Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.548
Peer-review started: November 23, 2018
First decision: December 15, 2018
Revised: December 29, 2018
Accepted: January 30, 2019
Article in press: January 30, 2019
Published online: March 6, 2019
Processing time: 104 Days and 15.3 Hours
Myocarditis is an important cause of morbidity and mortality in children, leading to long-term sequelae including chronic congestive heart failure, dilated cardiomyopathy, heart transplantation, and death. The initial diagnosis of myocarditis is usually based on clinical presentation, but this widely ranges from the severe sudden onset of a cardiogenic shock to asymptomatic patients. Early recognition is essential in order to monitor and start supportive treatment prior to the development of severe adverse events. Of note, many cases of fulminant myocarditis are usually misdiagnosed as otherwise minor conditions during the weeks before the unexpected deterioration.
To provide diagnostic clues to make an early recognition of pediatric myocarditis. To investigate early predictors for poor outcomes.
We conducted a retrospective cross-sectional single-center study from January 2008 to November 2017 at the Pediatric Department of our institution, including children < 18-years-old diagnosed with myocarditis. Poor outcome was defined as the occurrence of any of the following facts: death, heart transplant, persistent left ventricular systolic dysfunction or dilation at hospital discharge (early poor outcome), or after 1 year of follow-up (late poor outcome). We analyzed different clinical features and diagnostic test findings in order to provide diagnostic clues for myocarditis in children. Multivariable stepwise logistic regression analysis was performed using all variables that had been selected by univariate analysis to determine independent factors that predicted a poor early or late outcome in our study population.
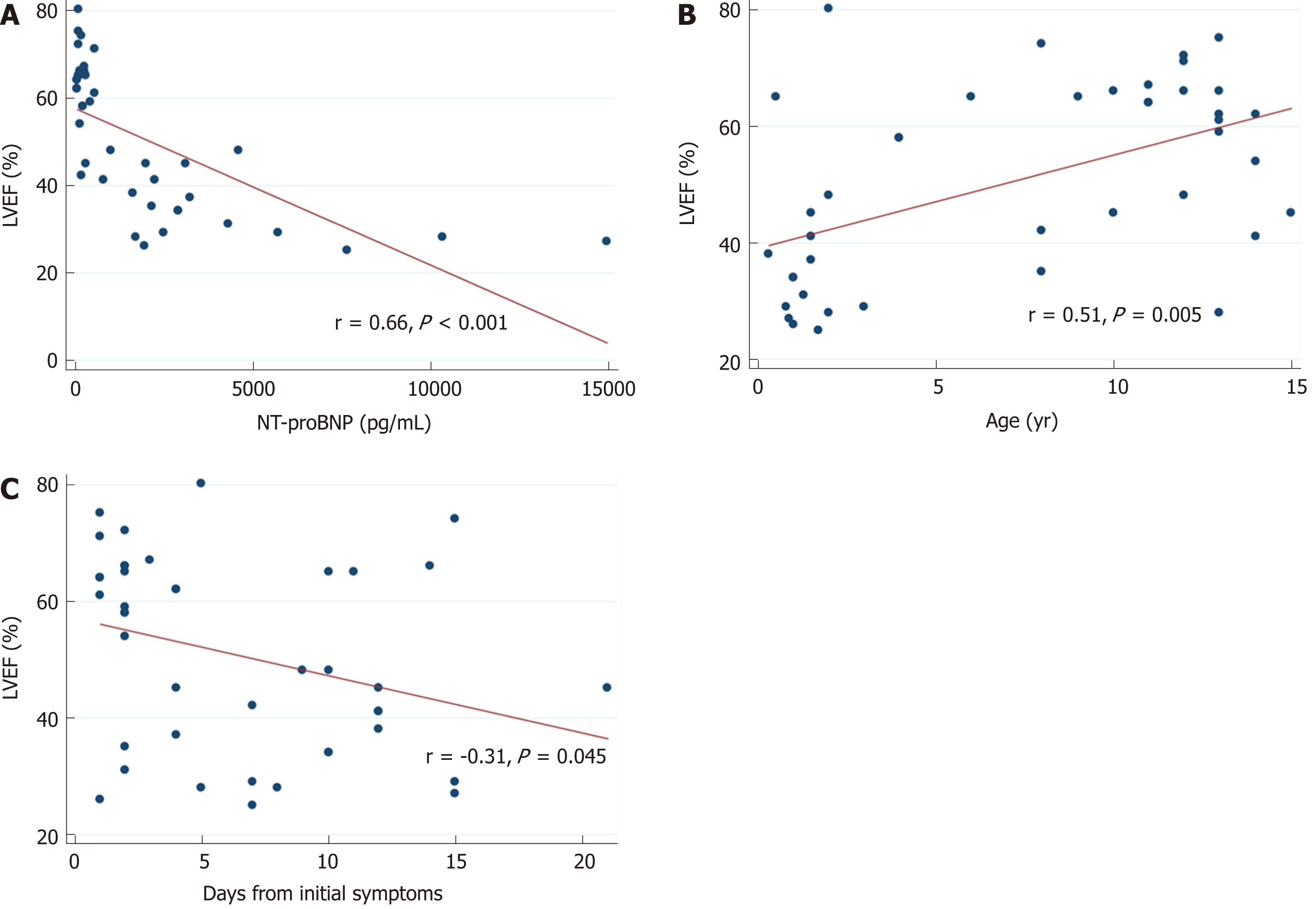
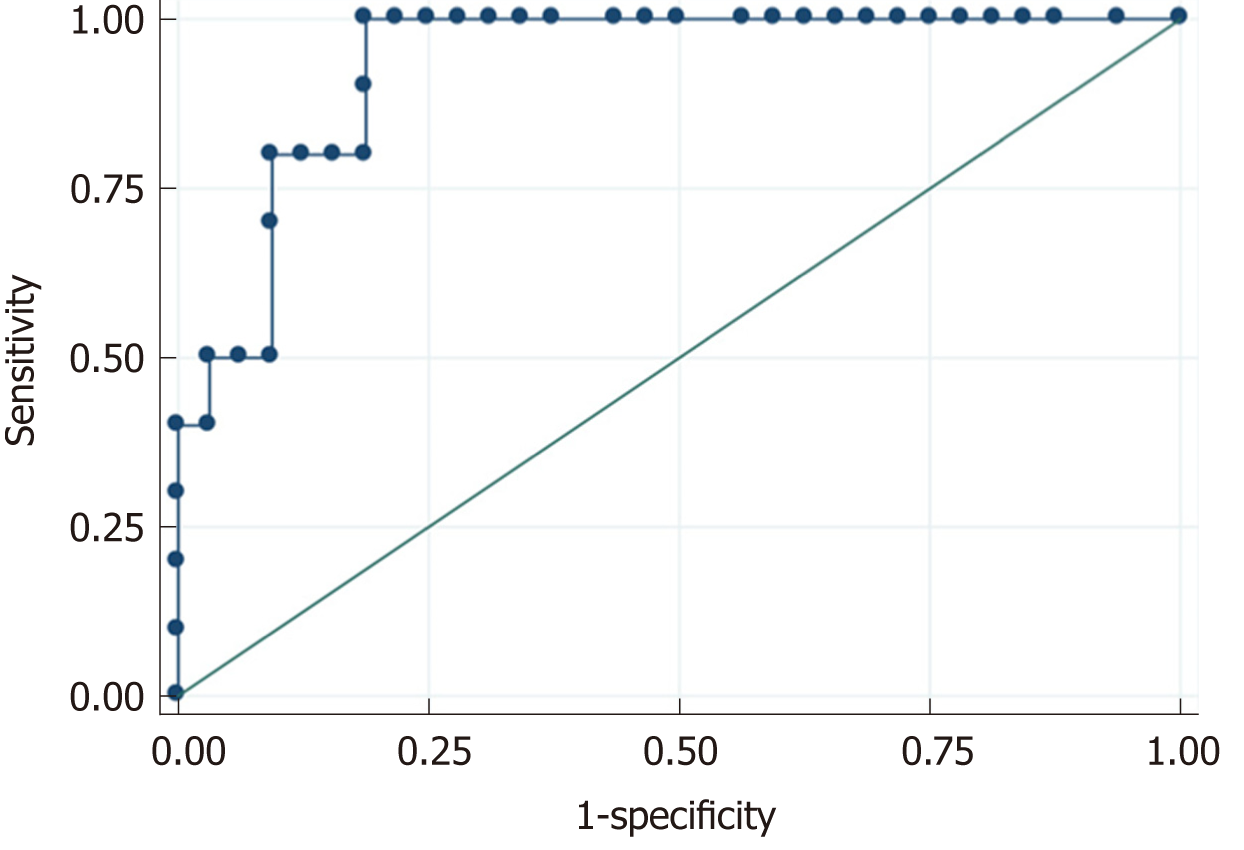
A total of 42 patients [69% male; median age of 8 (1.5-12) years] met study inclusion criteria. Chest pain (40%) was the most common specific cardiac symptom. Respiratory tract symptoms (cough, apnea, rhinorrhea) (38%), shortness of breath (35%), gastrointestinal tract symptoms (vomiting, abdominal pain, diarrhea) (33%), and fever (31%) were the most common non-cardiac initial complaints. Tachycardia (57%) and tachypnea (52%) were the most common signs on the initial physical exam followed by nonspecific signs of respiratory tract infection (44%) and respiratory distress (35%). Specific abnormal signs of heart failure such as heart murmur (26%), systolic hypotension (24%), gallop rhythm (20%), or hepatomegaly (20%) were less prevalent. Up to 43% of patients presented an early poor outcome, and 16% presented a late poor outcome. In multivariate analysis, an initial left ventricular ejection fraction (LVEF) < 30% remained the only significant predictor for early [odds ratio (OR) (95%CI) = 21 (2-456), P = 0.027) and late [OR (95%CI) = 8 (0.56-135), P = 0.047) poor outcome in children with myocarditis. LVEF correlated well with age (r = 0.51, P = 0.005), days from the initiation of symptoms (r = -0.31, P = 0.045), and N-terminal pro-brain natriuretic peptide levels (r = 0.66, P < 0.001), but not with troponin T (r = -0.05, P = 0.730) or C-reactive protein levels (r = -0.13, P = 0.391). N-terminal pro-brain natriuretic peptide presented a high diagnostic accuracy for LVEF < 30% on echocardiography with an area under curve of 0.931 (95%CI: 0.858-0.995, P < 0.001). The best cut-off point was 2000 pg/mL with a sensitivity of 90%, specificity of 81%, positive predictive value of 60%, and negative predictive value of 96%.
The diagnosis of myocarditis in children is challenging due to the heterogeneous and unspecific clinical presentation. The presence of LVEF < 30% on echocardiography on admission was the major predictor for poor outcomes. Younger ages, a prolonged course of the disease, and N-terminal pro-brain natriuretic peptide levels could help to identify these high-risk patients.
Core tip: In this retrospective study involving 42 children with myocarditis, we delineated the heterogeneous and unspecific clinical presentation of this condition in order to provide clinical clues to improve its early recognition. We found that the presence of left ventricle ejection fraction < 30% on echocardiography on admission was the major predictor for poor early outcomes. Because echocardiography is not widely available at emergency departments, we found that younger ages (< 2-years-old), a prolonged course of the disease (> 7 d), and increased N-terminal pro-brain natriuretic peptide levels (> 5000 pg/mL) could help to identify these high-risk patients.
- Citation: Rodriguez-Gonzalez M, Sanchez-Codez MI, Lubian-Gutierrez M, Castellano-Martinez A. Clinical presentation and early predictors for poor outcomes in pediatric myocarditis: A retrospective study. World J Clin Cases 2019; 7(5): 548-561
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/548.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.548
Myocarditis is an inflammatory condition characterized by leukocyte infiltration and subsequent fibrosis and necrosis of the myocardium. Multiple causes can produce it, but most cases are thought to be associated with viral infections[1,2]. The real prevalence of pediatric myocarditis is still unknown, but it is considered a rare disease that accounts for 0.05% of pediatric hospital discharges[3-5].
Despite its rarity, myocarditis is an important cause of morbidity and mortality in children. A high percentage of patients presents with congestive heart failure (CHF) and require hospitalization, intensive care admission, mechanical ventilation, cardiac medication, and inotropic or mechanical circulatory support[3-6]. Remarkably, the overall mortality rate in the acute phase is reported to be 7%-15%[3,5] with an increased likelihood of death early in the illness trajectory, and it has been found as the cause of up to 17% of cases of sudden cardiac death in children younger than 16-years-old[4]. Also, most patients with myocarditis develop a left ventricular (LV) dysfunction or dilation in the acute phase that does not recover completely leading to long-term sequelae including chronic CHF, dilated cardiomyopathy (DCM), and death[7,8]. Thus, myocarditis is one of the leading causes for DCM (27%) and for heart transplantation (80%) in children without congenital heart diseases[4,9].
The definitive diagnostic is made by established histological, immunological, and immunohistochemical findings in myocardial tissue samples obtained through endomyocardial biopsy (EMB)[10,11]. However, EMB has many inherent risks, is not widely available in most centers that initially treat patients with myocarditis, and is not routinely performed. Therefore, the initial diagnosis of myocarditis is usually based on clinical presentation, but it widely ranges from severe sudden onset of cardiogenic shock to asymptomatic patients[12-15]. Of note, many cases of fulminant myocarditis (FM) are usually misdiagnosed as otherwise minor conditions during the weeks prior to the unexpected and severe deterioration[13].
In this context, we sought to describe the clinical presentation and diagnostic tests´ findings of our cases of pediatric myocarditis in order to provide diagnostic clues that would help clinicians recognize these patients early. Also, we aimed to investigate the existence of early predictors for poor outcomes.
We conducted a retrospective cross-sectional single-center study from January 2008 to November 2017 at the Pediatric Department of our institution, a tertiary university-affiliated hospital with 25000 visits per year to our pediatric emergency department and 1200 admissions excluding the neonatal unit. The study was performed according to the requirements of our Institutional Review Board.
Patients were included if they were children up to 18-years-old, and they were diagnosed with myocarditis based on the International Classification of Diseases, Tenth Revision. In all cases, the final diagnosis was made by an expert pediatric cardiologist based on the context of possible myocardial injury with (probable acute myocarditis) or without (possible subclinical myocarditis) cardiovascular symptoms, and at least one of the following criteria[7]: (1) raised biomarkers of cardiac injury (troponin T); (2) ECG findings suggestive of cardiac injury[13,16-21]; (3) abnormal LV function or LV dilation on echocardiogram[22,23] or cardiac magnetic resonance imaging (cMRI); and (4) evidence of inflammation on cMRI (late gadolinium enhancement sequence)[24]. Because EMB was not available at our institution during the study period, no cases of definitive or confirmed myocarditis are presented. Patients were excluded if any etiology of DCM or CHF different from myocarditis (congenital heart diseases, Kawasaki disease, sepsis, cardiotoxic drugs, an inborn error of metabolism, neuromuscular disorders, malformation syndromes, or familial syndromes) was identified, and if there were incomplete medical records.
A standardized data collection form was used to collect data from inpatient and outpatient medical records. Inpatient medical records were reviewed to obtain initial data at the time of evaluation at the emergency department when the patient was admitted with the clinical suspicion of myocarditis. Outpatient medical records were reviewed to obtain a follow-up data up to 1 yr following initial hospitalization. Data obtained from each patient included demographics, presenting symptoms, physical examination findings, diagnostic test results, treatment received, and outcomes.
LV systolic dysfunction was defined as left ventricle ejection fraction (LVEF) < 50% and severe LV systolic dysfunction as LVEF < 30%. LV dilation was defined as left ventricle diastolic diameter > 2 Z score for body surface area[25]. Based on their clinical presentation, patients were retrospectively classified under four different cardiac syndromes: (1) FM (abrupt cardiogenic shock or sudden cardiac death); (2) CHF without hemodynamic instability; (3) Dysrhythmia (palpitations associated to any abnormal rhythm documented on ECG)[18,19]; and (4) Acute coronary syndrome-like (ACS-like) (chest pain, ECG findings suggestive of myocardial ischemia, and raised troponin T)[17,26-29]. Increased CPR, troponin T, and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were defined as > 60 mg/L, > 10 ng/L, and > 600 pg/mL, respectively. Poor outcome was defined as the occurrence of any of the following facts: death, heart transplant, or persistent LV systolic dysfunction or dilation at hospital discharge (early poor outcome) or after 1 year of follow-up (late poor outcome).
We analyzed different variables in order to characterize the clinical presentation and to find diagnostic clues for myocarditis in children. Also, patients were divided into two groups based on the presence of poor early (at hospital discharge) and late (1 yr after admission) outcomes. Then, we looked for risk factors for poor outcomes at the presentation in our pediatric myocarditis cohort.
Data are presented descriptively using frequency and percentage for qualitative variables. Quantitative variables were expressed using the mean (± SD) or the median and interquartile range according to the variable’s distribution (tested with Shapiro-Wilk normality test). For bivariate analysis, unpaired two-tailed Student’s t-test or Mann-Whitney U-test was used depending on the distribution of the studied variables. Chi-squared test was used to compare categorical variables (Fisher’s exact test was used when the expected frequency was less than 5). The relation among quantitative variables was explored through estimation of Pearson or Spearman correlation coefficients. Multivariable stepwise logistic regression analysis was performed using all variables that had been selected by univariate analysis to determine independent factors that predicted a poor early or late outcome in our population study, and the results were expressed as an odds ratio (OR) with a 95%CI. Significance level was considered as P < 0.05. A 95%CI that did not include 1.0 was interpreted to indicate statistical significance. Analyses were performed using the Stata 13.0. (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX, United States: StataCorp LP).
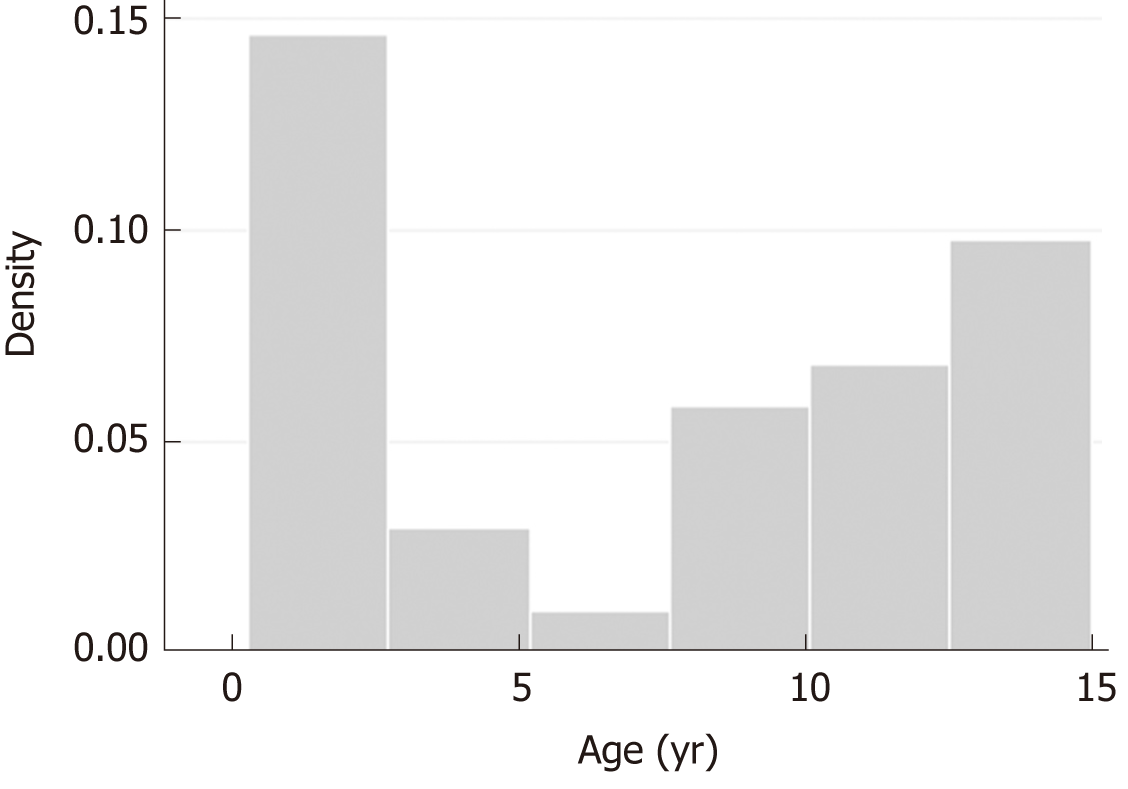
A total of 42 patients (69% male) met study inclusion criteria. The median age was 8 (1.5-12) years with a bimodal age presentation: most cases were children < 2-years-old and > 12-year-old (35% for each group; Figure 1). Table 1 provides the baseline data of the patients enrolled.
| Clinical and demographic data | n = 42 |
| Age | Median (IQR) yr: 8 (1.5-12) |
| Male:Female gender | 2.23 |
| Evolution | Median (IQR) days from initial symptoms: 5 (2-10) |
| Visits to ED previously before diagnosis of myocarditis | Median (IQR) visits prior to admission: 2 (1-2); 1 visit (41), 2 visits (36), 3 visits (9), more than 3 visits (14) |
| Presenting symptoms (%) | Previous viral infection (69); Chest pain (40); Respiratory tract symptoms (cough, apnea, rhinorrhea) (38); Shortness of breath (35); Gastrointestinal tract symptoms (vomiting, abdominal pain, diarrhea) (33); Fever (31); Weakness, exercise or feeding intolerance (21); Palpitations (16); Lethargy (12); Syncope (4) |
| Physical exam (%) | Tachycardia (57); Tachypnea (52); Evidence of respiratory tract infection (44); Respiratory distress (35); Abnormal lung auscultation (31); Murmur (26); Systolic hypotension (24); Poor perfusion or diminished pulses (21); Gallop rhythm (20); Hepatomegaly (20); Edema (7); Cyanosis (2) |
| Cardiac syndrome (%) | ACS-like (34); Fulminant myocarditis (29); Congestive heart failure (23); Dysrhythmia (14) |
| Complementary exams | |
| Laboratory (%) | CRP > 60 mg/L (16); Troponin T > 10 ng/L (62); NT-proBNP > 600 pg/mL (40) |
| Chest X-Ray (%) | Cardiomegaly (35); Pulmonary edema (28); Pulmonary infiltrate (4%); Pleural effusion (2.5%) |
| ECG (%) | Abnormal ECG (93); Sinus tachycardia (61); Ischemic changes (57); Low voltage (50); SVT (2.5); VT (7); AVB (2.5); prolonged QT interval (2.5%) |
| Echocardiography (%) | Abnormal echocardiography (88): LV systolic dysfunction (50): severe (14), moderate (16), mild (20); Biventricular systolic dysfunction (10); Segmental wall motion abnormalities (38); LV dilation (43); Mitral regurgitation (69); Pericardial effusion (59) |
| Cardiac MRI (%) | MRI performed (50); Median days to realization from admission, 5 (3-9); Lake Louis criteria positive (86), equivocal (10), negative (4) |
| Microbiology (%) | Positive microbiology (47): Coxsakie (30); Parvovirus B19 (20); Adenovirus (15); EBV (15); CMV (10); Mycoplasma (10) |
| Treatment (%) | Any treatment (71): Diuretics (69); ACEI (69); Beta-blockers (64); Digoxin (14) Spironolactone (14); Antiarrhythmic (5); Inotropic support (35); Mechanical Ventilation (26); ECMO/VAD (2.5); Pacemaker (2.5) |
| Outcomes (%) | Hospitalization length of stay (d): Median 6 (IQR 3-13); Death (5), transplant (0) |
| Poor early outcomes: Death, transplant, or LV systolic dysfunction/dilation at discharge (43) | |
| Poor late outcomes: Death, transplant or LV systolic dysfunction/dilation after 1 yr of follow-up (16); Spontaneous LV function recovery during first year after diagnosis (69) | |
The diagnosis of myocarditis was made after a median of 5 (2-10) d from the initial symptoms, and the patients consulted to the ED a median of 2 (1-2) times prior to their admission. The definitive diagnosis of myocarditis was made during the first visit at our ED in 41% of patients, 34% of cases were children > 10-years-old with the ACS-like presentation, and 7% were infants with FM. Most patients (59%) required two or more visits to the ED before the diagnosis of myocarditis. A previous diagnosis different from myocarditis was made in 52% of cases; 31% were categorized as respiratory infection (bronchiolitis, pneumonia, and upper respiratory tract infection), 17% as gastrointestinal infection, 2% as urinary tract infection, and 2% as infantile colic. The majority of patients (69%) presented a viral infection prior to the diagnosis, and a positive microbiologic study by serology or blood-PCR was observed in 47% of patients, with Coxsackie (30%) and Parvovirus B19 (20%) as the most prevalent agents.
Most patients had overlapping signs and symptoms at presentation. Chest pain (40%) was the most common specific cardiac symptom. Other specific cardiac symptoms such as palpitations (16%) or syncope (4%) were less prevalent. Respiratory tract symptoms (cough, apnea, rhinorrhea) (38%), shortness of breath (35%), gastrointestinal tract symptoms (vomiting, abdominal pain, diarrhea) (33%), and fever (31%) were the most common non-cardiac initial complaints. Tachycardia (57%) and tachypnea (52%) was the most common sign on the initial physical exam, followed by nonspecific signs of respiratory tract infection (44%) and respiratory distress (35%). Specific abnormal signs of heart failure such as heart murmur (26%), systolic hypotension (24%), gallop rhythm (20%), or hepatomegaly (20%) were less prevalent. ACS-like was the most common cardiac syndrome (34%), followed by FM (29%), CHF (23%), and dysrhythmia (14%). Patients with ACS-like were older (median age 12.5 (11-13) years vs 2 (1.5-8) years; P < 0.001), presented earlier [median 2 (1-3) d vs 8.5 (4-12) d, P = 0.005], and with normal LV systolic function [median LVEF of 65% (62-77) vs 41% (30-53), P < 0.001] than patients with the rest of the cardiac syndromes. Conversely, patients with FM were younger [median age of 1.15 years [0.95-1.85 vs 11 years (6-13), P < 0.001], and presented later [median 9 (4.5-11) d vs 4 (2-10) d, P = 0.017] and with depressed LV systolic function [median LVEF of 32% (27-36) vs 62% (45-66), P < 0.001] compared to patients with other cardiac syndromes (Table 2).
| Variable | ACS-like (n = 14; 33%) | No ACS-like (n = 28; 66%) | P value | FM (n = 12; 28%) | No FM (n = 30; 78%) | P value |
| Age (yr), median (IQR) | 11 (5-12.5) | 1.6 (1-8) | < 0.001 | 10 (2-13) | 1.7 (0.9-3) | < 0.001 |
| Male sex, n (%) | 19 (71) | 19 (67) | 0.813 | 9 (75) | 20 (66) | 0.598 |
| Days from initial symptoms, median (IQR) | 2 (1-3) | 8.5 (4-12) | 0.005 | 9 (4.5-11) | 4 (2-10) | 0.017 |
| Visits prior to admission, median (IQR) | 1 (1-1) | 2 (2-3) | < 0.001 | 2.5 (2-3) | 1 (1-1) | < 0.001 |
| Viral prodromal, n (%) | 9 (31) | 5 (38) | 0.637 | 9 (31) | 3 (23) | 0.598 |
| Altered ECG, n (%) | 14 (100) | 25 (89) | 0.204 | 12 (100) | 27 (90) | 0.256 |
| CPR (mg/dl), median (IQR) | 5 (2.5-188) | 3 (2-144) | 0.179 | 29 (3-203) | 3 (2-144) | 0.095 |
| Troponin T (ng/mL), median (IQR) | 466 (89-800) | 51 (4-353) | 0.041 | 134 (71-431) | 70 (4-160) | 0.308 |
| NT-proBNP (pg/mL), median (IQR) | 137 (78-234) | 1960 (272-3175) | < 0.001 | 2900 (2207-6125) | 225 (105-561) | < 0.001 |
| LVEF (%), median (IQR) | 65 (62-67) | 41 (30-53) | < 0.001 | 32 (27-36) | 62 (45-66) | < 0.001 |
| LGE in cMRI (n = 21), n (%) | 10/14 (71) | 5/7 (71) | 1.000 | 0/1 (0) | 15/20 (75) | 0.105 |
| Positive Microbiology, n (%) | 9 (64) | 18 (64) | 1.000 | 8 (66) | 19 (63) | 0.839 |
| Poor outcomes, n (%) | 0 (0) | 7 (25) | 0.041 | 5 (41) | 2 (7) | 0.006 |
Troponin T plasma levels were assessed as a biomarker for myocardial damage in 90% of patients with median levels of 91 (5-550) pg/mL and were increased in 62% of cases. NT-proBNP as a biomarker for CHF was determined in 81% of patients with median levels of 482 (151-2500) pg/mL and was increased in 40% of cases. The systemic inflammatory response was assessed by C-reactive protein (CPR) in all patients with median levels of 4 (2-144) mg/dL and was increased in 16% of cases. An abnormal ECG was observed in most patients (93%) with sinus tachycardia (61%) and signs suggestive of ischemia (57%) as the most common features. Life-threatening arrhythmias (ventricular tachycardia or acute complete atrioventricular block with cardiac arrest) were observed in 9.5% of patients. Chest X-ray revealed cardiomegaly in 35% and pulmonary edema in 28% of patients. Echocardiography was abnormal in 88% of patients. LV systolic dysfunction was observed in up to 50% of the entire cohort, 14% with severe LV dysfunction and 10% with biventricular dysfunction. Segmental wall motion abnormalities were observed in 38% of cases, most of them (76%) children with ACS-like and normal LVEF. cMRI was performed in 50% of the population study after a median of 4 (3-6) d of hospitalization, most of them (95%) children > 10-year-old without FM presentation confirming the presence of myocardial inflammation in 86% of cases.
Most patients (73%) received cardiac medications during the admission, mostly diuretics (69%), angiotensin-converting-enzyme inhibitors (69%), and beta-blockers (64%). No treatment with gammaglobulin or steroids was administered. Up to 35% of cases required inotropic support, whereas advanced circulatory support with extracorporeal membrane oxygenation or pacemaker implantation was required in 2.5% of cases. Up to 43% of patients were classified as having an early poor outcome and 16% as having a late poor outcome. The mortality rate secondary to cardiac causes was 5%, all cases during the first hospitalization with no cases of heart transplantation. The median length of hospitalization was 6 (3-13) d. LV dysfunction or dilation persisted in up to 38% and 12% of patients, respectively after the hospital discharge and after 1 year of follow-up. The spontaneous recovery rate of LV function was 69% during the first year of follow-up. Only one patient presented a permanent and significant non-cardiac complication, which was cerebral palsy due to prolonged cardiac arrest after acute atrioventricular block.
Upon univariate analysis (Table 2 and Table 3), age < 2 years, diagnosis > 7 d from initial symptoms, NT-proBNP admission levels > 5000 pg/mL, and LVEF < 30% predicted both early and late poor outcome. In multivariate analysis (Table 4 and Table 5), an initial LVEF < 30% remained the only significant predictor for early and late poor outcome in children with myocarditis.
| Variable | Early outcome (hospital discharge) | Late outcome (1 yr after admission) | ||||
| Good outcome(n = 24; 57%) | Poor outcome(n = 18; 43%) | P value | Good outcome (n = 35; 84%) | Poor outcome (n = 7; 16%) | P value | |
| Age (years), median (IQR) | 11 (5-12.5) | 1.6 (1-8) | 0.026 | 10 (2-13) | 1.7 (0.9-3) | 0.014 |
| Male sex, n (%) | 14 (78) | 15 (62) | 0.289 | 24 (68) | 5 (71) | 0.881 |
| Evolution (days from initial symptoms), median (IQR) | 2.5 (2-10) | 7 (4-12) | 0.036 | 4 (2-10) | 7 (7-15) | 0.043 |
| Cardiac syndrome, n (%) | ||||||
| FM | 2 (8) | 10 (55) | < 0.001 | 7 (20) | 5 (71) | < 0.001 |
| CHF | 6 (25) | 4 (22) | 0.834 | 8 (23) | 2 (28) | 0.746 |
| Dysrhythmia | 3 (12) | 3 (16) | 0.793 | 6 (17) | 0 (0) | 0.237 |
| ACS-like | 13 (54) | 1 (5) | 0.001 | 14 (40) | 0 (0) | 0.041 |
| Viral prodromal, n (%) | 15 (62) | 14 (68) | 0.289 | 23 (65) | 6 (85) | 0.296 |
| Altered ECG, n (%) | 22 (91) | 17 (94) | 0.729 | 32 (91) | 7 (100) | 0.421 |
| CPR (mg/dL), median (IQR) | 4 (2-147) | 4 (3-125) | 0.443 | 4 (2-144) | 33 (3-156) | 0.415 |
| Troponin T (ng/mL), median (IQR) | 118 (5-580) | 91 (32-196) | 0.789 | 103 (5-610) | 52 (32-177) | 0.447 |
| NT-proBNP (pg/mL), median (IQR) | 291 (92-300) | 2700 (1955-4320) | < 0.001 | 300 (123-1955) | 5700 (2500-10321) | 0.002 |
| LVEF (%), median (IQR) | 65 (58-66) | 34 (28-41) | < 0.001 | 59 (41-66) | 29 (27-31) | < 0.001 |
| LVDD Z score > 2, n (%) | 9 (37) | 17 (94) | < 0.001 | 19 (54) | 7 (100) | 0.023 |
| Dyskinesia, n (%) | 11 (46) | 5 (27) | 0.233 | 15 (43) | 1 (14) | 0.155 |
| LGE in cMRI (n =21), n (%) | 12 (75) | 3 (60) | 0.517 | 14 (70) | 1 (100) | 0.517 |
| Positive Microbiology, n (%) | 12 (50) | 8 (44) | 0.307 | 16 (45) | 4 (57) | 0.666 |
LVEF correlated well with age (r = 0.51, P = 0.005), days from initial symptoms (r = -0.31; P = 0.045), and NT-proBNP levels (r = 0.66, P = 0.001), but not with troponin T (r = -0.05, P = 0.730) or CRP levels (r = -0.13, P = 0.391) (Figure 2). NT-proBNP presented a high diagnostic accuracy for severe LV systolic dysfunction on echocardiography, with an area under the curve of 0.931 (95%CI: 0.858-0.995, P = 0.001) (Figure 3). The best cut-off point was 2000 pg/mL with a sensitivity of 90%, specificity of 81%, positive predictive value of 60%, and negative predictive value of 96%.
In this retrospective study, we described the clinical presentation and diagnostic method findings of 42 children with a clinical diagnosis of myocarditis. Also, we provided clues to improve the early recognition of myocarditis and define possible early predictors for poor outcome in these patients.
The identification of pediatric patients with myocarditis in the early phases of the disease is essential in order to start monitoring and supportive treatment in a timely manner. However, the diagnosis may be challenging at initial stages in milder cases before the development of severe adverse events. Our results showed a heterogeneous and unspecific clinical presentation of myocarditis in children. Most patients presented with a preceding viral illness involving the respiratory or gastrointestinal tracts that was not associated with myocarditis initially. Of note, the most common specific cardiac symptom (chest pain) was observed in a similar proportion with shortness of breath and nonspecific respiratory or gastrointestinal symptoms that could mimic the clinical presentation of benign viral infections[2,13-15]. Besides, the physical examination revealed specific cardiac signs only in a minority of cases, and the most common alterations found on physical examination (tachycardia, tachypnea, respiratory distress, and abnormal lung auscultation) were non-specific cardiac signs. Therefore, the early identification of pediatric myocarditis based on clinical presentation and physical examination is challenging. We characterized four specific cardiac presentations in our pediatric myocarditis cohort that included FM, ACS-like, dysrhythmia, and CHF. Remarkably, we observed that the clinical presentation was associated with the age and the time of evolution of symptoms. Thus, older children (> 10-years-old) with ACS-like were diagnosed early after the initial symptoms usually at the time of the first consultation. Conversely, infants (< 2-years-old) were diagnosed late usually after two or more consultations with FM or CHF. This may be explained by the capacity of the older children to describe their symptoms in detail leading to prompt medical consultation and diagnosis. Conversely, younger infants are not able to express their symptoms, and clinicians are more reliant on parents’ observations. Therefore, they only seek medical attention when they observe late symptoms related to heart failure[6,24-26]. Also, the influence of the age on the immunologic response to viral infection of the myocardium might lead to different clinical courses and outcomes[7,29]. Our finding that most patients were consulted to the ED two or more times prior to the final diagnosis and that many patients had a previous diagnosis different from myocarditis, reinforces that a high index of suspicion is needed for infants with prolonged viral infections (overall with respiratory signs or symptoms) that do not improve and lead to multiple medical consultations.
Another reason that diagnosing myocarditis is difficult is the limited diagnostic accuracy of most available noninvasive diagnostic tests. There are no accurate serum biomarkers for the diagnosis of myocarditis[1]. CPR can be elevated in the acute phase, but it is neither sensitive nor specific to determine the presence or absence of active myocardial inflammation. We observed elevated CRP levels only in 16% of our population, so normal values do not exclude a myocardial inflammatory process. Myocytes destruction also occurs in the acute phase of myocarditis; thus serum biomarkers of myocardial damage can be elevated in some cases. However, the sensitivity and negative predictive of elevated troponin I level in patients with biopsy-proven myocarditis were reported to be low[30-32]. We observed elevated troponin T levels in up to 65% of our population. Therefore, increased troponin T levels can reinforce the clinical suspicion of myocarditis in children, but a normal result does not exclude the diagnosis[1]. Natriuretic peptides are secreted by ventricular myocytes in response to volume or pressure overload to counteract the renin-angiotensin-aldosterone and sympathetic nervous system actions in the context of heart failure. Thus, natriuretic peptides can be elevated in myocarditis with LV dysfunction and heart failure[33]. We observed increased NT-proBNP levels only in 40% of patients, and therefore a normal value does not discard the diagnosis of myocarditis. Of note, increased NT-proBNP levels may aid in distinguishing a cardiac from a non-cardiac reason for respiratory symptoms in children[34,35]. This feature seems to be very useful to identify patients with a cardiac process in the context of nonspecific respiratory symptoms, as we observed in our young patients with myocarditis.
ECG and chest X-ray also have limited value for the diagnosis of myocarditis. Although ECG is virtually always abnormal in children with myocarditis[13], the abnormalities observed were widely variable, and there was not one specific abnormality that occurs with enough frequency to be a specific marker[16-19]. Also, chest X-ray revealed more specific cardiac findings such as cardiomegaly and pulmonary in a minority of patients.
Echocardiography remains the more useful diagnostic test in cases with clinical suspicion of myocarditis[22]. Most children had echocardiographic alterations on admission. The most typical findings associated with myocarditis were LV dilatation and reduced global LVEF. Another echocardiographic sign that can help make the diagnosis is the presence of segmental wall motion abnormalities mimicking ischemic cardiomyopathy[23], which were observed mostly in older children with ACS-like. This feature can be explained by the focal distribution of areas of inflammation frequently seen in myocarditis. Mitral regurgitation and pericardial effusion may also be observed and help make a diagnosis. Similar to the clinical presentation, echocardiographic findings depend on both the manner and timing of a patient’s presentation. Thus, young patients with FM and more days of evolution presented the most depressed LVEF whereas older patients that were consulted early in the course of the disease with ACS-like usually presented a normal LVEF and segmental wall motion alterations.
cMRI is a promising diagnostic method for myocarditis. The major strength of cMRI is its capacity to detect inflammation, edema, necrosis, and fibrosis within myocardial tissue through several imaging sequences. The use of a mixed cMRI method (Lake Louis criteria) is preferred compared to EMB in clinically stable patients suspected of having myocarditis. Nevertheless, the presence of two of the three characteristics listed in the Lake Louise criteria leads to a low-moderate sensitivity (67%-78%) and negative predictive value (69%), and high specificity (91%) and positive predictive value (91%)[24]. Therefore, a negative test does not discard the clinical suspicion of myocarditis. The sensitivity of cMRI for the diagnosis of myocarditis is likely to be limited in patients with less inflammation and a prolonged duration of symptoms; thus cMRI may be more helpful in the diagnosis of acute myocarditis if performed within 14 d of the onset of symptoms[30]. Also, cMRI is not widely available in all centers, and it is difficult to carry out in non-stable patients and infants, which represent the most common clinical picture of myocarditis. Consistent with this, we performed cMRI early in the course of the disease (median of 4 d after admission) in 50% of our population, most of them teenagers with an ACS-like presentation, confirming the diagnosis of suspected myocarditis in 86%. The only cMRI in an infant with FM was performed after 14 d of diagnosis when the patient was stable and was negative. Although cMRI is a promising technology, its sensitivity for the diagnosis of myocarditis must be improved and used timely in the appropriate candidates.
Myocarditis is a significant etiology of CHF, often leading to DCM, need for heart transplantation, and death. Poor outcomes were observed in up to 43% of patients at the time of hospital discharge and 16% after 1 year of follow-up. The mortality rate was 5%, and all cases of death occurred during the initial hospitalization. We observed LV dilation in 43% and LV dysfunction in 50% (14% severe) of cases on admission, reflecting the potential of myocarditis to produce important alterations in the early phases of the disease. Acute myocarditis in patients who present with LV systolic dysfunction can recover in weeks to months. Of note, we found a spontaneous complete recovery rate of initial LV dysfunction or dilation of 69% during the first year of follow-up. These outcomes are similar to those observed in two recent large prospective multicentric studies involving pediatric myocarditis[6,9] and highlight a contemporary good prognosis of acute myocarditis in children that could reflect the advances in the recognition and management of these patients. Also, this reinforces the need for aggressive initial management in the acute phase of the disease waiting for a possible recovery in most patients. Nevertheless, we found that myocarditis in children is still associated with a high rate of CHF, hospitalization, intensive care unit stay, use of cardiac medications, and inotropic support or mechanical circulatory support at the time of diagnosis. Also, 12% still suffered from LV dysfunction or dilation after 1 year. Hence, it would be useful to identify these high-risk patients in the early phase of the disease. The main finding of this study was that a severe depressed LV systolic function (LVEF < 30%) on admission was the only independent predictor for poor outcomes in pediatric myocarditis. However, in the univariate analysis we identified some features that could help clinicians detect these patients on admission. These factors were age < 2-years-old, > 7 d from the initial symptoms, and NT-proBNP > 5000 pg/mL. Of note, LVEF was associated with all these factors. As echocardiography is not widely available at emergency departments and requires some training and expertise, we think that the above mentioned clinical and biochemical factors could be useful to identify high-risk patients. Specifically, NT-proBNP levels seem to be very useful for the screening of LV dysfunction upon admission in children with myocarditis. We found that the diagnostic accuracy of NT-proBNP levels on admission for severe LV dysfunction on echocardiography was high and that those patients with concentrations less than 2000 pg/mL at admission probably will not have a severe LV dysfunction on echocardiography, and therefore they will have a good outcome.
This study had several limitations. The retrospective nature without the establishment of inclusion criteria prior to the beginning of the study and the use of clinically diagnosed myocarditis could lead to the inclusion of patients who did not have myocarditis. The absence of cases of heart transplantation could be because our center does not have a heart transplant program. Therefore, it is possible that more severe cases of myocarditis were referred directly to hospitals with a heart transplant program. Although our mortality rate was low and consistent with the recent literature, it could also be influenced by this fact, and this could limit the risk factors provided. Also, the time of follow-up was short, and this could prevent the analysis of risk factors for long-term outcomes. Finally, none of our patients received immunomodulatory therapy, a promising therapeutic strategy as suggested by several randomized trials. This could impact the outcomes observed in our study, but this kind of therapy remains investigational mainly at this time and requires further elucidation. Of note, despite not using these treatments, our outcomes were similar to those studies that used them.
The timely diagnosis of myocarditis in children is challenging due to the heterogeneous and unspecific clinical presentation and the low diagnostic accuracy of most non-invasive laboratory tests. Myocarditis should be considered among the differential diagnosis in infants with prolonged viral infections (overall with respiratory signs or symptoms) that does not improve and leads to multiple medical consultations. Echocardiography remains the most useful diagnostic tool for myocarditis, and the presence of severe LV dysfunction at admission was the primary predictor for poor outcomes. Younger ages, a prolonged course of the disease, and NT-proBNP levels are some factors that could help to identify these high-risk patients in ED.
Pediatric myocarditis constitutes an important cause of morbidity and mortality in the form of death, heart transplant, and dilated cardiomyopathy. Pediatric myocarditis diagnosis is usually challenging. It is mainly due to its heterogeneous and unspecific clinical presentation, and the low accuracy of the currently available non-invasive diagnostic tests. Also, the gold standard for diagnosis, the endomyocardial biopsy has inherent risks and is not widely available. Of note, a diagnostic delay can lead to a subsequent elevated morbimortality.
We aimed to investigate the clinical presentation and the results of the non-invasive tests utilized in the diagnostic work-up of pediatric myocarditis at presentation.
Our main purpose was to provide clinical, biochemical, or echocardiographic clues in order to improve the early recognition of pediatric myocarditis at presentation in the emergency department. Also, we sought to identify predictors for poor outcome in pediatric myocarditis in order to identify high-risk patients that will need a more intensive therapy and close follow-up.
We carried out a retrospective cross-sectional single-center study. A total of 42 patients between 2008 and 2017 were enrolled. Patients were divided into two groups: poor outcome and no poor outcome. We delineated the clinical presentation of our patients with myocarditis. Also, different clinical and diagnostic test variables were analyzed in order to find possible poor outcome predictors.
The clinical presentation of pediatric myocarditis was heterogeneous, ranging from asymptomatic to cardiogenic shock cases. Of note, pediatric myocarditis clinical presentation can mimic benign viral infections in children, usually in those younger patients that are not able to verbalize symptoms and debut as fulminant myocarditis. Conversely older patients presented with acute coronary syndrome-like myocarditis. There was no single non-invasive diagnostic test that led to rule out or rule in the diagnosis of pediatric myocarditis. Cardiac magnetic resonance imaging presented a high diagnostic accuracy, but was mostly useful only for older children with acute coronary syndrome-like presentation. Severe depressed left ventricle systolic function on echocardiography was the only independent predictor for poor outcome (death, transplant, or dilated cardiomyopathy). The presence of age < 2-years-old, a clinical course of more than 7 d from initial symptoms, or N-terminal pro-brain natriuretic peptide levels > 5000 pg/mL was associated with a severe depressed LV systolic function.
A high grade of clinical suspicion is needed to make an early diagnosis of pediatric myocarditis, especially in infants who cannot verbalize their symptoms. This clinical suspicion must be used in combination with several findings of different non-invasive diagnostic tests in order to improve the prompt recognition of pediatric myocarditis. This study provided some clinical pictures that can help clinicians in this context. Echocardiography is a reliable diagnostic tool for pediatric myocarditis. Patients that present with a severe depressed LV systolic function have a poor outcome. Age younger than 2 years, prolonged course of disease, and higher N-terminal pro-brain natriuretic peptide levels are predictors of poor outcome that could be useful even in centers where echocardiography is not available. The combined use of these predictors can lead to an early detection of high-risk patients in order to initiate adequate treatment and monitoring.
This article reflects the challenge to diagnose pediatric myocarditis. Future studies should prospectively collect multicenter data on epidemiology, clinical presentation, and diagnostic value of currently available diagnostic tools in children with myocarditis to establish clinically meaningful criteria for the diagnosis of myocarditis. This will enhance the early recognition and subsequently the prognosis of these patients.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Karatza AA, Ciccone MM S- Editor: Dou Y L- Editor: Filipodia E- Editor: Bian YN
| 1. | Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Böhm M. Update on myocarditis. J Am Coll Cardiol. 2012;59:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 672] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 2. | Shekerdemian L, Bohn D. Acute viral myocarditis: Epidemiology and pathophysiology. Pediatr Crit Care Me. 2006;7:S2. [DOI] [Full Text] |
| 3. | Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 4. | Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 545] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 5. | Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, Slonim AD. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatr Cardiol. 2010;31:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Butts RJ, Boyle GJ, Deshpande SR, Gambetta K, Knecht KR, Prada-Ruiz CA, Richmond ME, West SC, Lal AK. Characteristics of Clinically Diagnosed Pediatric Myocarditis in a Contemporary Multi-Center Cohort. Pediatr Cardiol. 2017;38:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet. 2012;379:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 548] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Levine MC, Klugman D, Teach SJ. Update on myocarditis in children. Curr Opin Pediatr. 2010;22:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Messroghli DR, Pickardt T, Fischer M, Opgen-Rhein B, Papakostas K, Böcker D, Jakob A, Khalil M, Mueller GC, Schmidt F, Kaestner M, Udink Ten Cate FEA, Wagner R, Ruf B, Kiski D, Wiegand G, Degener F, Bauer UMM, Friede T, Schubert S; MYKKE Consortium. Toward evidence-based diagnosis of myocarditis in children and adolescents: Rationale, design, and first baseline data of MYKKE, a multicenter registry and study platform. Am Heart J. 2017;187:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Elamm C, Fairweather D, Cooper LT. Pathogenesis and diagnosis of myocarditis. Heart. 2012;98:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 12. | Freedman SB, Haladyn JK, Floh A, Kirsh JA, Taylor G, Thull-Freedman J. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics. 2007;120:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 13. | Chang YJ, Chao HC, Hsia SH, Yan DC. Myocarditis presenting as gastritis in children. Pediatr Emerg Care. 2006;22:439-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, Yasukochi S, Arakaki Y, Joo K, Nakazawa M. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. 2012;76:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Durani Y, Egan M, Baffa J, Selbst SM, Nager AL. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009;27:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Checchia PA, Kulik TJ. Acute viral myocarditis: Diagnosis. Pediatr Crit Care Me. 2006;7:S8. [DOI] [Full Text] |
| 17. | Kern J, Modi R, Atalay MK, Kochilas LK. Clinical myocarditis masquerading as acute coronary syndrome. J Pediatr. 2009;154:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Batra AS, Epstein D, Silka MJ. The clinical course of acquired complete heart block in children with acute myocarditis. Pediatr Cardiol. 2003;24:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Ichikawa R, Sumitomo N, Komori A, Abe Y, Nakamura T, Fukuhara J, Matsumura M, Miyashita M, Kanamaru H, Ayusawa M, Mugishima H. The follow-up evaluation of electrocardiogram and arrhythmias in children with fulminant myocarditis. Circ J. 2011;75:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Balaji S, Wiles HB, Sens MA, Gillette PC. Immunosuppressive treatment for myocarditis and borderline myocarditis in children with ventricular ectopic rhythm. Br Heart J. 1994;72:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Dello Russo A, Pieroni M, Santangeli P, Bartoletti S, Casella M, Pelargonio G, Smaldone C, Bianco M, Di Biase L, Bellocci F, Zeppilli P, Fiorentini C, Natale A, Tondo C. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011;8:1915-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A, Silvestri F, Camerini F. Echocardiographic findings in myocarditis. Am J Cardiol. 1988;62:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 172] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Angelini A, Calzolari V, Calabrese F, Boffa GM, Maddalena F, Chioin R, Thiene G. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000;84:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1951] [Cited by in RCA: 1743] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 25. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8282] [Cited by in RCA: 8796] [Article Influence: 462.9] [Reference Citation Analysis (0)] |
| 26. | Dec GW, Waldman H, Southern J, Fallon JT, Hutter AM, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Hoyer MH, Fischer DR. Acute myocarditis simulating myocardial infarction in a child. Pediatrics. 1991;87:250-253. [PubMed] |
| 28. | Kühl U, Pauschinger M, Bock T, Klingel K, Schwimmbeck CP, Seeberg B, Krautwurm L, Poller W, Schultheiss HP, Kandolf R. Parvovirus B19 infection mimicking acute myocardial infarction. Circulation. 2003;108:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Drossner DM, Hirsh DA, Sturm JJ, Mahle WT, Goo DJ, Massey R, Simon HK. Cardiac disease in pediatric patients presenting to a pediatric ED with chest pain. Am J Emerg Med. 2011;29:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, Klingel K, Kandolf R, Schuler G, Gutberlet M, Thiele H. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 32. | Lippi G, Salvagno GL, Guidi GC. Cardiac troponins in pediatric myocarditis. Pediatrics. 2008;121:864; author reply 864-864; author reply 865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Koulouri S, Acherman RJ, Wong PC, Chan LS, Lewis AB. Utility of B-type natriuretic peptide in differentiating congestive heart failure from lung disease in pediatric patients with respiratory distress. Pediatr Cardiol. 2004;25:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Welisch E, Norozi K, Rauch R. N-terminal pro-brain natriuretic peptide level as a screening tool for cardiac involvement in paediatric diseases of extracardiac origin. Clin Res Cardiol. 2011;100:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Hammerer-Lercher A, Geiger R, Mair J, Url C, Tulzer G, Lechner E, Puschendorf B, Sommer R. Utility of N-terminal pro-B-type natriuretic peptide to differentiate cardiac diseases from noncardiac diseases in young pediatric patients. Clin Chem. 2006;52:1415-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |