Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.839
Peer-review started: January 16, 2019
First decision: March 10, 2019
Revised: March 14, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 6, 2019
Processing time: 80 Days and 2.8 Hours
Unstable carotid atherosclerotic plaques are prone to cause ischemic stroke. Contrast-enhanced ultrasound (CEUS) is the primary method of assessing plaque stability, but CEUS cannot be a method for screening for unstable plaque. The emergence of superb micro-vascular imaging (SMI) offers the possibility of clinically screening for unstable plaque
To investigate the value of SMI in predicting ischemic stroke in patients with carotid atherosclerotic plaques.
Patients with carotid atherosclerotic plaques (luminal stenosis of 50%-70%) were enrolled into the present study. All patients received conservative medication. The patient's clinical baseline data, serological data, CEUS and SMI data were analyzed. All patients underwent a 3-year follow-up. The follow-up endpoint was the occurrence of ischemic stroke and patients were divided into stroke group and non-stroke group according to whether the prognosis occurred or not. Subsequently, the difference in clinical data was compared, the correlation of SMI and CEUS was analyzed, and multiple Cox regression and receiver operating characteristic curve were applied to investigate the value of SMI and CEUS in predicting cerebral arterial thrombosis in three years.
In this study, 43 patients were enrolled in the stroke group and 82 patients were enrolled in the non-stroke group. Cox regression revealed that SMI level (P = 0.013) and enhancement intensity (P = 0.032) were the independent factors influencing ischemic stroke. There was a positive correlation between SMI level and enhancement intensity (r = 0.737, P = 0.000). The area under curve of SMI level predicting ischemic stroke was 0.878. The best diagnostic point was ≥ level II, and its sensitivity and specificity was 86.05% and 79.27%. The area under curve of enhancement intensity predicting ischemic stroke was 0.890. The best diagnostic point was 9.92 db, and its sensitivity and specificity was 88.37% and 89.02%. As the SMI level gradually increased, the incidence of ischemic stroke increased gradually (X2 = 108.931, P = 0.000).
SMI can be used as a non-invasive method of screening for unstable plaques and may help prevent ischemic stroke.
Core tip: Unstable carotid plaques are easily ruptured leading to the occurrence of ischemic stroke. Contrast-enhanced ultrasound is currently the primary means of assessing plaque stability, but it cannot be used for screening for unstable plaques. Superb micro-vascular imaging is able to check for low-speed blood flow in the plaque and is expected to be a screening tool for unstable plaques. This study found that superb micro-vascular imaging shows promise in predicting ischemic stroke.
- Citation: Yang DB, Zhou J, Feng L, Xu R, Wang YC. Value of superb micro-vascular imaging in predicting ischemic stroke in patients with carotid atherosclerotic plaques. World J Clin Cases 2019; 7(7): 839-848
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/839.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.839
Effective preventive measures are important ways to prevent ischemic stroke[1,2]. Studies have revealed that carotid atherosclerotic plaque is an independent risk factor for ischemic stroke[3,4]. Carotid plaques cause stenosis of the lumen, and unstable plaques are also prone to rupture and to form thrombi[5,6]. Thrombosis can block distal intracranial blood vessels leading to ischemic stroke[7,8]. Researchers have found that neovascularization in the plaque is closely related to the stability of a plaque[9]. Contrast-enhanced ultrasound (CEUS) can sensitively detect neovascularization in the plaque and then determine the stability of the plaque. It is currently the main clinical detection method for assessing plaque stability[10,11]. However, patients must be injected with contrast agents to perform CEUS, which requires the assistance of a nurse. The operation is more complicated than conventional ultrasound examination and requires more examination time. Therefore, it cannot be a method for screening unstable plaques. With the development of new ultrasound technology, the emergence of superb micro-vascular imaging (SMI) provides a more sensitive method in detecting low-velocity blood flow than color Doppler, providing the possibility of clinical screening for unstable plaques[12,13]. Therefore, the present study compared the accuracy of SMI and CEUS in predicting ischemic stroke and explored the potential value of SMI in predicting ischemic stroke.
Carotid atherosclerosis patients with a lumen area stenosis of 50%-70% who were confirmed by conventional X-ray angiography in Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences from March 2013 to March 2015 were recruited. These patients underwent carotid ultrasound and at least one side of carotid atherosclerotic plaque was confirmed. In total, 130 patients with complete clinical data were included in the study. Patients with hemorrhagic stroke, brain trauma, poisoning, post-epileptic state, hypertensive encephalopathy, abnormal blood glucose, encephalitis and vital organ function were excluded. All patients provided informed consent, and the study was supported by the Ethics Committee of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences.
Clinical data records: The clinical data of each patient, including age, gender, blood lipid index (cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, lipoprotein a), smoking history, diabetes history, hypertension history, and high-sensitivity C-reactive protein, were recorded.
Ultrasound examination: Toshiba Aplio500 ultrasound (PLT-1005BT linear array probe, 5-10 MHz) equipped with SMI imaging software was used in the present study. GE Logiq E8 ultrasound (9L4 probe, 4-9 MHz) equipped with ultrasound contrast imaging was also used in the present study. The patient was placed in a supine position with the head biased to the opposite side, and the neck was fully exposed. The carotid artery was continuously scanned longitudinally and laterally to observe the presence of plaque and plaque echo. The position, length and thickness of the target plaque were recorded. If the patient had multiple plaques, then the thickest hypoechoic or mixed echo plaques were selected as the target plaque.
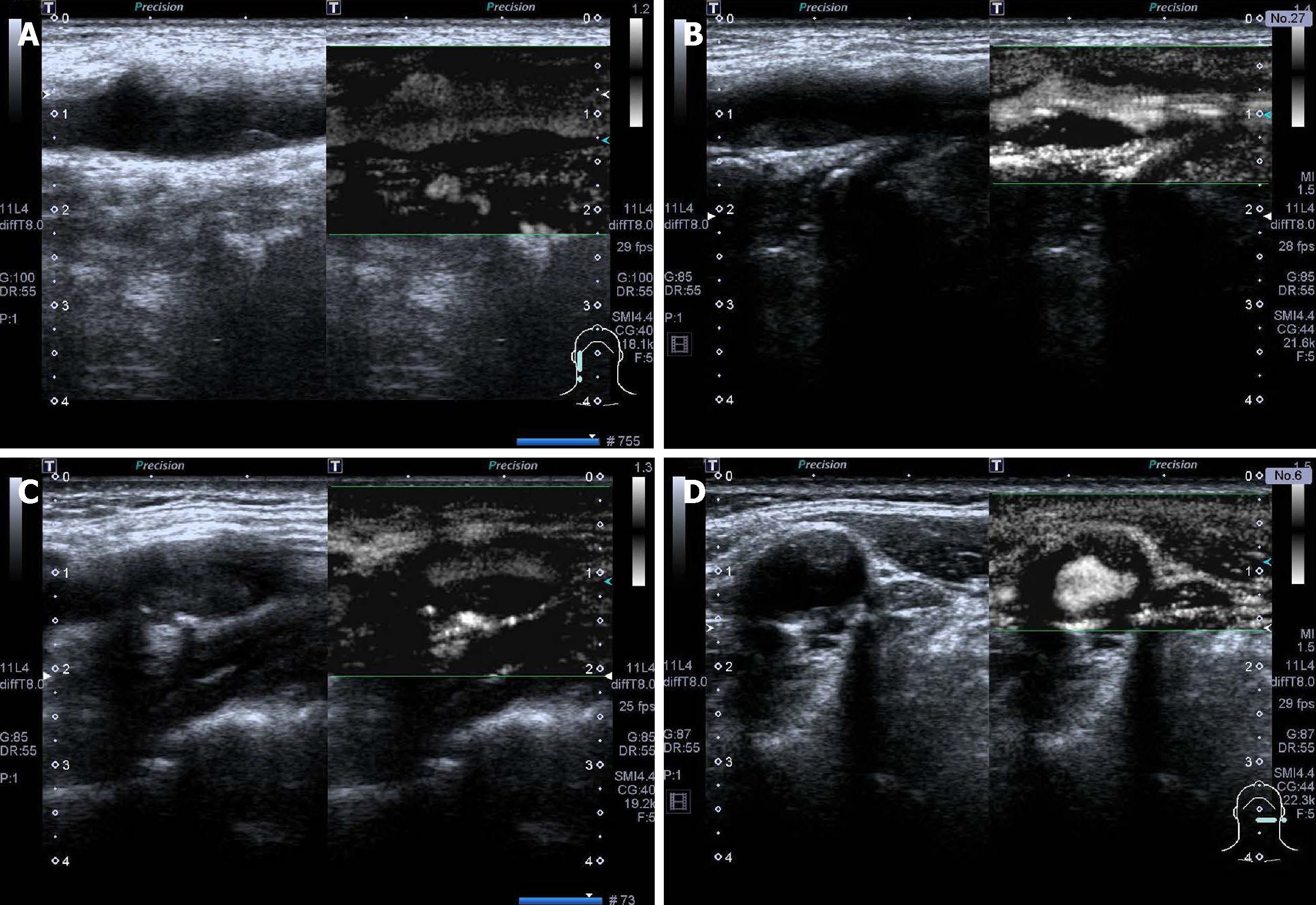
SMI examination: After clearly displaying the two-dimensional ultrasound image of the plaque, the patient was instructed to continue to breathe calmly. The target plaque was observed using the transverse and longitudinal sections of the SMI imaging mode. The video of the target plaque was recorded. After the scan was finished, the video was played back and was observed whether there were new blood vessels in the plaque. The blood vessels in the plaque were categorized as follows[14]: level 0: no blood flow signal is found in the plaque; level I: one or several dot blood vessels were found in the plaque; level II: dot blood vessels and 1-2 linear blood vessels are found; and level III: Multiple linear blood vessels are visible in the plaque, and most of them penetrate the plaque.
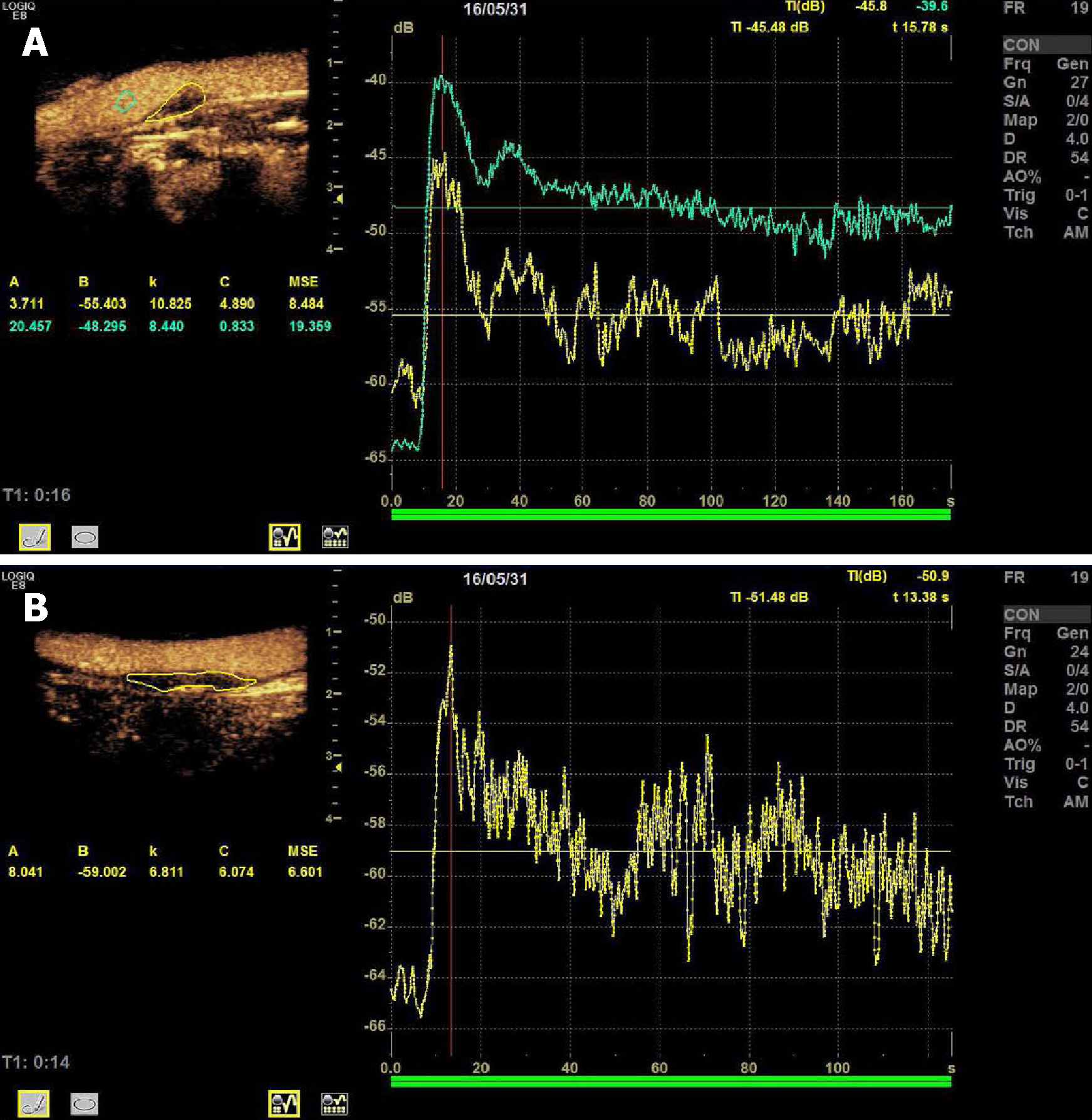
CEUS examination: Sonovue microbubble contrast agent (Bracco, Italy) and 5 mL of physiological saline were used to prepare a suspension. The truncated median vein was punctured and injected with 2.0 mL of contrast agent. After the injection, 5 mL of physiological saline was injected immediately, and the contrast mode was started. The enhancement of the plaque was observed, and the video was retained for later analysis. The contrast video was played back and the contrast perfusion of the plaque was observed. The image was analyzed by contrast analysis software, and the region of interest was manually drawn. The time-intensity curve was plotted to obtain the contrast parameters: time-to-peak and plaque enhancement intensity (EI)[15,16].
A total of 130 patients who participated in the study were followed up for 3 years. The first follow-up was performed at 3 mo after the end of treatment. Subsequently, follow-up was performed every 6 mo by phone. The endpoint was defined as ischemic stroke occurring during patient follow-up. Patients’ refusal, midway exit, and accidental death were defined as loss of follow-up. Based on the follow-up results, patients with endpoint events were defined as the stroke group and the remaining patients were defined as the non-stroke groups. The baseline data, imaging parameters, blood lipids (cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, lipoprotein a), high-sensitivity C-reactive protein, smoking history, diabetes history, hypertension history, SMI and CEUS diagnostic parameters were analyzed.
Statistical analysis was performed using Statistical Product and Service Solutions and Medcalc software. The measurement data was expressed as x ± s, and the count data was expressed in frequency. The comparison between the two groups of mea-surement data was performed using an independent sample t test, the count data was applied by chi-square test, and the graded data was performed using Subparameter test. Correlation analysis between SMI level and EI was performed using Spearman correlation analysis. Multivariate Cox proportional regression was used to analyze the risk of stroke in patients. The receiver operating characteristic (ROC) curve was used to evaluate the accuracy of the potential indicators in predicting ischemic stroke. The incidence of ischemic stroke in patients with different SMI levels was analyzed by Kaplan-Meier, and the difference was analyzed by log-rank. P < 0.05 was considered statistically significant.
By the end of the follow-up, five patients were lost to follow-up and forty-three patients were diagnosed with ischemic stroke and placed in the stroke group. There were 82 patients in the non-stroke group. The general information for the two groups of patients was presented in Table 1. Comparison of the clinical data between the two groups found that the age, gender, plaque thickness, lipoprotein a, high-density lipoprotein, high-sensitivity C-reactive protein, cholesterol, triglyceride, low-density lipoprotein, smoking history, diabetes history and hypertension history in the two groups were similar. There was no significant difference between the two groups (P > 0.05).
| Stroke group (n=43) | Non-stroke group (n=82) | X2/t/Z | P | |
| Gender, male (%) | 25 (58.14) | 46 (56.10) | 0.018 | 0.892 |
| Age | 70.04 ± 10.82 | 67.06 ± 8.59 | 1.672 | 0.095 |
| Triglyceride (mmol/L) | 1.68 ± 0.91 | 1.72 ± 0.67 | 0.279 | 0.780 |
| Cholesterol (mmol/L) | 4.50 ± 1.03 | 4.31 ± 0.88 | 1.080 | 0.282 |
| Lipoprotein (a) (mmol/L) | 235.67 ± 285.37 | 179.56 ± 184.67 | 1.243 | 0.216 |
| High-density lipoprotein (mmol/L) | 1.09 ± 0.24 | 1.15 ± 0.33 | -1.086 | 0.279 |
| Low-density lipoprotein (mmol/L) | 3.13 ± 0.69 | 2.96 ± 0.72 | 1.272 | 0.206 |
| High-sensitivity C-reactive protein (mg/L) | 7.57 ± 4.74 | 6.23 ± 3.98 | 1.673 | 0.097 |
| History of hypertension | 22 (51.16) | 40 (48.78) | 0.064 | 0.800 |
| History of diabetes | 26 (60.47) | 48 (55.54) | 0.043 | 0.835 |
| History of smoking | 20 (46.51) | 47 (57.32) | 1.324 | 0.250 |
| Plaque thickness (mm) | 3.38 ± 0.83 | 3.30 ± 0.90 | 0.534 | 0.594 |
| Superb micro-vascular imaging level | ||||
| Level 0 | 1 (2.32) | 17 (20.73) | 56.678 | < 0.001 |
| Level I | 6 (13.95) | 48 (58.53) | ||
| Level II | 17 (39.53) | 14 (17.07) | ||
| Level III | 19 (44.19) | 3 (3.66) | ||
| Ultrasound contrast | ||||
| Time-to-peak (s) | 12.47 ± 1.88 | 12.98 ± 1.54 | 1.618 | 0.108 |
| Enhancement intensity (db) | 10.67 ± 1.52 | 8.82 ± 1.03 | 7.526 | 0.000 |
The number of SMI vascular grading cases in the stroke and non-stroke groups were as follows: level 0: 1 case and 17 cases, respectively (Figure 1A); level I: 6 cases and 48 cases, respectively (Figure 1B); level II: 17 cases and 14 cases, respectively (Figure 1C); and level III: 19 cases and 3 cases, respectively (Figure 1D). Patients in the stroke group were mainly in level II and III, while patients in the non-stroke group were mainly in level 0 and I. There were statistically significant differences between the two groups (Z = 56.678, P < 0.001).
There was no statistically significant difference in plaque time-to-peak between the stroke group and the non-stroke group (t = 1.618, P = 0.108). EI in the stroke group (Figure 2A) was larger than that in the non-stroke group (Figure 2B), and the difference was statistically significant (t = 7.526, P = 0.000).
Multivariate analysis of SMI level and EI showed that SMI level (P = 0.013) and EI (P = 0.032) were the independent influencing factors affecting ischemic stroke (Table 2).
| Factors | B | SE | Wald | P-value | RR | 95%CI | |
| Lower limit | Upper limit | ||||||
| Superb micro-vascular imaging level | 0.640 | 0.312 | 4.112 | 0.013 | 1.896 | 1.971 | 6.696 |
| Enhancement intensity | 0.601 | 0.226 | 1.428 | 0.032 | 1.824 | 1.171 | 2.840 |
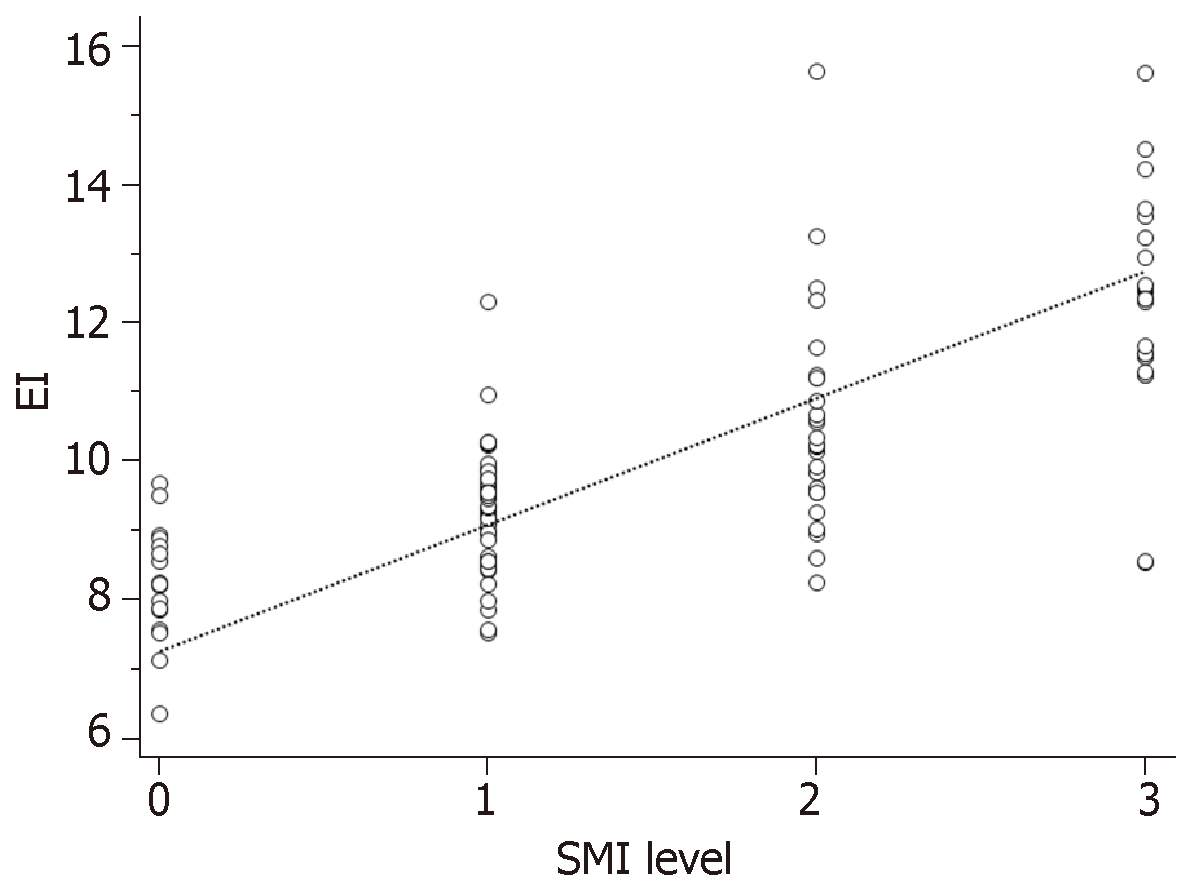
Spearman correlation analysis revealed a positive correlation between SMI level and EI (r = 0.737, P = 0.000; Figure 3).
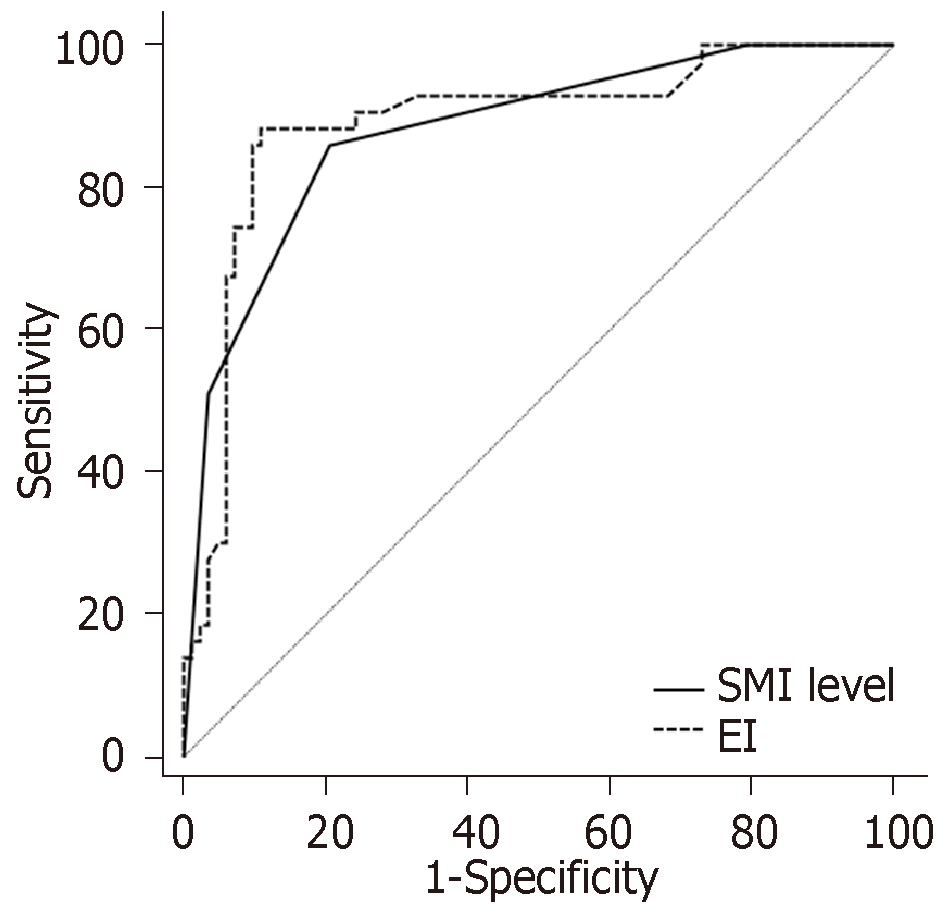
According to the ROC analysis, the area under curve (AUC) of SMI level predicting ischemic stroke in patients with plaque was 0.878. The best diagnostic point was ≥ level II. The sensitivity was 86.05%, and the specificity was 79.27%. The AUC of EI predicting ischemic stroke in patients with plaque was 0.890. The best diagnostic point was 9.92 db. The sensitivity was 88.37%, and the specificity was 89.02%. The difference was similar and there was no statistical significance between them (Z = 0.336, P = 0.737; Figure 4).
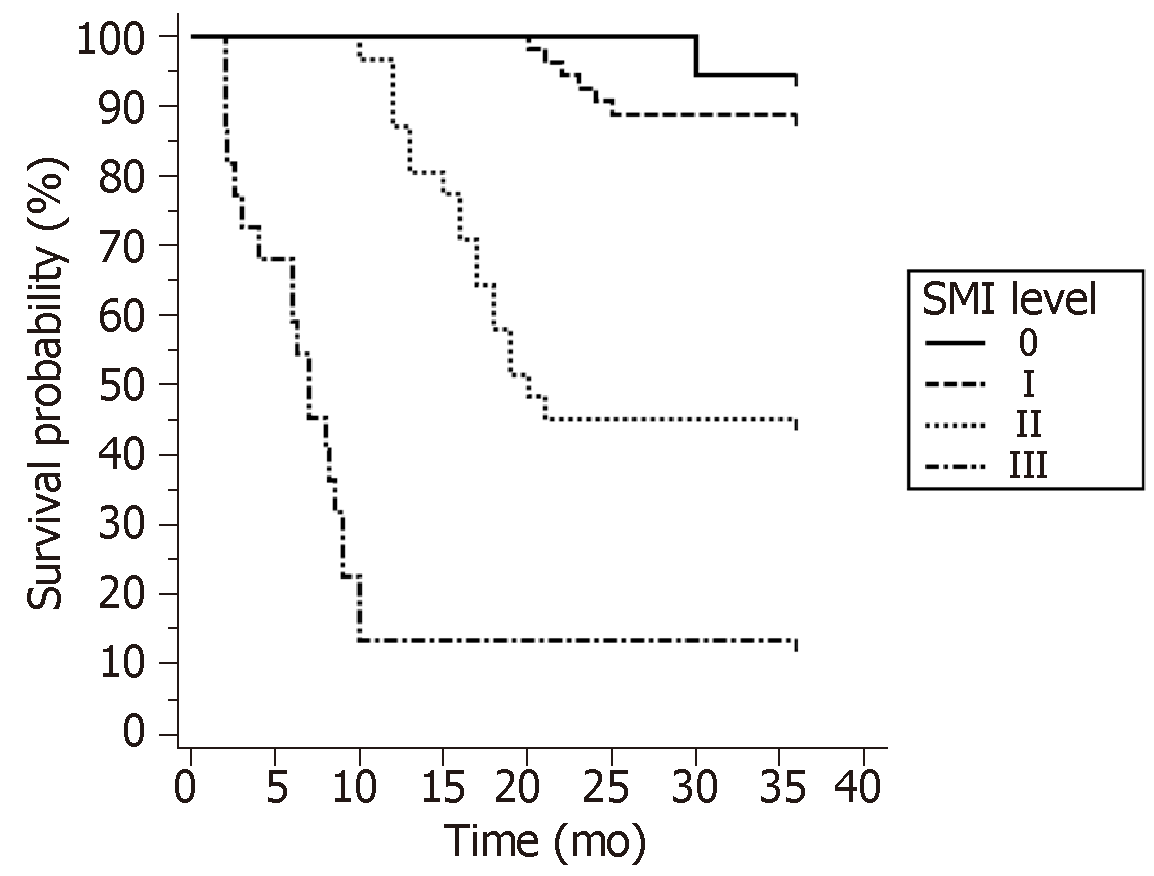
Survival curve analysis of patients with ischemic stroke under different SMI classification was plotted by Kaplan-Meier. The results showed that with the increase of SMI level, the incidence of ischemic stroke was gradually increased. The log-rank test revealed a statistically significant difference (X2 = 108.931, P = 0.000). The incidence rate of SMI level 0 and level I was similar. The incidence rate of SMI level II was significantly higher than level I. Therefore, it is feasible to use the SMI level II as a predictor of ischemic stroke (Figure 5).
Carotid atherosclerotic plaques are prone to cause ischemic stroke. Studies have shown that plaque formation is an independent risk factor for stroke[17,18]. Plaques gradually block blood vessels and cause stroke as the disease progresses. In addition, unstable plaques are prone to rupture, resulting in the blockage of distal blood vessels and subsequent ischemic stroke. Studies have found that neovascularization in plaques is an important indicator of plaque stability[19]. Therefore, timely detection and effective assessment of neovascularization in plaques have potential value in predicting patients with ischemic stroke. Recent studies have shown that CEUS is of great value in the detection and assessment of neovascularization in plaques[20,21]. The operation of CEUS is complex and requires the support of contrast agents and nurses. Therefore, it cannot be used as a screening tool for finding unstable plaques. SMI is a novel technique for detecting blood flow in micro-vessels, enabling the detection of micro-vessels at a much lower speed[13,22-24]. However, there are few studies on the assessment of neovascularization in plaque, and it is not clear whether it can be used to predict ischemic stroke in patients with carotid plaque. Therefore, the present study aimed to determine the ability of SMI in detecting neovascularization of carotid atherosclerotic plaque and analyzed its value in predicting ischemic stroke in patients with plaques.
The subjects recruited in this study needed to meet two conditions. First, the degree of vascular stenosis needed to be between 50% and 70%. Second, the patients were only treated with conservative drugs in order to prevent ischemic stroke caused by excessive plaque growth. These conditions controlled to the best of our ability that an ischemic stroke in the study subject was caused by the rupture of an unstable plaque.
In this study, baseline data analysis was performed on patients who underwent 3 years of follow-up. The results showed that the clinical data of age, gender, serum index, and medical history were not significantly different between the two groups (P > 0.05). It indicates that the difference in patient baseline data is small. However, SMI level and EI were different between the two groups. The results showed that patients in the stroke group had higher SMI grades and higher EI values. Multivariate COX regression analysis found that SMI level and EI were independent factors influencing ischemic stroke. This initially suggests that SMI levels and CEUS markers can be used to detect neovascularization in plaques of patients with carotid atherosclerosis and to assess the occurrence of ischemic stroke. Previous studies have reported that unstable plaques are prone to rupture and cause distal vascular obstruction to cause ischemic stroke. The abundant neovascularization in the plaque is associated with rupture of the plaque[25,26]. Therefore, SMI and CEUS are good tools for dynamic observation of blood flow distribution in the plaque, which can be used to assess the occurrence of ischemic stroke .
Studies have shown that contrast EI in plaques have a good correlation with histological micro-vessel density[27]. It has been clinically established that CEUS can be used to analyze the stability of plaques[20,28]. Therefore, we used correlation analysis to evaluate the value of SMI in detecting low-velocity blood flow in plaques. Spearman correlation analysis showed that SMI level was positively correlated with EI, with a correlation of 0.737, suggesting SMI can evaluate blood flow in plaque like CEUS.
In order to investigate the value of CEUS and SMI in predicting ischemic stroke caused by plaque rupture, the ROC curve was used for analysis, and it was found that both could achieve an AUC of approximately 90%. This indicates that both are excellent predictors of stroke. SMI is more convenient and easier to promote than CEUS, suggesting that the clinical use of SMI can be applied for the large-scale screening of unstable plaques. In addition, the ROC curve revealed that the best diagnostic point of SMI was level II. Furthermore, level 0 and I suggested a negative result, while level II and III suggested a positive result. Based on the survival curve of patients, which compared the incidence of ischemic stroke in different SMI levels, it was found that patients with SMI level 0 and I had a similar incidence rate, but the incidence of level II and III significantly increased. This suggests that SMI level II can be used as one of the diagnostic criteria in clinic.
This study has certain limitations. Although we have screened patients with a stenosis degree between 50% and 70% through strict inclusion and exclusion criteria, we still cannot fully guarantee that the reason for ischemic stroke during follow-up was due to plaque rupture. Some patients may have had a stroke due to plaque clogging, resulting in false positive results. To expand our findings, we plan to establish a rabbit carotid plaque model with anatomical results as a standard for plaque rupture to accurately compare the diagnostic efficacy of SMI and CEUS.
In summary, SMI can be used as a non-invasive method for screening unstable plaques. It has a high diagnostic accuracy and can be applied for the clinical diagnosis and prevention of ischemic stroke.
Patients with carotid atherosclerotic plaque are prone to ischemic stroke because plaques gradually block blood vessels and cause stroke. Secondly, unstable plaques are prone to rupture and form thrombi, which in turn leads to ischemic stroke. Contrast-enhanced ultrasound (CEUS) can be used to assess unstable plaques because it can detect blood flow in the plaque. However, CEUS is complicated, requires the use of contrast agents and requires the support of nurses, so it cannot be used as a large-scale screening tool for unstable plaques.
Superb micro-vascular imaging (SMI) is a new technique for detecting micro-vascular blood flow, enabling the detection of low-speed micro-vessels. It is expected to be a screening tool for unstable plaques. However, there are few reports on the evaluation of neovascularization in plaques by SMI. Whether it can be used to predict ischemic stroke in patients with carotid plaque is still inconclusive.
In this study, the distribution of micro-vascular blood flow in carotid atherosclerotic plaques was detected by the SMI technique. The aim of this study is to compare the accuracy of SMI and CEUS in predicting ischemic stroke, and to explore the value of SMI in predicting ischemic stroke in patients with unstable carotid plaque.
Patients with carotid plaques (luminal stenosis of 50%-70%) were recruited as the subjects in the present study. The clinical baseline data, serological data, CEUS and SMI data were recorded. All patients were followed up for 3 years. During the follow-up period, patients only underwent conservative drug treatment to prevent an ischemic stroke caused by excessive plaque growth within 3 years. According to whether or not ischemic stroke occurred during the follow-up period, the patients were divided into the stroke group and the non-stroke group. The differences in clinical data between the two groups were compared. The correlation between SMI and CEUS was analyzed. The value of SMI and CEUS in predicting ischemic stroke in patients with carotid atherosclerotic plaque within 3 years was explored.
In the present study, there were 43 patients in the stroke group and 82 patients in the non-stroke group. Patients in the stroke group were mainly in SMI level II and III, while patients in the non-stroke group were mainly in SMI level 0 and I. Cox regression showed that SMI level and enhancement intensity were the independent factors influencing ischemic stroke. SMI level was positively correlated with enhancement intensity. The area under curve of SMI level and enhancement intensity predicting ischemic stroke was 0.878 and 0.890. It indicated that SMI was as good as CEUS in predicting ischemic stroke in patients with unstable carotid plaque. As the SMI level gradually increased, the incidence of ischemic stroke gradually increased. SMI level II revealed the highest accuracy of predicting ischemic stroke in patients with carotid plaque.
SMI can assess neovascularization in unstable plaques just like CEUS, but SMI is non-invasive and simple to operate. Hence, it can be used as a non-invasive tool for screening for unstable plaques. It can be applied to the clinical diagnosis and prevention of ischemic stroke.
This study further plans to establish a rabbit carotid atherosclerotic plaque model. The anatomical results of experimental rabbits will be used as criteria for plaque rupture, and the value of SMI and CEUS for predicting unstable plaque rupture will be more accurately analyzed.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dediulia T, Ko E S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17:1423-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Convertino VA, Wirt MD, Glenn JF, Lein BC. The Compensatory Reserve For Early and Accurate Prediction Of Hemodynamic Compromise: A Review of the Underlying Physiology. Shock. 2016;45:580-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Shu JE, Ying ML, Chen XR, Hua JJ, Fu JT, Xia XM, Pan YH, Jiang Y. Prognostic value of high-resolution magnetic resonance imaging in evaluating carotid atherosclerotic plaque in patients with ischemic stroke. Medicine (Baltimore). 2017;96:e8515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Nasr N, Ruidavets JB, Farghali A, Guidolin B, Perret B, Larrue V. Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke. 2011;42:3616-3618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 385] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 6. | Baradaran H, Myneni PK, Patel P, Askin G, Gialdini G, Al-Dasuqi K, Kamel H, Gupta A. Association Between Carotid Artery Perivascular Fat Density and Cerebrovascular Ischemic Events. J Am Heart Assoc. 2018;7:e010383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Gong CX. From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cell Mol Neurobiol. 2015;35:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 9. | Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Du J, Wang H, Wang H, Jiang J, Zhao J, Lu H. Comparison of diagnostic values of ultrasound micro-flow imaging and contrast-enhanced ultrasound for neovascularization in carotid plaques. Exp Ther Med. 2017;14:680-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Saha SA, Gourineni V, Feinstein SB. The Use of Contrast-enhanced Ultrasonography for Imaging of Carotid Atherosclerotic Plaques: Current Evidence, Future Directions. Neuroimaging Clin N Am. 2016;26:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Ma Y, Li G, Li J, Ren WD. The Diagnostic Value of Superb Microvascular Imaging (SMI) in Detecting Blood Flow Signals of Breast Lesions: A Preliminary Study Comparing SMI to Color Doppler Flow Imaging. Medicine (Baltimore). 2015;94:e1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Oura K, Kato T, Ohba H, Terayama Y. Evaluation of Intraplaque Neovascularization Using Superb Microvascular Imaging and Contrast-Enhanced Ultrasonography. J Stroke Cerebrovasc Dis. 2018;27:2348-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Hagiwara Y, Sasaki R, Shimizu T, Soga K, Hatada C, Miyauchi M, Okamura T, Sakurai M, Akiyama H, Hasegawa Y. The utility of superb microvascular imaging for the detection of deep vein thrombosis. J Med Ultrason (2001). 2018;45:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Polak JF, Shemanski L, O'Leary DH, Lefkowitz D, Price TR, Savage PJ, Brant WE, Reid C. Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology. 1998;208:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Huang PT, Chen CC, Aronow WS, Wang XT, Nair CK, Xue NY, Shen X, Li SY, Huang FG, Cosgrove D. Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol. 2010;2:89-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Bai L, Shi M, Lu H, Wu Y, Tu J, Ni J, Wang J, Cao L, Lei P, Ning X. Features and risk factors of carotid atherosclerosis in a population with high stroke incidence in China. Oncotarget. 2017;8:57477-57488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | van Hinsbergh VW, Eringa EC, Daemen MJ. Neovascularization of the atherosclerotic plaque: interplay between atherosclerotic lesion, adventitia-derived microvessels and perivascular fat. Curr Opin Lipidol. 2015;26:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Akkus Z, Hoogi A, Renaud G, van den Oord SC, Ten Kate GL, Schinkel AF, Adam D, de Jong N, van der Steen AF, Bosch JG. New quantification methods for carotid intra-plaque neovascularization using contrast-enhanced ultrasound. Ultrasound Med Biol. 2014;40:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Schmidt C, Fischer T, Rückert RI, Oberwahrenbrock T, Harms L, Kronenberg G, Kunte H. Identification of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS One. 2017;12:e0175331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | D'Oria M, Chiarandini S, Pipitone MD, Fisicaro M, Calvagna C, Bussani R, Rotelli A, Ziani B. Contrast Enhanced Ultrasound (CEUS) Is Not Able to Identify Vulnerable Plaques in Asymptomatic Carotid Atherosclerotic Disease. Eur J Vasc Endovasc Surg. 2018;56:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Ohno Y, Fujimoto T, Shibata Y. A New Era in Diagnostic Ultrasound, Superb Microvascular Imaging: Preliminary Results in Pediatric Hepato-Gastrointestinal Disorders. Eur J Pediatr Surg. 2017;27:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Gabriel M, Tomczak J, Snoch-Ziółkiewicz M, Dzieciuchowicz Ł, Strauss E, Pawlaczyk K, Wojtusik D, Oszkinis G. Superb Micro-vascular Imaging (SMI): a Doppler ultrasound technique with potential to identify, classify, and follow up endoleaks in patients after Endovascular Aneurysm Repair (EVAR). Abdom Radiol (NY). 2018;43:3479-3486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Hamada S, Kashiwazaki D, Yamamoto S, Akioka N, Kuwayama N, Kuroda S. Impact of Plaque Composition on Risk of Coronary Artery Diseases in Patients with Carotid Artery Stenosis. J Stroke Cerebrovasc Dis. 2018;27:3599-3604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Vorkas PA, Shalhoub J, Lewis MR, Spagou K, Want EJ, Nicholson JK, Davies AH, Holmes E. Metabolic Phenotypes of Carotid Atherosclerotic Plaques Relate to Stroke Risk: An Exploratory Study. Eur J Vasc Endovasc Surg. 2016;52:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Vavuranakis M, Sigala F, Vrachatis DA, Papaioannou TG, Filis K, Kavantzas N, Kalogeras KI, Massoura C, Toufektzian L, Kariori MG, Vlasseros I, Kallikazaros I, Stefanadis C. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa. 2013;42:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Rodriguez-Granillo GA, Carrascosa P, Bruining N, Waksman R, Garcia-Garcia HM. Defining the non-vulnerable and vulnerable patients with computed tomography coronary angiography: evaluation of atherosclerotic plaque burden and composition. Eur Heart J Cardiovasc Imaging. 2016;17:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |