Published online Feb 26, 2019. doi: 10.12998/wjcc.v7.i4.525
Peer-review started: October 10, 2018
First decision: November 27, 2018
Revised: December 22, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: February 26, 2019
Processing time: 139 Days and 19 Hours
Primary hepatic leiomyosarcoma is rare and reported sporadically, with less than 40 such cases have been reported in the English-language literature. Although it is reported to be associated with acquired immune deficiency syndrome, Epstein-Barr virus infection, Hodgkin’s lymphoma, immunosuppression after organ transplantation, and hepatitis C virus-related liver cirrhosis, the precise steps leading to leiomyosarcoma have not been fully identified. Therapeutic strategies include liver wedge resection or lobectomy, chemotherapy, radiotherapy and liver transplantation; however, the prognosis of primary hepatic leiomyosarcoma is dismal.
We describe here the first case of primary hepatic leiomyosarcoma successfully treated by transcatheter arterial chemoembolization (TACE). The patient was a 68-year-old woman who presented with right upper quadrant pain and weight loss over the past 5 wk before admission. Abdominal computed tomography (commonly known as CT) and ultrasonography showed a mixed echoic mass measuring about 10 cm × 7 cm occupying the right lobe of the liver. Exploratory laparotomy was performed 1 wk after admission. The tumor was unresectable and biopsy was performed. Based on rapid frozen-section and histopathological examination, a final diagnosis of primary hepatic leiomyosarcoma was established. TACE was performed 2 wk later. The postoperative course was uneventful and the patient was discharged on day 7 after the operation. Contrast-enhanced CT showed that the tumor significantly shrunk with satisfactory lipiodol deposition. The patient has been followed up for 82 mo until now, and no progressive enlargement of the tumor or distal metastasis was observed.
TACE is a safe and effective treatment for primary hepatic leiomyosarcoma. The therapeutic effect of TACE combined with surgical resection should be further assessed.
Core tip: Primary hepatic leiomyosarcoma is rare and reported sporadically. We here describe the first case of primary hepatic leiomyosarcoma successfully treated with transcatheter arterial chemoembolization (commonly known as TACE). The postoperative course was uneventful and the patient was discharged on day 7 after the operation. Contrast-enhanced computed tomography showed that the tumor significantly shrunk with satisfactory lipiodol deposition. The patient has been followed up for 82 mo until now, and no progressive enlargement of the tumor or distal metastasis was observed. This case indicates that TACE is a safe and effective treatment for primary hepatic leiomyosarcoma.
- Citation: Zhu KL, Cai XJ. Primary hepatic leiomyosarcoma successfully treated by transcatheter arterial chemoembolization: A case report. World J Clin Cases 2019; 7(4): 525-531
- URL: https://www.wjgnet.com/2307-8960/full/v7/i4/525.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i4.525
Primary hepatic leiomyosarcoma is rare and only reported sporadically[1], with less than 40 cases having been reported to date. It appears to be associated with acquired immune deficiency syndrome (commonly known as AIDS)[2], Epstein–Barr virus[3], Hodgkin’s lymphoma[4], immunosuppression after organ transplantation[5], and hepatitis C virus (commonly known as HCV)-related liver cirrhosis[6], the precise mechanisms leading to leiomyosarcoma have yet been fully identified. Surgical resection is recommended for a curative treatment, while diagnosis of primary hepatic leiomyosarcoma is challenging and often delayed until it reaches a size that results in dismal prognosis.
We herein describe the first case of primary hepatic leiomyosarcoma successfully treated by transcatheter arterial chemoembolization (TACE). The patient has survived for 82 mo with no tumor enlargement and distant metastasis detected at postoperative follow-up.
A 68-year-old woman was referred to our hospital on December 2011, due to right upper quadrant pain and a 5-pound weight loss.
Other symptoms were not presented and she did not drink alcohol, smoke or have a history of surgery.
Physical examination revealed a soft abdomen, no tenderness, no rebound tenderness, and no palpable lymph nodes or abdominal mass.
The following laboratory data were recorded: hemoglobin of 125 g/L; white cell count of 5.5 × 109/L, with 72.5% neutrophils; platelet count of 234 × 109 L; alanine aminotransferase of 25 U/L; serum total protein of 75 g/L; albumin of 37.2 g/L; total bilirubin of 11 μmol/L; direct bilirubin of 9.5 μmol/L; α-fetoprotein of 5.23 ng/mL; and carcinoembryonic antigen of 4.5 ng/L. Serological markers for hepatitis B virus and HCV were negative. The indocyanine green retention rate at 15 min was 2.2%.
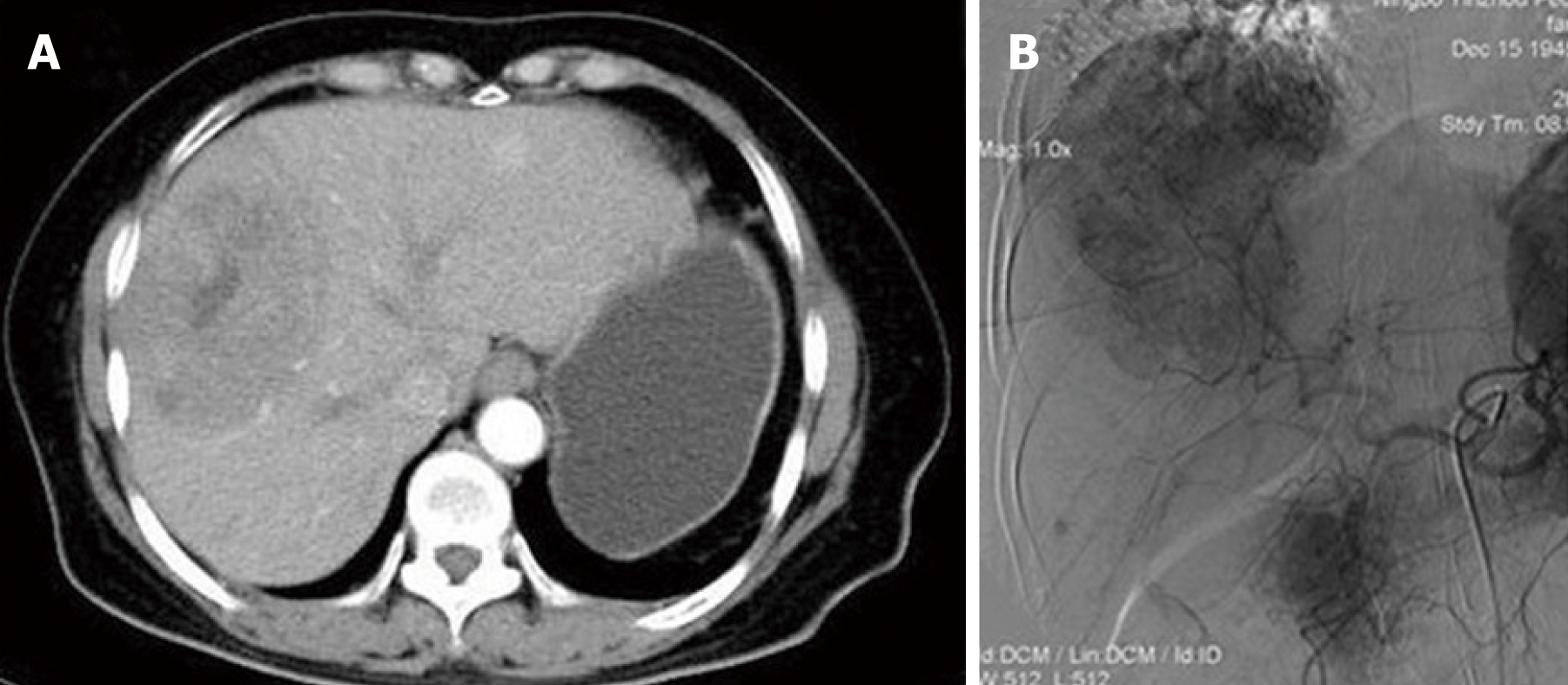
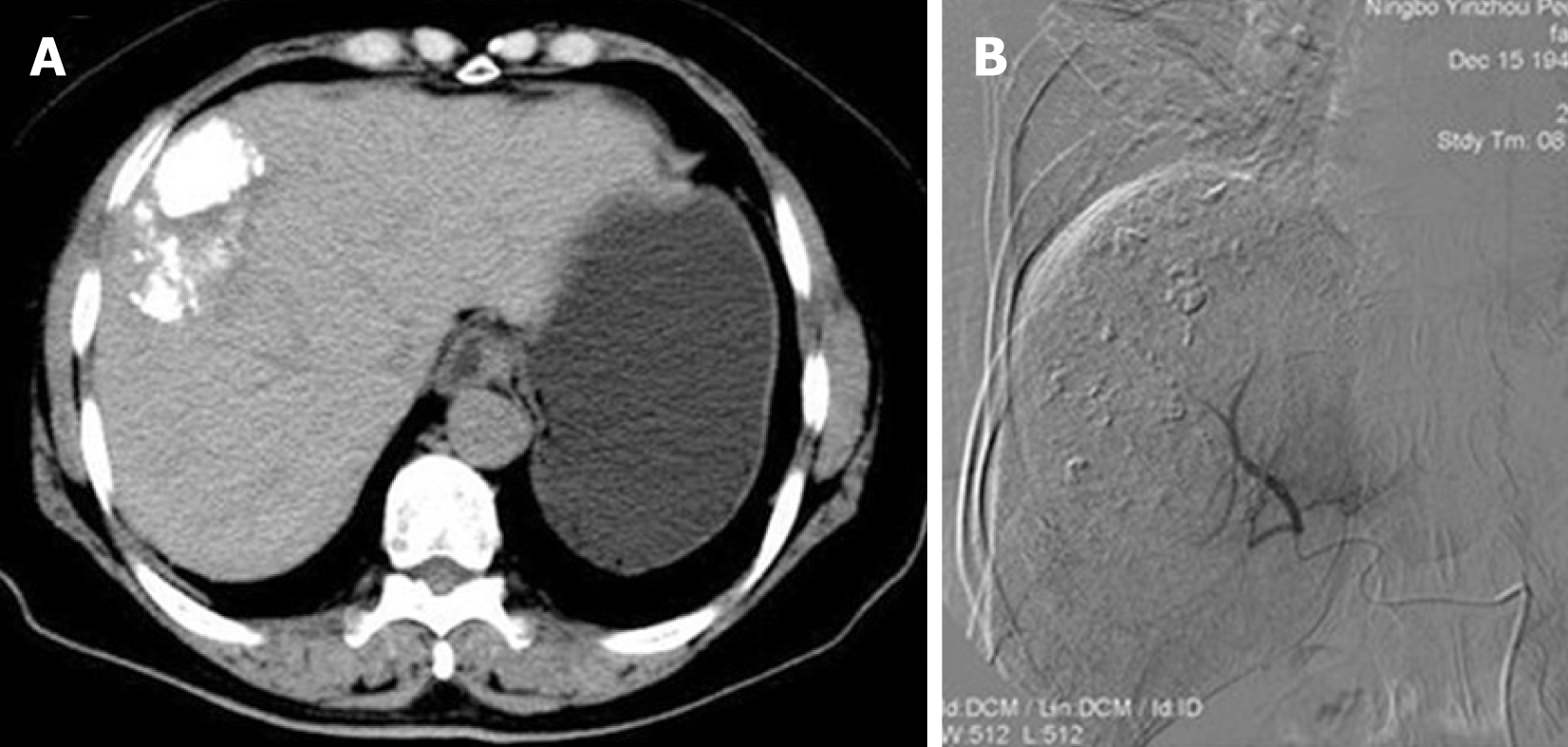
Abdominal ultrasonography showed there was a mixed echoic mass measuring around 10 cm × 7 cm in the right hepatic lobe. Abdominal computed tomography (CT) showed a similar finding, that the tumor was inhomogeneous density, with mild delayed enhancement, and had central necrosis (Figure 1A). Gastrointestinal endoscopy and colonoscopy showed negative findings. Chest CT showed no mass over the lung.
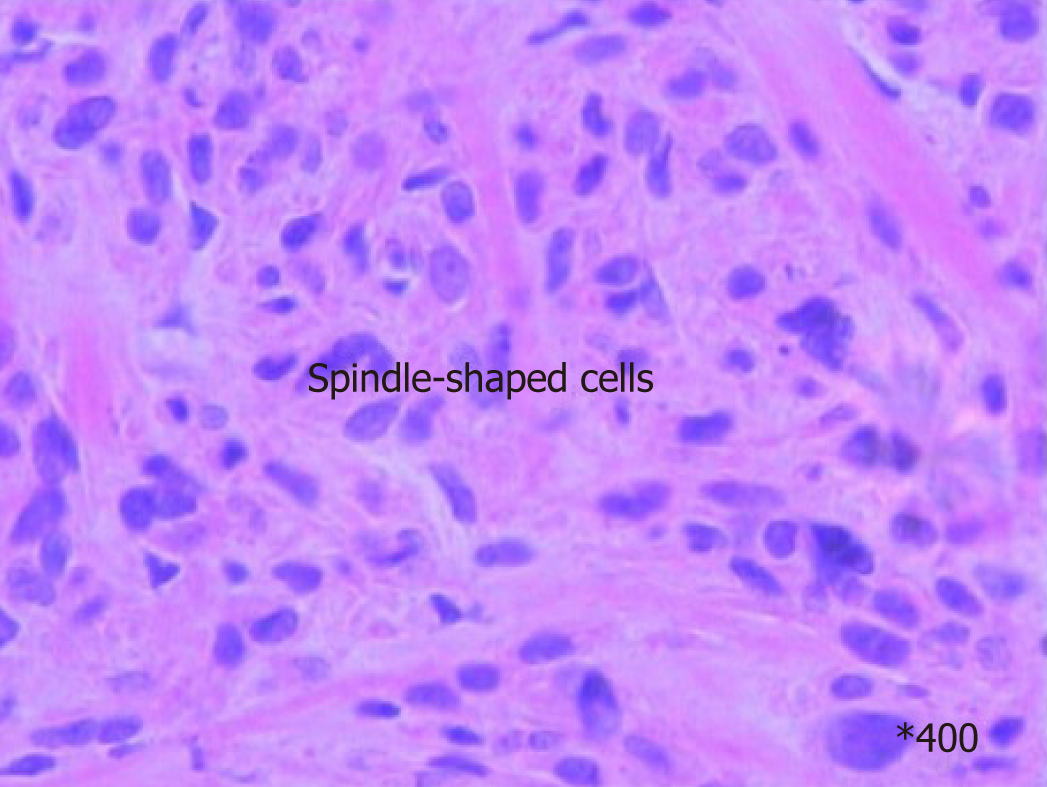
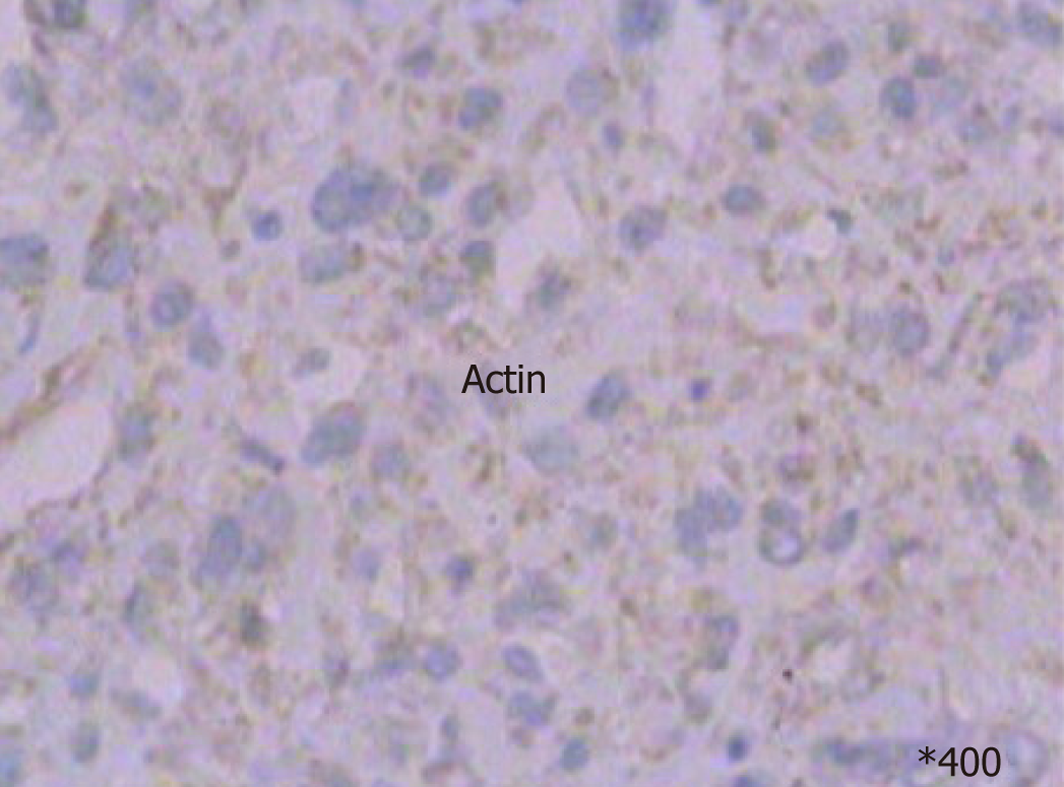
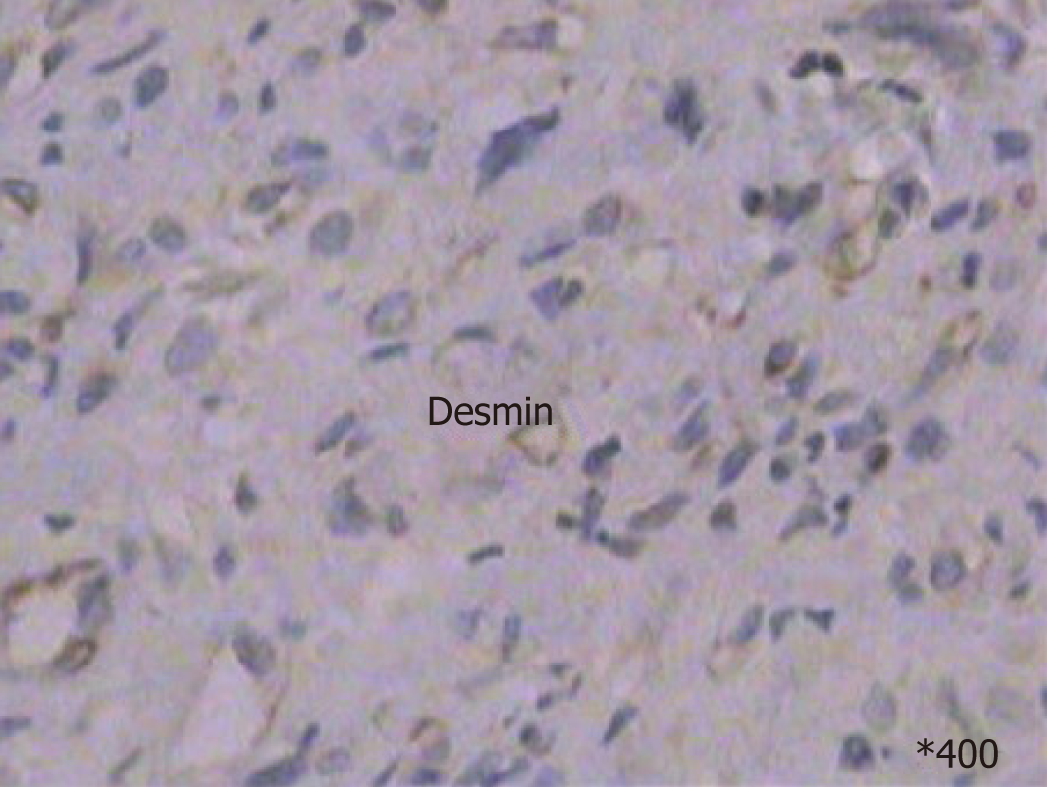
Preoperative diagnosis was unconfirmed, exploratory laparotomy was performed 1 wk after admission, and no obvious effusion was found in the abdominal cavity. The entire liver VIII was occupied by a creamy white, firm mass measuring about 10 cm × 7 cm, which protruded into the abdominal cavity and appeared to invade the diaphragm and middle hepatic vein , making the tumor unresectable (Figure 1); thus, a biopsy was performed. Rapid frozen-section biopsy analysis considered the diagnosis of leiomyosarcoma. Histopathological examination showed a hepatic mass that consisted of spindle-shaped cells with mitotic figures and nuclear atypia (Figure 2). Immunohistochemical staining showed that spindle-shaped cells were positive for anti-smooth muscle actin (Figure 3) and desmin (Figure 4) but negative for keratin, S-100, CD34 and CD117. No obvious lesions were observed in the bilateral adnexa and uterus, and no palpable mass was detected in the superficial body.
Taken together, a final diagnosis of primary hepatic leiomyosarcoma was established.
TACE was performed 2 wk postoperatively. Superior mesenteric angiography showed no significant abnormality. Common hepatic angiography demonstrated a single mass with hypervascularity, tumor staining and dilated feeding arteries in the anterior segment of the right liver lobe (Figure 1B). A catheter was inserted into the feeding artery of the tumor, and the emulsion containing 50 mg epirubicin and Lipiodol (Lipiodol Ultra-Fluid; Guerbet Japan, Tokyo, Japan) was injected, followed by embolization with gelatin sponge particles (Gelpart; Nihonkayaku, Tokyo, Japan). The postoperative course was uneventful and the patient was discharged on day 7 after the operation. The patient underwent three courses of TACE, and the latest treatment was performed in October 2016. There were no newly emerging lesions observed in the liver (Figure 5B).
Routine follow-up was continued in the hepatobiliary clinic. Contrast-enhanced CT in 2018 showed that the tumor had shrunk significantly and was associated with satisfactory lipiodol deposition (Figure 5A). The patient was followed up for 82 mo until now, and no progressive enlargement of the tumor or distal metastasis was observed.
Primary hepatic leiomyosarcoma is extremely rare, and less than 40 such cases have been reported in the English-language literature. Primary hepatic sarcoma comprises only 1%-2% of all malignant hepatic tumors[7], and leiomyosarcoma is particularly rare. Hepatic leiomyosarcoma may arise from intrahepatic vascular structures[8], bile ducts[9] or ligamentum teres[10], and is reported to be associated with AIDS[2], Epstein–Barr virus[3], Hodgkin’s lymphoma[3], immunosuppression after organ transplantation[4], and HCV-related liver cirrhosis[5]. However, the precise steps leading to leiomyosarcoma have not been fully identified.
Leiomyosarcoma has no specific clinical manifestations. It is often asymptomatic until it becomes large and produces nonspecific symptoms, or is unexpectedly found during physical examination. Some patients may have a wide spectrum of symptoms, including abdominal pain, abdominal mass, weight loss, nausea, vomiting, anorexia, abdominal distension, jaundice, and, rarely, acute intra-abdominal bleeding secondary to tumor rupture.
CT findings of primary hepatic leiomyosarcoma have been described as an enlarged, heterogeneous, hypodense mass with peripheral enhancement and evidence of central necrosis, whereas magnetic resonance imaging usually displays the homogeneous or heterogeneous hypointensity tumor on T1-weighted images and hyperintensity on T2-weighted images. Diagnosis of primary hepatic leiomyosarcoma is challenging due to the nonspecific nature of symptoms and lack of serological markers. The clinical manifestations and laboratory and imaging examinations have limited value for diagnosis and differential diagnosis. The definitive diagnosis of leiomyosarcoma mainly depends on pathological and immunohistochemical examinations. The pathological features of leiomyosarcoma include spindle-shaped cells, abundant cytoplasm, nuclear atypia, and presence of mitotic figures. Immunohistochemical staining of tumors is positive for vimentin, desmin and actin, and negative for α-fetoprotein, CD34, CD117, cytokeratin, and hepatocytes.
Surgical resection is considered to be the only potentially curative treatment. Patients with primary hepatic leiomyosarcoma mostly have no history of hepatitis B and cirrhosis. Therefore, complete resection of the tumor, such as regular hepatic lobectomy, hemihepatectomy and trisegmentectomy, may achieve a satisfactory therapeutic outcome. Matthaei et al[11] reported three cases with > 10 yr survival after hepatectomy. Liver transplantation for primary hepatic leiomyosarcoma has remained controversial until now. The outcome for liver transplantation has varied among cases. Liang et al[12] described a patient with primary hepatic leiomyosarcoma who survived for 34 mo after undergoing liver transplantation. In contrast, Saintpaul et al[13] reported a case with only 15 d survival after surgery. The efficacy of chemotherapy and radiotherapy for primary hepatic leiomyosarcoma has not been confirmed. Adjuvant chemotherapy using doxorubicin and ifosfamide seems to slow progression and help to prolong survival after complete resection[14].
Currently, no effective treatments for unresectable primary hepatic leiomyosarcoma have been reported. Fujita et al[5] reported a patient with metastatic leiomyosarcoma who just received palliative and conservative therapy and only survived for 3 mo after diagnosis. For our case, the patient underwent three courses of TACE with emulsion of epirubicin and Lipiodol, followed by embolization with gelatin sponge particles. Postoperative contrast-enhanced CT showed significant tumor shrinkage associated with satisfactory lipiodol deposition. Routine follow-up was continued in the hepatobiliary clinic and there were no newly emerging lesions observed in the liver. The case has been followed up for 82 mo until now, and no progressive enlargement of the tumor or distal metastasis has been observed. Primary hepatic leiomyosarcoma is a tumor with rich blood supply, and tumor cells are intolerant to ischemia. The embolic agents injected into the hepatic artery induced ischemic tumor necrosis. This may be the main mechanism underlying the effectiveness of drug-eluting bead TACE (commonly known as DEB-TACE). Otherwise, the epirubicin was used to suppress tumor progression[15].
We have described the first case of primary hepatic leiomyosarcoma successfully treated by TACE, and demonstrated that TACE is safe and effective. Further studies are required to assess the therapeutic effect of TACE in combination with surgical resection in primary hepatic leiomyosarcoma.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Farshadpour F, Hann HW, Pallav K, Tajiri K, Vaudo G S- Editor: Yan JP L- Editor: Filipodia E- Editor: Tan WW
| 1. | Metta H, Corti M, Trione N, Masini D, Monestes J, Rizzolo M, Carballido M. Primary hepatic leiomyosarcoma--a rare neoplasm in an adult patient with AIDS: second case report and literature review. J Gastrointest Cancer. 2014;45 Suppl 1:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ross JS, Del Rosario A, Bui HX, Sonbati H, Solis O. Primary hepatic leiomyosarcoma in a child with the acquired immunodeficiency syndrome. Hum Pathol. 1992;23:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Brichard B, Smets F, Sokal E, Clapuyt P, Vermylen C, Cornu G, Rahier J, Otte JB. Unusual evolution of an Epstein-Barr virus-associated leiomyosarcoma occurring after liver transplantation. Pediatr Transplant. 2001;5:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Giuliante F, Sarno G, Ardito F, Pierconti F. Primary hepatic leiomyosarcoma in a young man after Hodgkin's disease: diagnostic pitfalls and therapeutic challenge. Tumori. 2009;95:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Fujita H, Kiriyama M, Kawamura T, Ii T, Takegawa S, Dohba S, Kojima Y, Yoshimura M, Kobayashi A, Ozaki S, Watanabe K. Primary hepatic leiomyosarcoma in a woman after renal transplantation: report of a case. Surg Today. 2002;32:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tsuji M, Takenaka R, Kashihara T, Hadama T, Terada N, Mori H. Primary hepatic leiomyosarcoma in a patient with hepatitis C virus-related liver cirrhosis. Pathol Int. 2000;50:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Maki HS, Hubert BC, Sajjad SM, Kirchner JP, Kuehner ME. Primary hepatic leiomyosarcoma. Arch Surg. 1987;122:1193-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Civardi G, Cavanna L, Iovine E, Buscarini E, Vallisa D, Buscarini L. Diagnostic imaging of primary hepatic leiomyosarcoma: a case report. Ital J Gastroenterol. 1996;28:98-101. [PubMed] |
| 9. | Holloway H, Walsh CB, Thomas R, Fielding J. Primary hepatic leiomyosarcoma. J Clin Gastroenterol. 1996;23:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Yamaguchi J, Azuma T, Fujioka H, Tanaka K, Furui J, Tomioka T, Kanematsu T. Leiomyosarcoma occurring in the ligamentum teres of the liver: a case report and a review of seven reported cases. Hepatogastroenterology. 1996;43:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, Rogiers X, Knoefel WT, Peiper M. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144:339-44; discussion 344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Liang X, Xiao-Min S, Jiang-Ping X, Jie-Yu Y, Xiao-Jun Z, Zhi-Ren F, Guo-Shan D, Rui-Dong L. Liver transplantation for primary hepatic leiomyosarcoma: a case report and review of the literatures. Med Oncol. 2010;27:1269-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Saint-Paul MC, Gugenheim J, Hofman P, Arpurt JP, Fabiani P, Michiels JF, Fujita N, Goubeaux B, Loubière R, Delmont J. [Leiomyosarcoma of the liver: a case treated by transplantation]. Gastroenterol Clin Biol. 1993;17:218-222. [PubMed] |
| 14. | Shivathirthan N, Kita J, Iso Y, Hachiya H, Kyunghwa P, Sawada T, Kubota K. Primary hepatic leiomyosarcoma: Case report and literature review. World J Gastrointest Oncol. 2011;3:148-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 15. | Pasquali S, Gronchi A. Neoadjuvant chemotherapy in soft tissue sarcomas: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |