Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.291
Peer-review started: November 19, 2018
First decision: December 9, 2018
Revised: December 12, 2018
Accepted: December 14, 2018
Article in press: December 15, 2018
Published online: February 6, 2019
Processing time: 70 Days and 18.8 Hours
The main clinical treatment for esophageal cancer is surgery. Since traditional open esophageal cancer resection has the disadvantages of large trauma, long recovery period, and high postoperative complication rate, its clinical application is gradually reduced. The current report of minimally invasive Ivor-Lewis esophagectomy (MIILE) is increasing. However, researchers found that patients with MIILE had a higher incidence of early delayed gastric emptying (DGE).
To investigate the influencing factors of postoperative early DGE after MIILE.
A total of 156 patients diagnosed with esophageal cancer at Deyang People's Hospital were enrolled. According to the criteria of DGE, patients were assigned to a DGE group (n = 49) and a control group (n = 107). The differences between the DGE group and the control group were compared. Multivariate logistic regression analysis was used to further determine the influencing factors of postoperative early DGE. The receiver operating characteristic (ROC) curve was used to assess potential factors in predicting postoperative early DGE.
Age, intraoperative blood loss, chest drainage time, portion of anxiety score ≥ 45 points, analgesia pump use, postoperative to enteral nutrition interval, and postoperative fluid volume in the DGE group were higher than those in the control group. Perioperative albumin level in the DGE group was lower than that in the control group (P < 0.05). Age, anxiety score, perioperative albumin level, and postoperative fluid volume were independent factors influencing postoperative early DGE, and the differences were statistically significant (P < 0.05). The ROC curve analysis revealed that the area under the curve (AUC) for anxiety score was 0.720. The optimum cut-off value was 39, and the sensitivity and specificity were 80.37% and 65.31%, respectively. The AUC for postoperative fluid volume were 0.774. The optimal cut-off value was 1191.86 mL, and the sensitivity and specificity were 65.3% and 77.6%, respectively. The AUC for perioperative albumin level was 0.758. The optimum cut-off value was 26.75 g/L, and the sensitivity and specificity were 97.2% and 46.9%, respectively.
Advanced age, postoperative anxiety, perioperative albumin level, and postoperative fluid volume can increase the incidence of postoperative early DGE.
Core tip: Esophageal cancer is one of the most common gastrointestinal cancers. Minimally invasive esophageal cancer resection has achieved good results in the early clinical application of esophageal cancer and some advanced esophageal cancer. However, studies have shown that patients with Ivor-Lewis type esophageal cancer resection have a higher incidence of early gastric emptying disorder. This study explored the factors that influence the early onset of delayed gastric emptying after minimally invasive Ivor-Lewis esophageal cancer resection. The results show that advanced age, postoperative anxiety, perioperative hypoalbuminemia, and postoperative hyper-remediation can increase postoperative gastric emptying disorder. The incidence of obstacles affects the quality of life after surgery.
- Citation: Huang L, Wu JQ, Han B, Wen Z, Chen PR, Sun XK, Guo XD, Zhao CM. Influencing factors of postoperative early delayed gastric emptying after minimally invasive Ivor-Lewis esophagectomy. World J Clin Cases 2019; 7(3): 291-299
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/291.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.291
Esophageal cancer is very common and its incidence is very high in China[1,2]. Surgery is the main approach to treat esophageal cancer[3]. Traditional open esophageal cancer resection is gradually reduced due to its large trauma, long recovery period, and high postoperative complication rate. After nearly 20 years of development, minimally invasive esophageal cancer resection has achieved good results in early and advanced stage esophageal cancer[4,5]. It has the advantages of small wounds, low postoperative infection rate, and short hospital stay[6,7]. In recent years, minimally invasive Ivor-Lewis esophagectomy (MIILE) has been increasingly reported. MIILE reduces the cardiopulmonary stimulation, intraoperative blood loss, and postoperative extrathoracic catheter indwelling time because it avoids intraoperative thoracotomy[8,9]. Meanwhile, due to the use of thoracic laparoscopy in the process of lymph node dissection, the exposure of related nerves is reduced, and the postoperative complications are reduced[10,11]. However, patients treated by MIILE have a high incidence of early delayed gastric emptying (DGE)[12]. Postoperative DGE not only prolongs hospital stay and recovery time, but also increases the incidence of aspiration pneumonia[13,14]. The present study investigated the influencing factors of postoperative early DGE after MIILE in order to take targeted measures to avoid postoperative DGE.
A total of 156 patients diagnosed with esophageal cancer by fiberoptic endoscopy at our hospital from January 2015 to October 2017 were enrolled. Among them, 112 patients were male and 44 were female. The average age was 56.64 ± 9.96 years old. The inclusion criteria were as follows: (1) esophageal cancer located in the middle or inferior esophagus; (2) preoperative clinical stage T:1-3, N:0-1, M:0; and (3) patients received MIILE. The exclusion criteria were as follows: (1) patients underwent neoadjuvant therapy or palliative surgery; (2) patients suffering from serious infectious diseases such as tuberculosis and/or cardiopulmonary infarction and other serious organic diseases; (3) patients with preoperative chronic gastrointestinal disease; and (4) patients with incomplete medical records. According to the diagnostic criteria for DGE, patients with postoperative early DGE (within one week) were assigned to a DGE group (n = 49), while patients without DGE were assigned to a control group (n = 107). The present study was approved by the Ethics Committee of our hospital, and each patient signed an informed consent form.
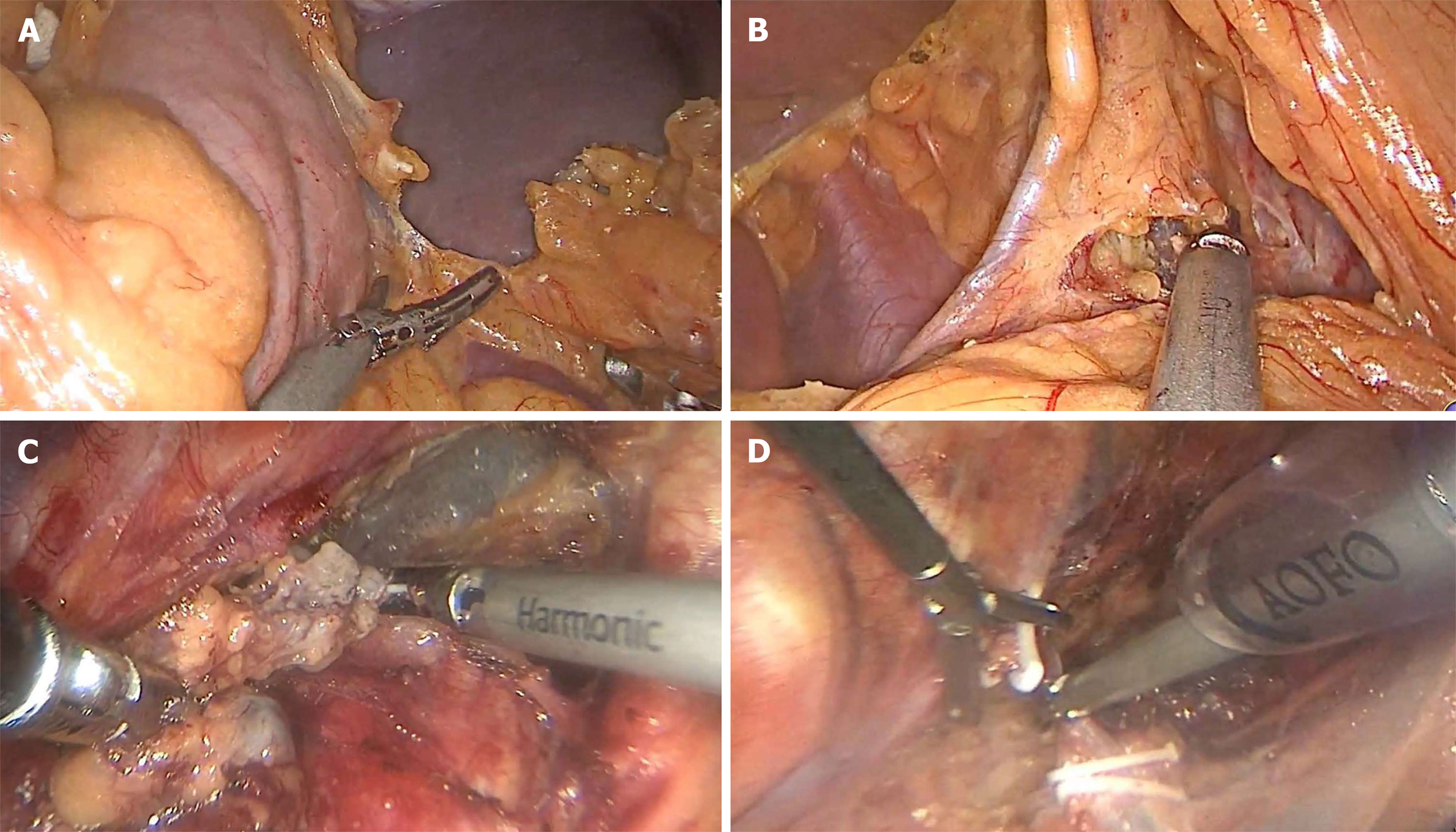
Laparoscopic surgery: Patients were placed in the supine position, and artificial pneumoperitoneum was established. A total of five ports were utilized. After the ultrasonic scalpel was placed, the omental tissue of the large curved side of the stomach was separated along the lateral side of the vascular arch of the gastric omentum (Figure 1A), and then the small curved omentum of the stomach was separated (Figure 1B). The stomach was separated up to 1-2 cm above the diaphragmatic esophageal hiatus, down to the beginning of the gastric retinal vascular arch. A linear cutting stapler was used to make a partial tubular stomach along the large curvature of the stomach. Subsequently, the stomach was put into the abdominal cavity in the original position.
Thoracoscopic surgery: Patients was placed in the left lateral position. A total of three ports were utilized. The lymph nodes near the right recurrent laryngeal nerve were cleared (Figure 1C). The odd vein bow was clipped with four HOME locks and the odd vein bow was severed (Figure 1D). The esophagus was separated and the surrounding lymph nodes were cleared. Finally, the esophageal tumor was removed and the intrathoracic lymph nodes were cleared.
Baseline data such as age, gender, bad habits (smoking and alcohol abuse), and underlying diseases (diabetes and hypertension) were collected. The perioperative albumin level was measured using an automatic biochemical analyzer. The operative time, intraoperative blood loss, chest drainage time, postoperative fluid volume, interval between surgery and enteral nutrition, use of analgesia pump, and postoperative complications (DGE, postoperative infection, anastomotic leakage, secondary surgery due to bleeding, and arrhythmia) were recorded. A self-evaluation scale was used to evaluate the anxiety of patients after MIILE.
Statistical analyses were performed using SPSS20.0. Measurement data are expressed as the mean ± SD. Data between the DGE group and control group were compared by the t-test and chi-square test. Multivariate logistic regression analysis was performed to further determine the influencing factors of early DGE. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the value of potential factors predicting early DGE after MIILE. P < 0.05 was considered statistically significant.
The general information of patients with esophageal cancer after MIILE revealed that the proportion of males was greater than females, and the majority of patients were under 60 years old. Most patients were in an anxiety state after MIILE. The incidence of DGE was higher compared to that of other postoperative complications (Table 1).
| Item | No. of cases (n = 156) |
| Gender | |
| Male | 112 (71.79) |
| Female | 44 (28.21) |
| Use of an analgesic pump | 61 (39.10) |
| Bad habits | |
| Smoking | 67 (42.95) |
| Alcohol abuse | 34 (21.79) |
| Underlying disease | |
| Diabetes | 34 (21.79) |
| Hypertension | 21 (13.46) |
| Age (yr) | |
| ≥ 60 | 32 (20.51) |
| < 60 | 124 (79.49) |
| Anxiety score | |
| ≥ 45 points | 111 (71.15) |
| < 45 points | 45 (28.85) |
| Postoperative complication | |
| DGE | 49 (31.41) |
| Postoperative infection | 8 (5.13) |
| Anastomotic leakage | 11 (7.05) |
| Secondary surgery due to bleeding | 7 (4.49) |
| Arrhythmia | 9 (5.77) |
Comparison between DGE group and the control group showed that the differences of age, intraoperative blood loss, chest drainage time, anxiety score, analgesia pump use, perioperative albumin level, postoperative to enteral nutrition interval and postoperative fluid volume were statistically significant (P < 0.05). Among them, age, the intraoperative blood loss, chest drainage time, portion of anxiety score ≥ 45 points, portion of analgesia pump use, postoperative to enteral nutrition interval, and postoperative fluid volume in the DGE group were higher than those in the control group. The perioperative albumin level in the DGE group was lower than that in the control group. The differences of gender, surgery time, and diabetes were not statistically significant (P > 0.05, Table 2).
| Item | DGE group (n = 49) | Control group (n = 107) | t or χ2 | P |
| Age (yr) | 59.15 ± 9.85 | 50.98 ± 10.33 | 4.651 | 0.000 |
| Gender (male/female) | 35/14 | 77/30 | 0.005 | 0.945 |
| Intraoperative blood loss (mL) | 196.53 ± 70.91 | 176.26 ± 50.17 | 2.046 | 0.042 |
| Chest drainage time (d) | 9.97 ± 4.06 | 8.01 ± 6.32 | 1.989 | 0.048 |
| Anxiety score (≥ 45 points/< 45 points) | 25/24 | 20/87 | 17.114 | 0.000 |
| Analgesic pump use (yes/no) | 29/20 | 32/75 | 12.098 | 0.001 |
| Perioperative albumin level (g/L) | 27.43 ± 8.56 | 34.12 ± 7.43 | -4.972 | 0.000 |
| Interval from surgery to enteral nutrition (d) | 3.42 ± 1.32 | 1.87 ± 0.96 | 8.281 | 0.000 |
| Operative time (min) | 276.15 ± 60.43 | 247.68 ± 57.31 | 1.438 | 0.152 |
| Postoperative fluid volume (mL) | 2034.56 ± 260.43 | 1544.96 ± 246.17 | 11.322 | 0.000 |
| Diabetes (yes/no) | 9/40 | 25/82 | 0.492 | 0.482 |
The multivariate logistic regression analysis revealed that chest drainage time, intraoperative blood loss, analgesia pump use, and interval between surgery and enteral nutrition were not independent factors influencing early postoperative DGE (P > 0.05). Age, anxiety score, perioperative albumin level, and postoperative fluid volume were independent factors influencing early postoperative DGE, and the differences were statistically significant (P < 0.05). Furthermore, according to the odds ratio value, the order of the indicators affecting the degree of early postoperative DEG was: anxiety score, postoperative fluid volume, age, and perioperative albumin level (Table 3).
| B | SE | Wald | Odd ratio | 95%CI | P | ||
| Lower limit | Upper limit | ||||||
| Age | 0.301 | 0.134 | 4.224 | 1.351 | 1.039 | 1.757 | 0.029 |
| Anxiety score | 0.702 | 0.197 | 4.678 | 2.017 | 1.371 | 2.968 | 0.033 |
| Analgesia pump use | 0.573 | 0.446 | 2.965 | 1.774 | 0.740 | 4.252 | 0.067 |
| Perioperative albumin level (g/L) | -0.186 | 0.115 | 4.115 | 0.830 | 0.663 | 1.040 | 0.041 |
| Chest drainage time (d) | 0.508 | 0.411 | 1.792 | 1.662 | 0.743 | 3.719 | 0.128 |
| Intraoperative blood loss (mL) | 0.116 | 0.428 | 1.003 | 1.123 | 0.485 | 2.598 | 0.259 |
| Interval from surgery to enteral nutrition (d) | 0.490 | 0.344 | 2.522 | 1.632 | 0.832 | 3.203 | 0.078 |
| Postoperative fluid volume (mL) | 0.328 | 0.128 | 4.612 | 1.388 | 1.080 | 1.784 | 0.034 |
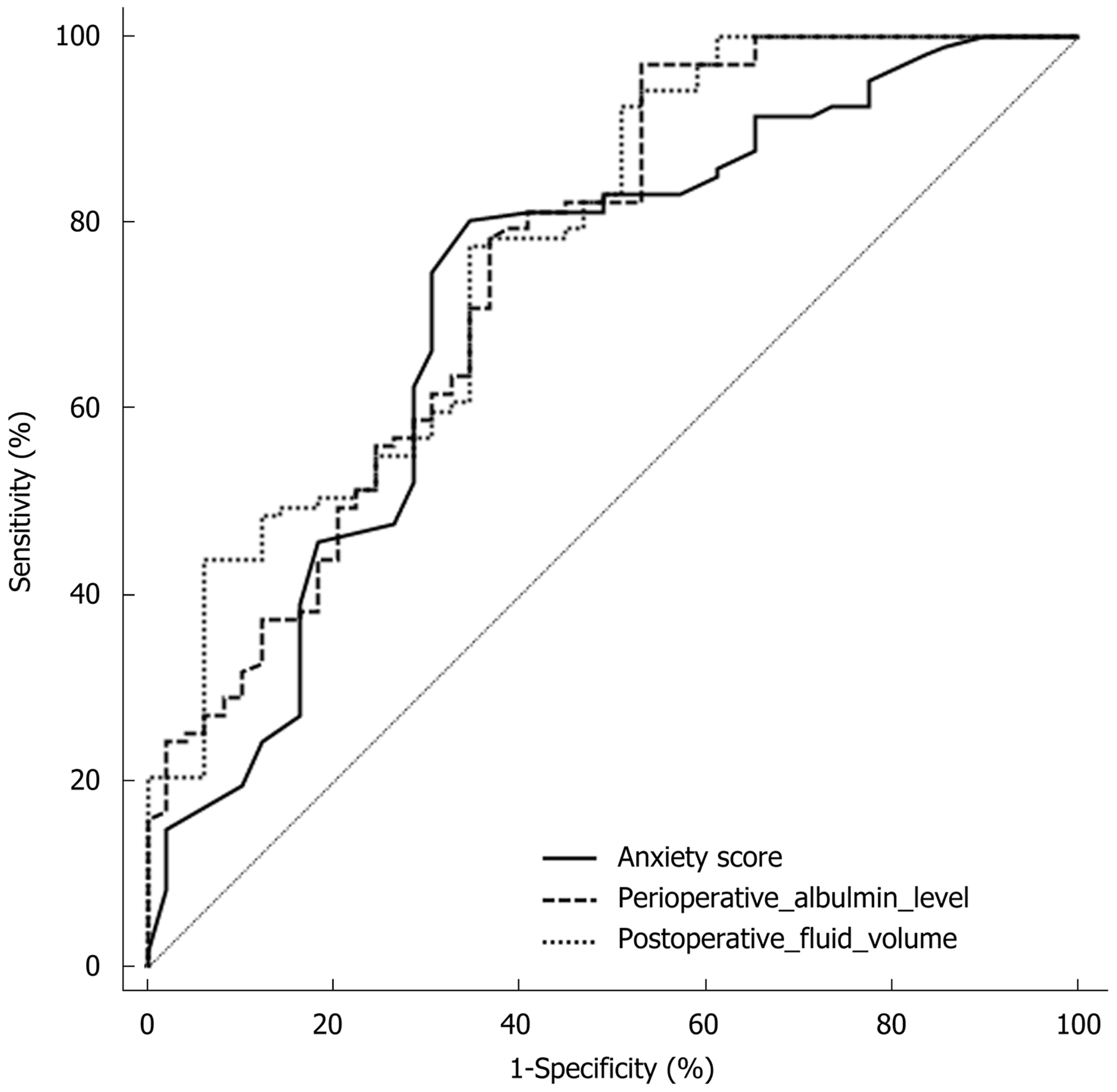
The ROC curve analysis revealed that the areas under the curves (AUCs) for anxiety score, postoperative fluid volume, and perioperative albumin level were 0.720, 0.774 and 0.758, respectively. The optimal cut-off value of anxiety score was 39, and the sensitivity and specificity were 80.37% and 65.31%, respectively. For postoperative fluid volume, the optimal cut-off value was 1191.86 mL, and the sensitivity and specificity were 65.3% and 77.6%, respectively. For perioperative albumin level, the optimum cut-off value was 26.75 g/L, and the sensitivity and specificity were 97.2% and 46.9%, respectively (Figure 2).
Esophageal cancer has a high incidence in China and is the second largest malignant tumor of the digestive tract after gastric cancer[15-18]. Currently, the surgical procedures for esophageal cancer are constantly improving and innovating[19-22]. MIILE has gradually become the main surgical procedure for advanced esophageal cancer in clinical treatment. However, in addition to the difficulty of surgery operation, MIILE also has a high incidence of postoperative early DGE. Postoperative early DGE is mainly a functional emptying disorder, which not only affects the enthusiasm of postoperative rehabilitation, but also increases the risk of other complications such as anastomotic leakage. Hence, it is important to identify the reason of postoperative early DGE and assess early intervention.
The pathogenesis of DGE remains unclear. Studies have found that preoperative and postoperative mental stress, and changes in hormones induced by trauma and surgical stress are the main mechanisms that lead to postoperative DGE[23-26]. In the present study, the postoperative mental state of the patients was analyzed by anxiety score. The anxiety score of the DGE group was significantly higher than that of the control group. Furthermore, the logistic regression analysis revealed that anxiety score was one of the independent factors influencing postoperative early DEG, suggesting that postoperative anxiety is likely to be the reason of DGE. The ROC curve analysis revealed that the AUC of anxiety score was 0.72. It indicated that the anxiety score could predict the occurrence of postoperative early DGE to some extent. Therefore, effective postoperative psychological counseling is helpful to prevent the occurrence of DGE. Epidemiological surveys show that esophageal cancer is common in middle-aged and elderly people, while elderly patients themselves are suffering from gastrointestinal dysfunction accompanied by aging, and have reduced tolerance and resilience to surgery. Therefore, postoperative observation of elderly patients should be more detailed.
A study conducted by Cheong et al[27] revealed that postoperative hypoalbuminemia can easily lead to anastomotic edema, cause local motor dysfunction, and thereby affect gastrointestinal function recovery. By comparing perioperative albumin levels between the DGE group and control group, it was found that albumin level was significantly lower in the DGE group. Furthermore, logistic regression analysis revealed that high perioperative albumin level was a protective factor for postoperative DGE. Therefore, timely enteral nutrition to improve albumin level is important for preventing DGE. Furthermore, the present study found that postoperative fluid volume also affected the occurrence of DGE, and postoperative rehydration was a risk factor for this complication. The ROC curve analysis revealed that the AUC of postoperative fluid volume was higher than that of perioperative albumin level. It indicates that the prediction ability of postoperative fluid volume is stronger. Moreover, the multivariate analysis revealed that the effect of postoperative fluid volume was higher. These imply that excessive fluid rehydration during enteral nutrition may promote the occurrence of DGE. Therefore, caution should be given for rehydration after surgery, and this should be based on the diagnosis of patient's vital signs.
Studies also revealed that the interval between surgery and enteral nutrition promotes DGE[28-30]. However, in the present study, the multivariate analysis result revealed that the interval between surgery and enteral nutrition had no significant effect on the occurrence of DGE. It implies that further exploration such as expanding the sample size may be needed.
In summary, the present study found that advanced age, postoperative anxiety, perioperative albumin level, and postoperative fluid volume can increase the incidence of postoperative early DGE. Evaluating the anxiety score, perioperative albumin level, and postoperative fluid volume can predict the occurrence of postoperative DGE. These findings help improve postoperative care to prevent postoperative complications.
Esophageal cancer is the second largest digestive tract malignancy after gastric cancer. The surgical procedures for esophageal cancer are constantly improving and innovating. Due to its minimally invasive and precise features, minimally invasive Ivor-Lewis esophagectomy (MIILE) significantly reduces the incidence of complications in patients undergoing surgery. It is superior to traditional open surgery and has gradually become the main surgical procedure for advanced esophageal cancer in clinical treatment.
MIILE also has the disadvantages that need to be overcome. In addition to the disadvantages of high surgical difficulty, MIILE has a relatively high incidence of complications such as postoperative early delayed gastric emptying (DGE). Postoperative DGE is a functional emptying disorder. It will not only affect the enthusiasm of postoperative rehabilitation, but also increase the risk of other complications such as anastomotic leakage. It may lead to patients undergoing secondary surgery. Therefore, it is the current top priority to find out the precise cause of early DGE and provide early intervention.
The present study aimed to compare the differences between patients with postoperative early DGE and those without, in order to explore the influencing factors of postoperative early DGE after MIILE.
A total of 156 patients with esophageal cancer diagnosed at our hospital were recruited. All patients were treated by MIILE. According to the DGE diagnostic criteria, patients were divided into a DGE group if DGE was found in the early postoperative period (within one week). While patients were divided into a control group if DGE was not found in the early postoperative period. Various data of the DGE group and the control group were recorded and compared, and single factor analysis was performed. Multivariate logistic regression analysis was performed to further determine the extent of these factors’ effect on early postoperative DGE. The ROC curve was used to analyze the accuracy of these factors in predicting the early postoperative DGE.
Multivariate logistic regression analysis showed that age, anxiety score, perioperative albumin level, and postoperative fluid volume were the independent factors influencing postoperative early DGE. The receiver operating characteristic curve analysis revealed that the area under the curve (AUC) for anxiety score was 0.720, and the sensitivity and specificity were 80.37% and 65.31%, respectively. The AUC, sensitivity, and specificity for postoperative fluid volume were 0.774, 65.3%, and 77.6%, respectively. Regarding perioperative albumin level, they were 0.758, 97.2%, and 46.9%, respectively. However, studies have shown that the time interval from surgery to enteral nutrition also contributes to the early postoperative DGE, but this study found that the time interval from surgery to enteral nutrition had no significant effect on postoperative early DGE. It implied that our research may have a limited sample size and further research is necessary.
The present study found that advanced age, postoperative anxiety, perioperative albumin level, and postoperative fluid volume were the independent factors influencing postoperative early DGE. These indicators are expected to be used to predict the occurrence of postoperative early DGE.
The findings of this study will help to further guide the care and treatment of postoperative patients, thereby preventing the occurrence of early postoperative DGE, and improving the quality of postoperative life of patients with esophageal cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Apisarnthanarax S, Currie IS, Rukavina M S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Song H
| 1. | Tan HZ, Lin WJ, Huang JQ, Dai M, Fu JH, Huang QH, Chen WM, Xu YL, Ye TT, Lin ZY, Lin XS, Cai JX, Dong YH, Luo HY, Chen SH, Huang YL, Yang J, Lin AX, Yuan XQ, Chen SY, Wang KS, Zhuang CY, Wang SC, Lin LL, Zou XF, Song ZH, Fang XH, Chen T, Zhang JH, Li KQ, Chen LH, Lin XP, Lin JM, Lin JN, Lin PL, Chen JT, Lin KM, Hong XC, Wang LD, Xu LY, Li EM, Zhang JJ. Updated incidence rates and risk factors of esophageal cancer in Nan'ao Island, a coastal high-risk area in southern China. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Soler M, Bosetti C, Franceschi S, Negri E, Zambon P, Talamini R, Conti E, La Vecchia C. Fiber intake and the risk of oral, pharyngeal and esophageal cancer. Int J Cancer. 2001;91:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Taioli E, Wolf AS, Camacho-Rivera M, Kaufman A, Lee DS, Bhora F, Flores RM. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol. 2016;113:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Honda M, Daiko H, Kinoshita T, Fujita T, Shibasaki H, Nishida T. Minimally invasive resection of synchronous thoracic esophageal and gastric carcinomas followed by reconstruction: a case report. Surg Case Rep. 2015;1:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Zhao L, Ge J, Li W, Luo Y, Chai Y. Minimally invasive esophageal resection and intrathoracic anastomosis for lower thoracic esophageal cancer with single position. J Thorac Dis. 2015;7:1486-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Guo W, Ma L, Zhang Y, Ma X, Yang S, Zhu X, Zhang J, Zhang Y, Xiang J, Li H. Totally minimally invasive Ivor-Lewis esophagectomy with single-utility incision video-assisted thoracoscopic surgery for treatment of mid-lower esophageal cancer. Dis Esophagus. 2016;29:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Wang J, Xu MQ, Xie MR, Mei XY. Minimally Invasive Ivor-Lewis Esophagectomy (MIILE): A Single-Center Experience. Indian J Surg. 2017;79:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Li B, Xiang J, Zhang Y, Li H, Zhang J, Sun Y, Hu H, Miao L, Ma L, Luo X, Chen S, Ye T, Zhang Y, Zhang Y, Chen H. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2015;150:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Akkerman RD, Haverkamp L, van Hillegersberg R, Ruurda JP. Surgical techniques to prevent delayed gastric emptying after esophagectomy with gastric interposition: a systematic review. Ann Thorac Surg. 2014;98:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Chung KH, Lee SH, Park JM, Lee JM, Shin CM, Ahn SH, Park do J, Kim HH, Ryu JK, Kim YT. Partially covered self-expandable metallic stent for postoperative benign strictures associated with laparoscopy-assisted gastrectomy. Gastric Cancer. 2016;19:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 12. | Zhou RM, Li Y, Wang N, Huang X, Cao SR. Phospholipase C ε-1 gene polymorphisms and prognosis of esophageal cancer patients from a high-incidence region in northern China. Mol Clin Oncol. 2018;8:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ghadirian P. Thermal irritation and esophageal cancer in northern Iran. Cancer. 1987;60:1909-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Nobis S, Morin A, Achamrah N, Belmonte L, Legrand R, Chan P, do Rego JL, Vaudry D, Gourcerol G, Déchelotte P, Goichon A, Coëffier M. Delayed gastric emptying and altered antrum protein metabolism during activity-based anorexia. Neurogastroenterol Motil. 2018;30:e13305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Marciani L, Pritchard SE, Hellier-Woods C, Costigan C, Hoad CL, Gowland PA, Spiller RC. Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: novel MRI insights. Eur J Clin Nutr. 2013;67:754-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Hanna MM, Gadde R, Tamariz L, Allen CJ, Meizoso JP, Sleeman D, Livingstone AS, Yakoub D. Erratum to: Delayed Gastric Emptying After Pancreaticoduodenectomy: Is Subtotal Stomach Preserving Better or Pylorus Preserving? J Gastrointest Surg. 2015;19:1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Limongelli P, Docimo L, Malleo G, Salvia R. Delayed Gastric Emptying after Pancreaticoduodenectomy: The Hunt Continues. J Am Coll Surg. 2018;226:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wee JO, Morse CR. Minimally invasive Ivor Lewis esophagectomy. J Thorac Cardiovasc Surg. 2012;144:S60-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2010;89:S2159-S2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, Turrado Rodriguez VT, van der Peet DL, Cuesta MA. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc. 2012;26:1795-1802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Liu Q, Chen J, Wen J, Yang H, Hu Y, Luo K, Tan Z, Fu J. Comparison of right- and left-approach esophagectomy for elderly patients with operable thoracic esophageal squamous cell carcinoma: a propensity matched study. J Thorac Dis. 2017;9:1883-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Li X, Wang W, Zhou Y, Yang D, Wu J, Zhang B, Wu Z, Tang J. Efficacy comparison of transcervical video-assisted mediastinoscopic lymphadenectomy combined with left transthoracic esophagectomy versus right transthoracic esophagectomy for esophageal cancer treatment. World J Surg Oncol. 2018;16:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, Ruurda JP, van Hillegersberg R, Soeters PB, Luyer MD. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr. 2015;34:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Farnes I, Johnson E, Johannessen HO. Management of gastric conduit retention following hybrid and minimally invasive esophagectomy for esophageal cancer: Two retrospective case series. Int J Surg Case Rep. 2017;41:505-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | van Workum F, Berkelmans GH, Klarenbeek BR, Nieuwenhuijzen GAP, Luyer MDP, Rosman C. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis. 2017;9:S826-S833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Tang H, Zheng H, Tan L, Shen Y, Wang H, Lin M, Wang Q. Neoadjuvant chemoradiotherapy followed by minimally invasive esophagectomy: is it a superior approach for locally advanced resectable esophageal squamous cell carcinoma? J Thorac Dis. 2018;10:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Cheong E. How minimally invasive esophagectomy was implemented at the Norfolk and Norwich University Hospital. J Thorac Dis. 2017;9:S879-S885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Bencini L, Moraldi L, Bartolini I, Coratti A. Esophageal surgery in minimally invasive era. World J Gastrointest Surg. 2016;8:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Fritz S, Feilhauer K, Schaudt A, Killguss H, Esianu E, Hennig R, Köninger J. Pylorus drainage procedures in thoracoabdominal esophagectomy - a single-center experience and review of the literature. BMC Surg. 2018;18:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Guo W, Yang S, Li H. Esophagectomy with gastric conduit reconstruction for benign disease: extreme but important. Ann Transl Med. 2018;6:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |