 2014;102:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Giulianotti PC, Sbrana F, Coratti A, Bianco FM, Addeo P, Buchs NC, Ayloo SM, Benedetti E. Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg. 2011;146:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Nota CL, Rinkes IHB, Hagendoorn J. Setting up a robotic hepatectomy program: a Western-European experience and perspective. Hepatobiliary Surg Nutr. 2017;6:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 771] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 29. | Magistri P, Tarantino G, Guidetti C, Assirati G, Olivieri T, Ballarin R, Coratti A, Di Benedetto F. Laparoscopic versus robotic surgery for hepatocellular carcinoma: the first 46 consecutive cases. J Surg Res. 2017;217:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Lai EC, Tang CN. Long-term Survival Analysis of Robotic Versus Conventional Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Comparative Study. Surg Laparosc Endosc Percutan Tech. 2016;26:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Han DH, Choi SH, Park EJ, Kang DR, Choi GH, Choi JS. Surgical outcomes after laparoscopic or robotic liver resection in hepatocellular carcinoma: a propensity-score matched analysis with conventional open liver resection. Int J Med Robot. 2016;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Kim JK, Park JS, Han DH, Choi GH, Kim KS, Choi JS, Yoon DS. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc. 2016;30:4756-4764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Montalti R, Berardi G, Patriti A, Vivarelli M, Troisi RI. Outcomes of robotic vs laparoscopic hepatectomy: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:8441-8451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc. 2016;30:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 35. | Salloum C, Lim C, Lahat E, Gavara CG, Levesque E, Compagnon P, Azoulay D. Robotic-Assisted Versus Laparoscopic Left Lateral Sectionectomy: Analysis of Surgical Outcomes and Costs by a Propensity Score Matched Cohort Study. World J Surg. 2017;41:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Shu J, Wang XJ, Li JW, Bie P, Chen J, Zheng SG. Robotic-assisted laparoscopic surgery for complex hepatolithiasis: a propensity score matching analysis. Surg Endosc. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Stewart CL, Ituarte PHG, Melstrom KA, Warner SG, Melstrom LG, Lai LL, Fong Y, Woo Y. Robotic surgery trends in general surgical oncology from the National Inpatient Sample. Surg Endosc. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Dong JH, Ye S, Duan WD, Ji WB, Liang YR. Feasibility of liver graft procurement with donor gallbladder preservation in living donor liver transplantation. Hepatol Int. 2015;9:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Su F, He E, Qian L, Zhu Z, Wei L, Zeng Z, Qu W, Xu R, Yi Z. Complication Follow-up With Ultrasonographic Analyses of 91 Cases With Donor Gallbladder Preservation in Living Donor Liver Transplantation of Left Lateral Sectionectomies. Transplant Proc. 2018;50:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |