Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.340
Peer-review started: October 13, 2018
First decision: October 18, 2018
Revised: November 11, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: February 6, 2019
Processing time: 109 Days and 9.6 Hours
Adrenal primitive neuroectodermal tumor (PNET) is an extremely rare malignant tumor with poor prognosis and of neural crest origin. Herein, we report a case of adrenal PNET and summarized its clinical and pathological characteristics on the basis of 16 patients reported recently.
A female patient aged 25 years presented with right lumbago for 12 mo, and pre-operative computed tomography showed a huge right adrenal mass. She received tumorectomy, and post-operative pathological examination showed adrenal PNET. After surgery, she underwent adjuvant chemotherapy and was followed up 31 mo after surgery. She received brachytherapy for right paracolic and hepatic metastases. She was alive and followed up for 60 mo. In available studies, only 57.14% (4/7) and 44.44% (4/9) were positive for the expression of neuron-specific enolase and synaptophysin, respectively, although CD99 expression was found in all the patients (100%; 10/10).
It is concluded that adrenal PNET is very rare and highly malignant, and histology is a golden standard in its diagnosis. Surgery and adjuvant therapy is the main treatment.
Core tip: Primitive neuroectodermal tumor (PNET), a member of small round blue cell tumors, is very rare and highly malignant. PNET originating from the adrenal is extremely rare and only a few cases have been reported. The outcome of adrenal PNET is poor, standard treatments have never been established, and the efficacy of adjuvant chemotherapy and radiotherapy after operation is still unclear. Herein, we reported a case of adrenal PNET in a female patient who was alive after 60 mo of follow-up. We hypothesize that early removal and chemotherapy provided the excellent outcome of the patient.
- Citation: Dai J, He HC, Huang X, Sun FK, Zhu Y, Xu DF. Long-term survival of a patient with a large adrenal primitive neuroectodermal tumor: A case report. World J Clin Cases 2019; 7(3): 340-346
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.340
Primitive neuroectodermal tumor (PNET), a member of small round blue cell tumors, is very rare and highly malignant. PNET is usually found in the chest wall, retroperitoneal, and paravertebral areas, but also reported in atypical regions (lung, uterus, testicle, ovary, and pancreas). According to the location, PNET can be classified as central and peripheral PNETs. PNET originating from the adrenal is extremely rare and only a few cases have been reported[1-4]. Currently, the diagnosis of adrenal PNET is mostly dependent on immunohistochemistry, but its specificity is still controversial. Moreover, the outcome of adrenal PNET is poor, standard treatments have never been established, and the efficacy of adjuvant chemotherapy and radiotherapy after operation is still unclear. Herein, we reported a case of adrenal PNET in a female patient, and summarized the clinicopathological characteristics of adrenal PNET on the basis of 16 cases available.
A 25-year-old female presented with right lumbago without palpitation, chest distress, and headache for 12 mo. On physical examination, palpation showed a giant mass without tenderness on the right upper abdomen; signs such as purple striae, moon face, and central obesity were not observed. The patient’s blood pressure was stable (120/80 mmHg).
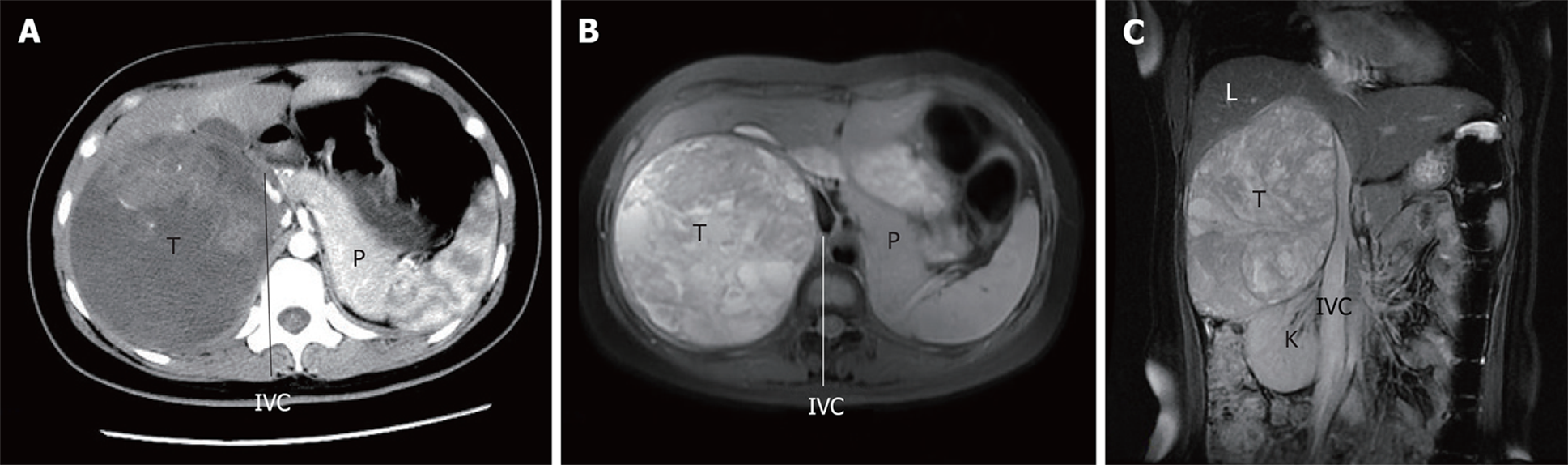
The renal function and metabolism of electrolytes were normal. Tumor markers such as carcinoembryonic antigen (3.14 ng/mL), alpha-fetoprotein (1.54 ng/mL), carbohydrate antigen (CA) 125 (12.8 U/mL), and CA199 (0.7 U/mL) and adrenal endocrinological examinations such as cortisol (7.56 µg/dL), aldosterone (162.7 pg/mL), metanephrine (34 pg/mL), and normetanephrine (69 pg/dL) were normal except for elevated serum β2-microglobulin (3986 ng/mL). The abdominal ultrasonography indicated a spherical solid mass (150 mm in diameter) in the right adrenal with a clear border and little blood flow signal. Abdominal magnetic resonance imaging (MRI) revealed a huge right adrenal tumor (181.5 mm × 115.0 mm × 107.2 mm), with diffused intralesional hemorrhage; the right lobe of the liver, right kidney, and inferior vena cava were significantly compressed (Figure 1).
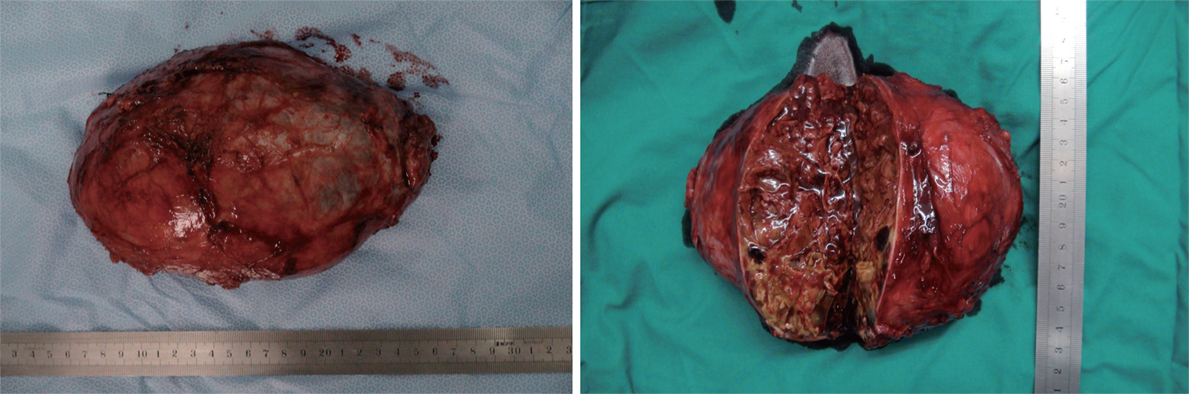
Surgery was performed under general anesthesia via a transabdominal L-shaped incision. The hypervascular tumor was adherent to the liver and tightly connected to the right kidney and inferior vena cava. The tumor was successfully removed and vital signs remained stable during operation. Macroscopically, the tumor was 200 mm × 160 mm in diameter and 1262 g in weight (Figure 2). The estimated intraoperative blood loss was 2000 mL. Severe complications were not observed and the patient was discharged 12 d after surgery.
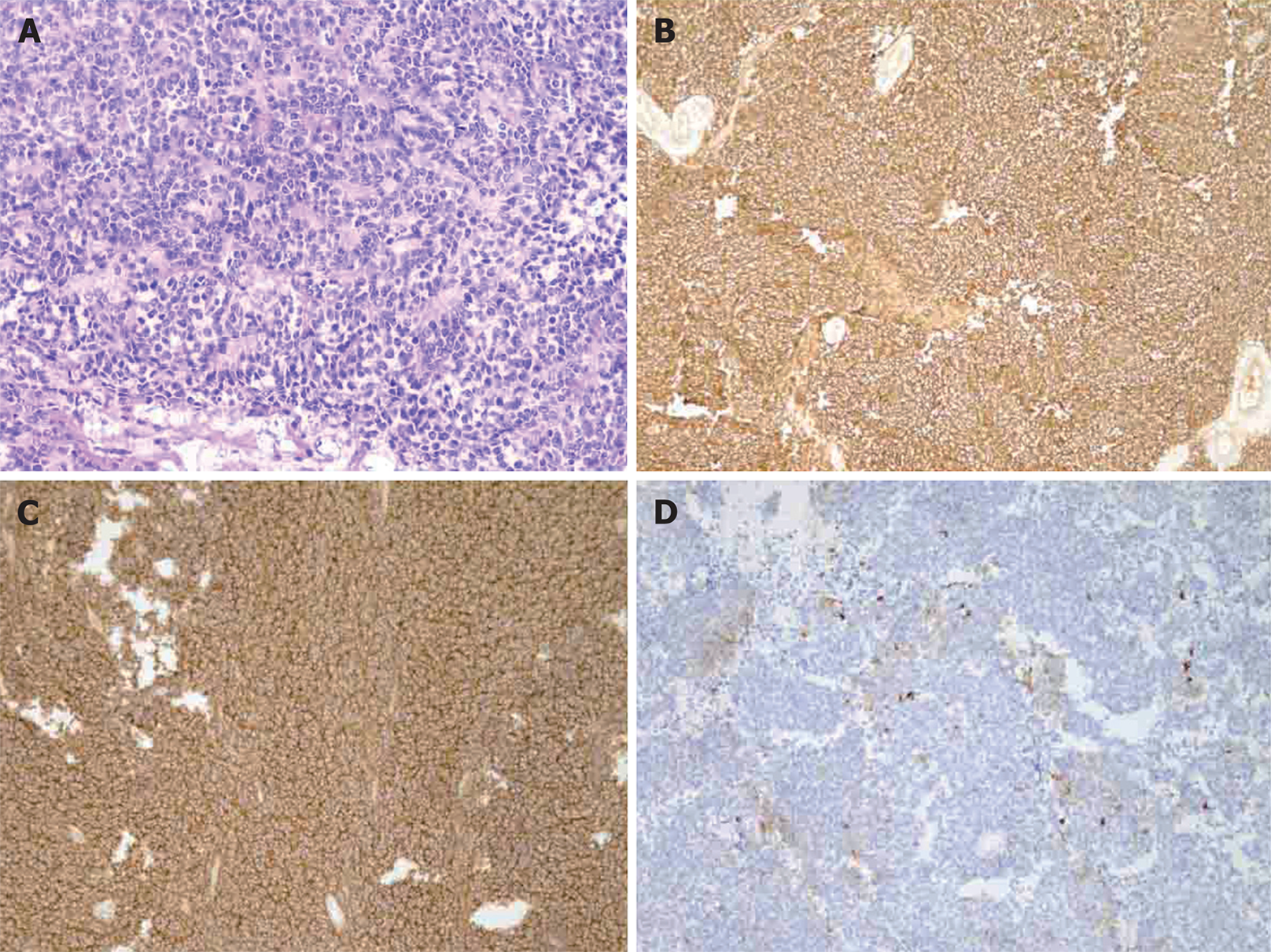
Post-operative pathological evaluation revealed the tumor cells were diffusively distributed and formed Homer-Wright rosettes. Necrosis and collagen fiber hyperplasia were found (Figure 3A). The tumor cells were small and round, had little endoplasm, irregular nuclei, fine and dusty chromatin, and invisible nucleoli. Many cells displayed caryokinesis. Immunohistochemistry showed tumor cells were positive for CD99, vimentin, neuron-specific enolase (NSE), synaptophysin, Bcl-2, MIB-1 (10%) (Figure 3B, 3C, and 3D), while negative for glial fibrillary acidic protein, Nestin, MyoD1, LCA, HMB45, melanin A, inhibin, CHG, CD56, PGM-1, S-100, CD34 and AE1/AE3. Thus, right adrenal PNET was diagnosed on the basis of above pathological findings.
Post-operatively, this patient received liposomal doxorubicin-based chemotherapy. Liposomal doxorubicin was administered intravenously at 60 mg once. The patient received chemotherapy for 6 courses with an interval of 2 wk between courses. Routine laboratory tests were normal. The patient was followed up post-operatively, and recurrence and metastasis were not observed within 30 mo after surgery.
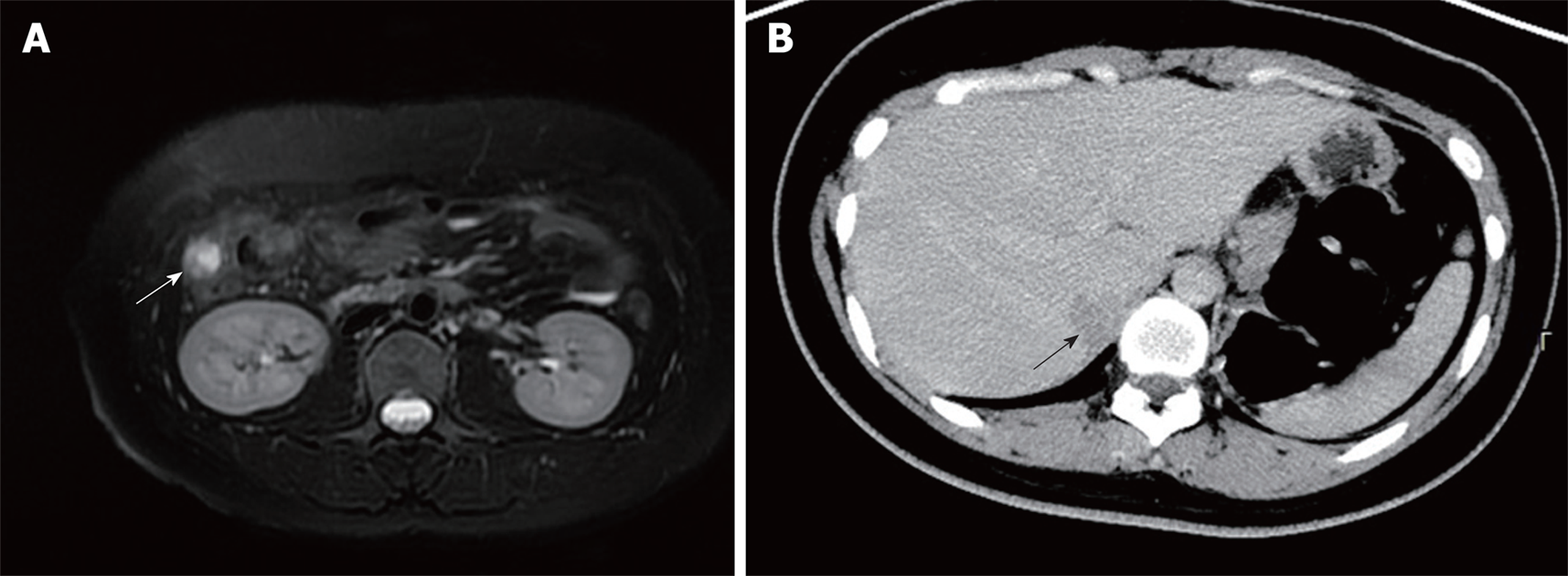
However, at 31 mo after surgery, computed tomography (CT) and MRI revealed a right paracolic nodule (14 mm × 11 mm) (Figure 4A), and biopsy confirmed the metastasis of PNET. The patient received iodine-125 brachytherapy (16965cGy). Five months later, CT showed an irregular low-density shadow in the liver (Figure 4B), and another brachytherapy (22380cGy) was administered. Currently, the patient survived after follow-up for 60 mo and the disease condition was stable.
We reviewed 16 available cases of adrenal PNET and summarized its clinicopathological features (Table 1). Adrenal PNET had a higher incidence in Asia than in other regions (n = 15 in Asian countries vs 1 in the United States). Among the patients reported, females were more likely to develop adrenal PNET (12 females vs 4 males). The mean age of these patients was 32.6 ± 13.0 years ranging from 16 years to 57 years, and there was no evident age predisposition. More cases of adrenal PNET were found in the left adrenal except for one bilateral adrenal PNET. Pain (flank pain, lumbodynia, and abdominal pain) was the major symptom. Endocrinal examination and tumor marker detection were often normal except for high 24-h urine cortisol and positive low-dose dexamethasone suppression test in one case. The tumor size ranged from 3 cm to 21 cm (median: 12 cm). More than half of the patients showed adjacent organ invasion or distant metastases. Thirteen received surgical treatment, half of who underwent further adjuvant therapy. The outcomes were poor in these patients, and the patient in our report received the longest follow-up (60 mo).
| Case | Year of publication | Gender | Age (yr) | Side | Symptom (local pain) | Endocrinal examination and tumor marker | Largest diameter of tumor (cm) | Adjacent invasions or distant metastasis | Surgery | Adjuvant therapy | Outcome (mo) |
| 1 | 2000 | M | 57 | R | - | - | 15 | + | + | N/A | N/A |
| 2 | 2002 | F | 16 | L | N/A | N/A | N/A | N/A | + | N/A | N/A |
| 3 | 2006 | F | 53 | R | N/A | - | 3 | N/A | + | - | 10 |
| 4 | 2006 | F | 25 | L | + | N/A | 15.2 | + | + | N/A | N/A |
| 5 | 2006 | F | 24 | N/A | + | N/A | 8.4 | N/A | N/A | N/A | N/A |
| 6 | 2010 | M | 30 | R | N/A | - | 12 | + | + | Radiotherapy | 8 (death) |
| 7 | 2010 | F | 21 | L | N/A | - | 10 | + | - | - | 6 (death) |
| 8 | 2010 | F | 24 | L | N/A | - | 9 | - | + | Chemotherapy | 13 (recurrence) |
| 9 | 2010 | M | 22 | L | N/A | - | 17 | + | + | Chemotherapy | Recurrence |
| 10 | 2011 | F | 17 | R | + | - | 5 | - | + | Chemotherapy + Radiotherapy | 14 |
| 11 | 2013 | F | 37 | L | + | High 24 h urine cortisol and low-dose dexamethasone test (+) | 12 | + | + | - | N/A |
| 12 | 2014 | F | 37 | L | + | - | 12 | + | + | Chemotherapy | 5 |
| 13 | 2014 | F | 40 | L | - | 14.6 | + | + | Chemotherapy | 8 | |
| 14 | 2016 | F | 25 | R | + | - | 21 | - | + | Chemotherapy + Brachytherapy | 37 |
| 15 | 2016 | M | 45 | B | + | - | 9.6 | + | - | Chemotherapy | N/A |
| 16 | 2016 | F | 48 | L | + | - | 13 | - | + | - | 53 (recurrence) |
PNET is very rare and highly malignant tumor arising from the neural crest that was first recognized by Arthur Purdy Stout in 1918. It shares a lot of features with Ewing’s sarcoma[1-5]. PNET can be categorized into two types: central (cPNET) and peripheral (pPNET). Both cPNETs and pPNETs are aggressive tumors, but they differ in their cell of origin. The cPNETs arise from a precursor cell of the subependymal matrix of the CNS or external granular layer of the cerebellum, pinealocytes, and subependymal cells of the ventricles whereas pPNETs derive from the neural crest located outside the CNS[6]. pPNET mainly occurs in the chest wall (also called Askin tumor), retroperitoneal, and paravertebral areas and other atypical regions like the lung, uterus, testicle, ovary, pancreas, bladder, parotid gland, skin, and subcutaneous tissues. Among them, the adrenal PNET is extremely rare, and only a small number of cases have been reported so far[1-4].
Adrenal PNET patients usually have no specific symptoms/signs on physical examination or special presentations on imaging exams and biochemical tests. Thus, most patients with adrenal PNET are in the advanced stage at the time of diagnosis[4]. The diagnosis of adrenal PNET is dependent on pathological examination and immunohistochemistry. Microscopically, PNET are mainly composed of small and round cells, which are diffusively distributed and form several lobulated structures. The Homer–Wright rosettes or rosettes of other styles can be found[4,7,8]. Immunohistochemistry indicates that tumor cells are positive for P30/P32MIC2 (CD99), which has high specificity and sensibility in the diagnosis of PNET because CD99 expression was found in all the available patients with PNET. In addition, tumor cells may be positive for other proteins related to neural differentiation, including NSE, S-100 protein, neurofilament, synaptophysin, and chromogranin A[4]. The genetic changes in PNET are similar to those in Ewing’s sarcoma, and both are believed to be due to a unique translocation (t (11;22)(q24q12): fusion gene designated EWS/FLI-1)[8] (Table 2).
| Case | CD99 | NSE | Syn | CgA | Vimentin | Fil-1 | Genetic analysis |
| 1 | Strong+ | + | + | Faible+ | N/A | N/A | t(11:22)(q24:q12) |
| 2 | N/A | N/A | N/A N/A | N/A | N/A | N/A | N/A |
| 3 | Strong+ | + | + | N/A | - | N/A | N/A |
| 4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 6 | ++ | - | - | - | - | N/A | N/A |
| 7 | ++ | - | - | - | + | N/A | N/A |
| 8 | + | + | - | - | + | N/A | N/A |
| 9 | + | - | (+/-) | + | - | N/A | N/A |
| 10 | + | N/A | N/A N/A | N/A | N/A | N/A | t(11:22)(q24:q12) |
| 11 | Strong+ | N/A | N/A | N/A | N/A | Faible+ | N/A |
| 12 | + | N/A | - | N/A | + | + | t(11:22)(q24:q12) |
| 13 | + | N/A | + | N/A | + | N/A | N/A |
| 14 | + | + | + | + | + | N/A | N/A |
| 15 | + | N/A | N/A N/A | N/A | N/A | N/A | N/A |
| 16 | + | N/A | N/A N/A | N/A | + | Faible+ | t(11:22)(q24:q12) |
Pre-operative biopsy is helpful for the diagnosis of adrenal PNET. Biopsy can be performed under the guidance of an imaging system, and the transcutaneous fine-needle aspiration (FNA) is the most common method used for biopsy. As a classic method, FNA can achieve only small amount of tissues, which is not beneficial for its differential diagnosis. Furthermore, FNA also increases the risk of tumor spread (0.003%-0.009%)[6]. For the retroperitoneal PNET, endoscopic ultrasonography guided fine needle aspiration (EUS-FNA) may reduce the risk for tumor spread as compared to transcutaneous FNA[9].
Adrenal PNET is very rare, and there are no well-established guidelines or treatment strategies for PNET[10]. Surgical intervention has been accepted as a preferred treatment for adrenal PNET[3]. However, radical resection cannot be guaranteed, and recurrences and metastases are commonly reported in available reports due to the high malignancy. Generally, adjuvant chemotherapy and/or radiotherapy are administered after surgical treatment. Because PNET and Ewing’s sarcoma have the same origin, the chemotherapy with CAV (CTX + ADM + VCR) or IE (Ifosfamide, IFO + Etoposide, ETO) is recommended for the treatment of PNET[4]. According to our experience, although the sensitivity of PNET to chemotherapy is relatively low, adjuvant chemotherapy is still recommended after surgery. Of note, the efficacy of adjuvant radiotherapy is still unclear due to lack of relevant studies. Recently, it has been noted that chemotherapy might be effective only for the first few cycles, and that the tumors develop resistance very quickly[4]. In our case, the brachytherapy with iodine-125 was performed, but its effectiveness should be further confirmed in future studies. Follow-up revealed that these tumors were aggressive. Because of this high rate of recurrence, intensive follow-up with regular CT scans (every 6 mo) has been advocated even after seemingly curative surgery[11].
The reported adrenal PNET is increasing in recent years, which might be ascribed to the augmented recognition of this disease by pathologists, especially in Asia. However, there is no epidemiological study investigating the incidence of adrenal PNET. On the basis of summary of available cases of PNET, tumor cells positive for NSE and synaptophysin were found in only 57.14% (4/7) and 44.44% (4/9) of patients, although being positive for CD99 was noted in all the patients (10/10)[12]. It has been confirmed that CD99 is mainly detectable in PNETs and Ewing’s sarcoma. NSE is a neuronal marker and can differentiate PNETs from other small round cell tumors, such as Ewing’s sarcoma and neuroblastoma[1,12,13]. Thus, there might be false positives in the immunohistochemistry, and the real incidence might be lower.
Because laboratory indicators are normal, and there are no obvious abnormalities in the laboratory reports in other literature, the prognosis of the tumor may not be closely related to the results of laboratory examinations. There are still no indicators predicting prognosis. As for the relatively excellent outcome in our patient versus other cases, we hypothesize that timely chemotherapy and close follow-up after operation are essential. Furthermore, salvage brachytherapy with iodine-125 is an effective method for small metastases detected in time. Besides, tumorectomy and the prevention of tumor damage are also essential.
In conclusion, the patient in this report was initially treated by surgery and adjuvant chemotherapy after surgery. Salvage brachytherapy with iodine-125 was employed when metastasis was confirmed. She was followed up for 60 mo, the longest time in available cases, and a good physical condition was observed currently in this patient. Continuous follow up is needed to assess its prognosis.
Adrenal PNET is very rare and highly malignant. Histology is the gold standard in diagnosis of adrenal PNET. Surgery and adjuvant therapy is the main treatment of adrenal PNET.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Taheri S S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Khong PL, Chan GC, Shek TW, Tam PK, Chan FL. Imaging of peripheral PNET: common and uncommon locations. Clin Radiol. 2002;57:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Komatsu S, Watanabe R, Naito M, Mizusawa T, Obara K, Nishiyama T, Takahashi K. Primitive neuroectodermal tumor of the adrenal gland. Int J Urol. 2006;13:606-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Pirani JF, Woolums CS, Dishop MK, Herman JR. Primitive neuroectodermal tumor of the adrenal gland. J Urol. 2000;163:1855-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Li H. Primitive neuroectodermal tumors of adrenal gland. Jpn J Clin Oncol. 2010;40:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Mani S, Dutta D, De BK. Primitive neuroectodermal tumor of the liver: a case report. Jpn J Clin Oncol. 2010;40:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kumar V, Singh A, Sharma V, Kumar M. Primary intracranial dural-based Ewing sarcoma/peripheral primitive neuroectodermal tumor mimicking a meningioma: A rare tumor with review of literature. Asian J Neurosurg. 2017;12:351-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Sofi AA, Thekdi AD, Nawras A. EUS-FNA for the Diagnosis of Retroperitoneal Primitive Neuroectodermal Tumor. Diagn Ther Endosc. 2011;2011:198029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Yu ZX, Parham DM. Paediatric soft tissue tumours: From histology to molecular diagnosis. Diagn Histopathol. 2009;15:524-530. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Fröstad B, Tani E, Brosjö O, Skoog L, Kogner P. Fine needle aspiration cytology in the diagnosis and management of children and adolescents with Ewing sarcoma and peripheral primitive neuroectodermal tumor. Med Pediatr Oncol. 2002;38:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Tsang YP, Lang BH, Tam SC, Wong KP. Primitive neuroectodermal adrenal gland tumour. Hong Kong Med J. 2014;20:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Marinova L. Retroperitoneal primitive neuroectodermal tumour (pnet). A case report and review of the literature. Rep Pract Oncol Radiother. 2009;14:221-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Tan Y, Zhang H, Ma GL, Xiao EH, Wang XC. Peripheral primitive neuroectodermal tumor: dynamic CT, MRI and clinicopathological characteristics--analysis of 36 cases and review of the literature. Oncotarget. 2014;5:12968-12977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kim MS, Kim B, Park CS, Song SY, Lee EJ, Park NH, Kim HS, Kim SH, Cho KS. Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. AJR Am J Roentgenol. 2006;186:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |