Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4391
Peer-review started: September 29, 2019
First decision: November 19, 2019
Revised: November 27, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: December 26, 2019
Processing time: 83 Days and 2.8 Hours
Castleman disease, also known as giant lymph node hyperplasia, was first reported in 1956. It is a rare benign proliferative pathological change of the lymph nodes.
The patient, a 33-year-old woman, had epigastric distension for half a year. Examinations were performed in a local hospital. Computed tomography scan showed round soft tissue nodules, about 5.45 cm in diameter, in the hepatic-gastric space. Endoscopic ultrasound and endoscopic ultrasound guided fine needle aspiration was performed on the patient. Rapid on-site evaluation, hematoxylin eosin staining and histopathology of the puncture smear was performed. According to the Diff-Quik staining and hematoxylin eosin staining results of preoperative endoscopic ultrasound guided fine needle aspiration puncture smears as well as the immunohistochemistry results, Castleman disease was highly suspected. A sufficient preoperative evaluation was made, and a precise surgical plan was developed. Postoperative pathology confirmed Castleman disease.
Endoscopic ultrasound guided fine needle aspiration can extract internal tissues of the tumor for histological and cytological examinations and provide accurate diagnosis as much as possible. Therefore, a sufficient preoperative evaluation can be made, and a precise surgical plan can be developed.
Core tip: Castleman disease, also known as giant lymph node hyperplasia, is a rare benign proliferative pathological change of the lymph nodes. This study reports a case of Castleman disease in a 33-year-old woman who was referred to our hospital for an enhanced computed tomography examination after prior examinations in a local hospital. Although enhanced computed tomography and magnetic resonance imaging scans have some characteristic manifestations, they still lack specificity. A gold standard for the pathological diagnosis is still required. Endoscopic ultrasound guided fine needle aspiration, which can extract internal tissues of the tumor for histological and cytological examinations, can provide an accurate diagnosis.
- Citation: Xu XY, Liu XQ, Du HW, Liu JH. Castleman disease in the hepatic-gastric space: A case report. World J Clin Cases 2019; 7(24): 4391-4397
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4391.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4391
Castleman disease (CD), also known as giant lymph node hyperplasia, was first reported by Castleman et al[1] in 1956. It is a rare benign proliferative pathological change of the lymph nodes, which maintains the basic morphology of lymph nodes with envelope formation and clear borders with adjacent structures[2]. The cause is unknown and may be related to a chronic inflammatory reaction, medications, autoimmune abnormalities or other factors[3]. CD can occur in any part of the body containing lymph nodes and is most common in the chest (accounting for about 70%), followed by the neck (14%), abdomen (12%) and axillae (4%)[4-6].
The main pathological change observed in CD is the hyperplasia of small blood vessel-like tissue and lymphoid tissue. It is pathologically classified into the hyaline vascular type, plasma cell type and mixed hyaline vascular and plasma cell type. The hyaline vascular type is the most common, accounting for about 80% to 90% with the pathological manifestation being intrafollicular or interfollicular lymphoid tissue hyperplasia. The follicular center contains many hyalinized capillaries, and the lymphoid tissue contains eosinophils and immunoblasts. The lesions are mostly focal and usually do not manifest any clinical symptoms. The main treatment adopted is surgery. The incidence of plasma cell type is relatively low, accounting for about 10%. Pathologically, it mostly presents as large follicles and interfollicular plasma cell infiltration, vascular proliferation being uncommon and a common diffuse distribution with about 50% of the cases exhibiting clinical symptoms, including superficial lymph node enlargement, anemia, weight loss and hepatosplenomegaly. It has a malignant tendency and requires comprehensive treatment. The mixed type has the characteristics of both the hyaline vascular type and plasma cell type[7,8].
The patient, a 33-year-old woman, had epigastric distension for half a year.
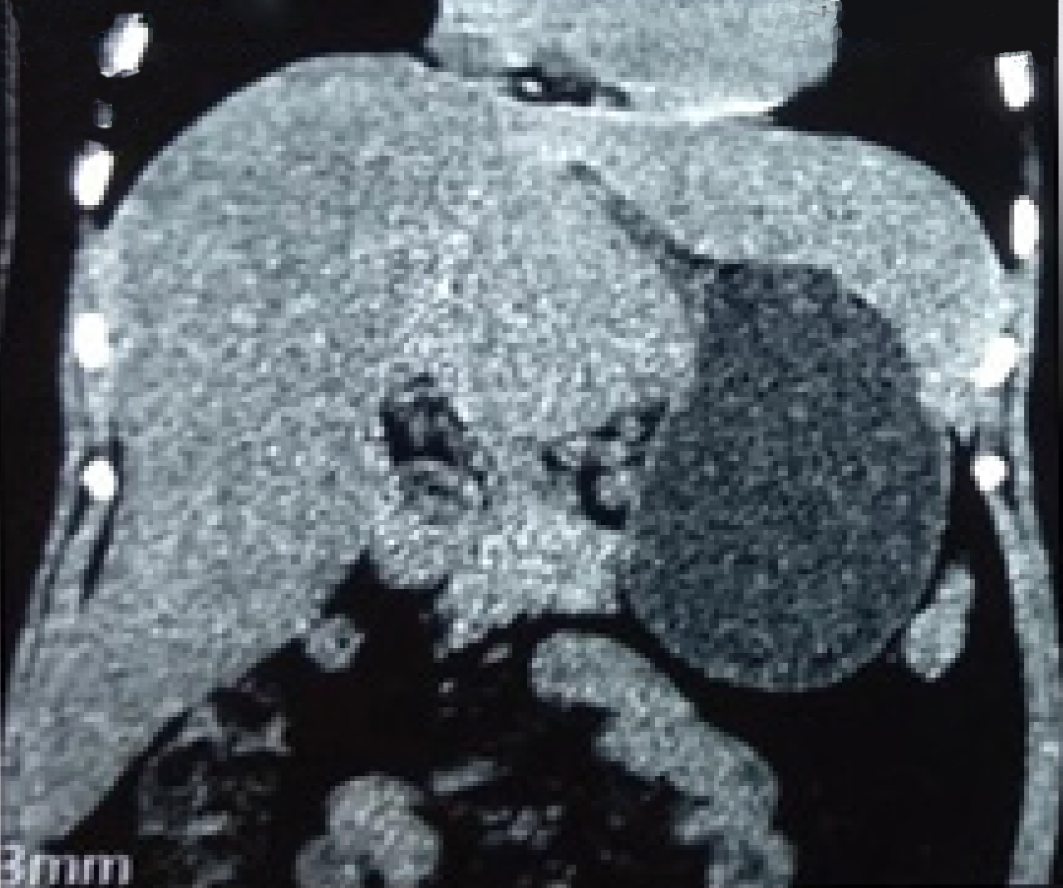
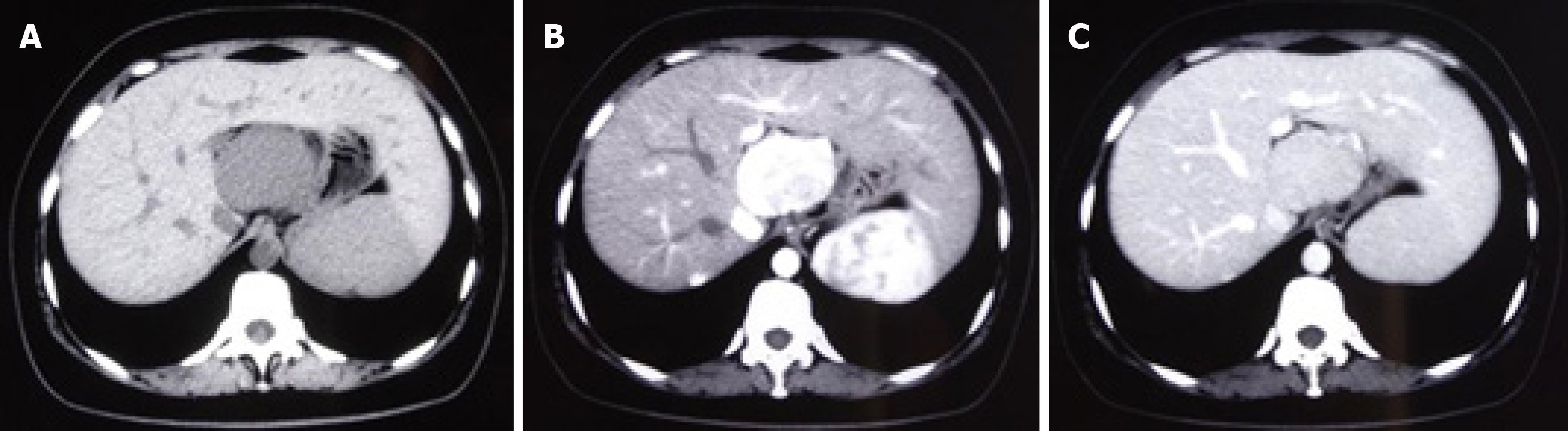
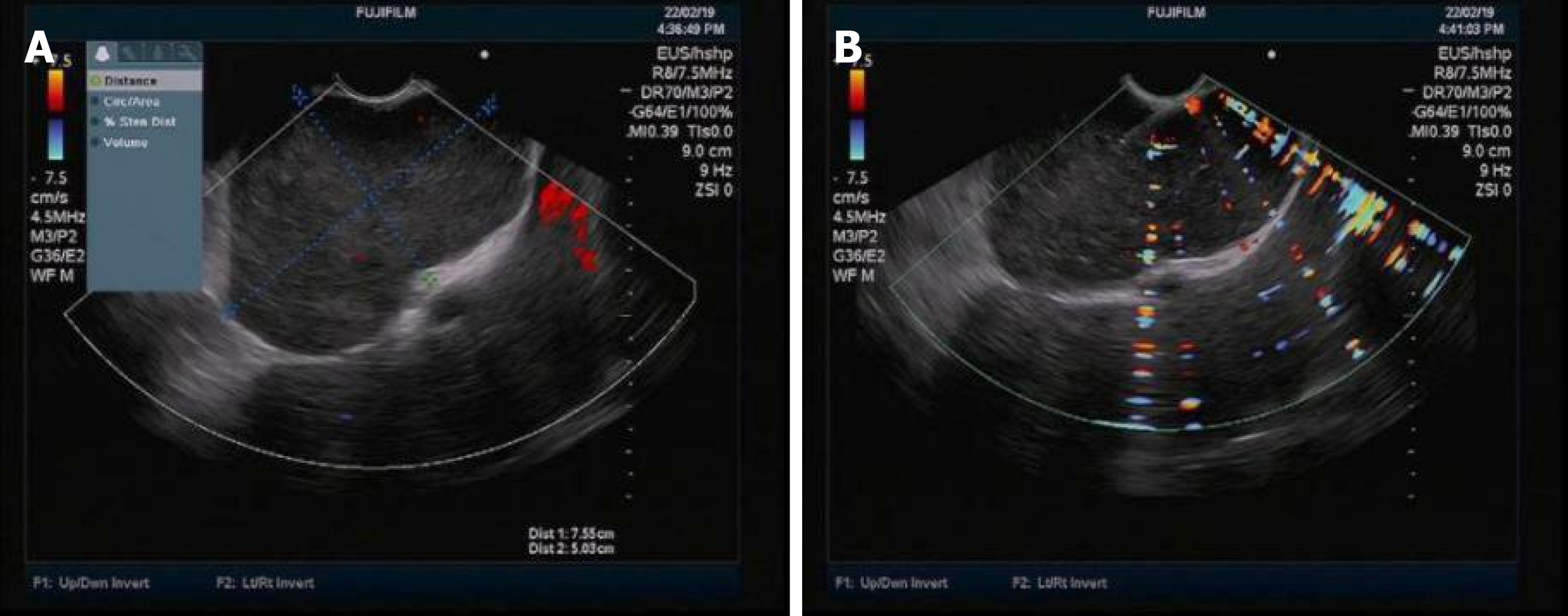
Examinations were performed in a local hospital. Gastroscopy revealed erythema exudative gastritis, and computed tomography (CT) scan showed round soft tissue nodules, about 5.45 cm in diameter, in the hepatic-gastric space (Figure 1). The patient was referred to our hospital for an enhanced CT examination. The CT values for the plain scan, arterial phase and venous phase were 42.9 HU, 109.6 HU and 82.4 HU, respectively (Figure 2). The disease was suspected to be CD according to enhanced CT scan findings. Endoscopic ultrasound (EUS) was performed on the patient using a linear array EUS EG530UT with the host Fujin Su-8000. The EUS findings demonstrated low echoes in the hepatic-gastric space, and scattered star-like hyperechoes were observed inside. The color Doppler indicated that there was no blood flow signal. In order to clarify the nature of the lesion, EUS guided fine needle aspiration (EUS-FNA), (puncture needle COOK-ECHO 19G) was used (Figure 3).
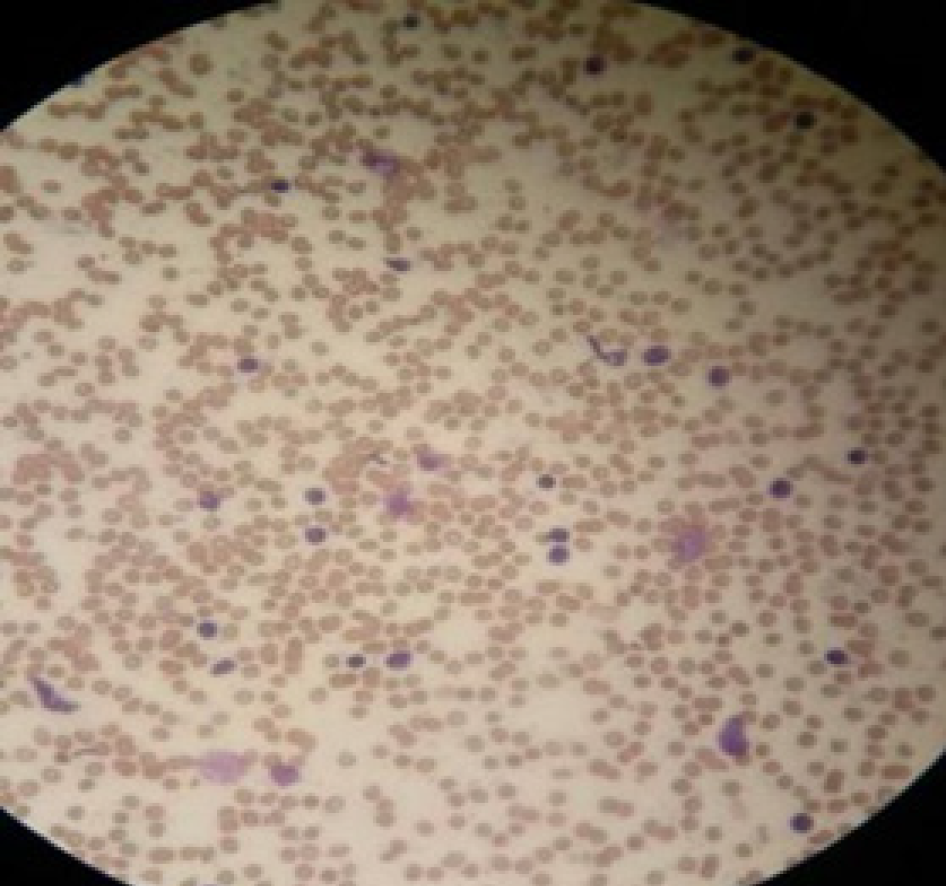
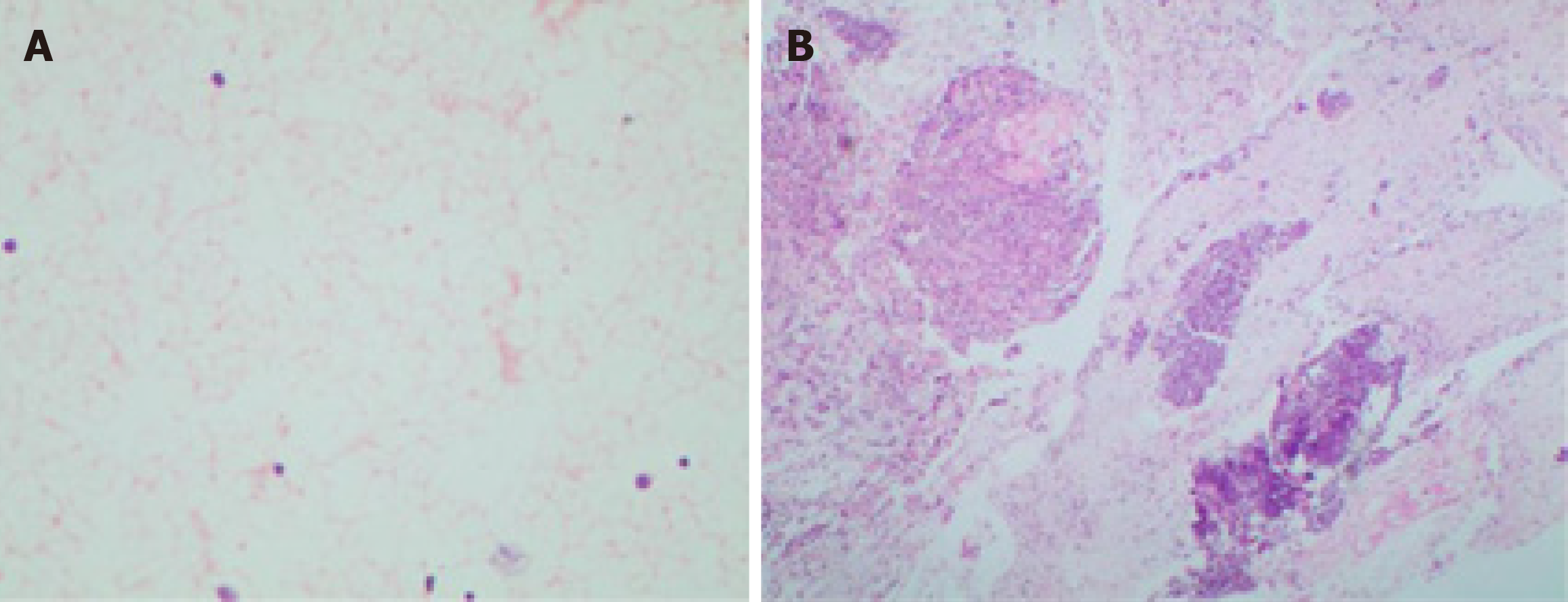
The puncture smear was subjected to Diff-Quik staining. Rapid on-site evaluation (ROSE) was performed, which showed a large number of scattered lymphocytes with normal morphology under a background of red blood cells (Figure 4). Hematoxylin eosin staining of the puncture smear showed scattered lymphocytes with normal morphology. Histopathology showed a large number of lymphocytes with normal morphology with B cells predominating. Immunohistochemistry results were as follows: CD117 (-), CD20 (+), CD3 (scattered +), CD30 (-), CD68 (scattered +), desmin (+), Ki-67 (+ < 30%), S-100 (-), SMA (+) and vimentin (+) (Figure 5). The possibility of CD was high.
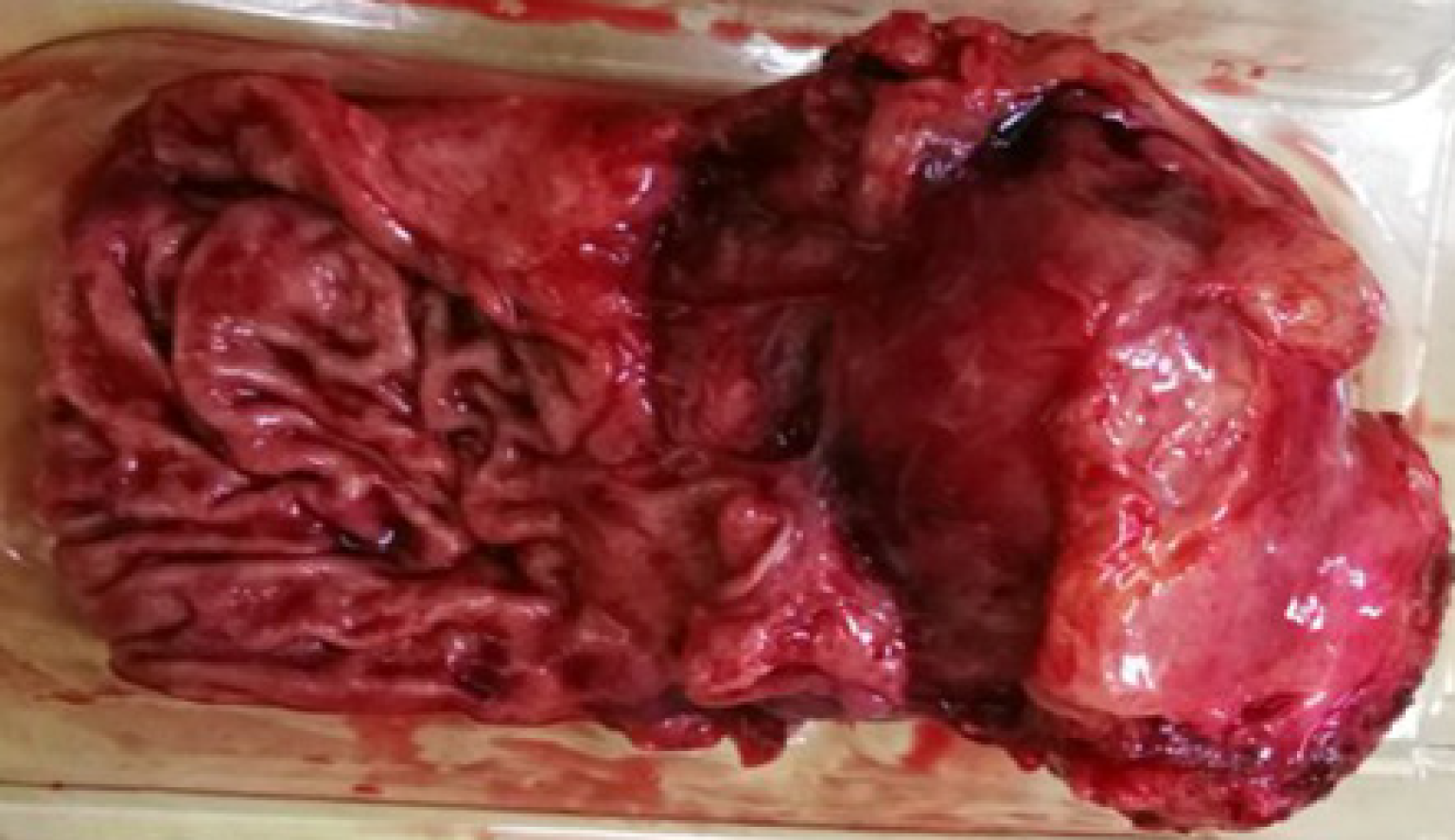
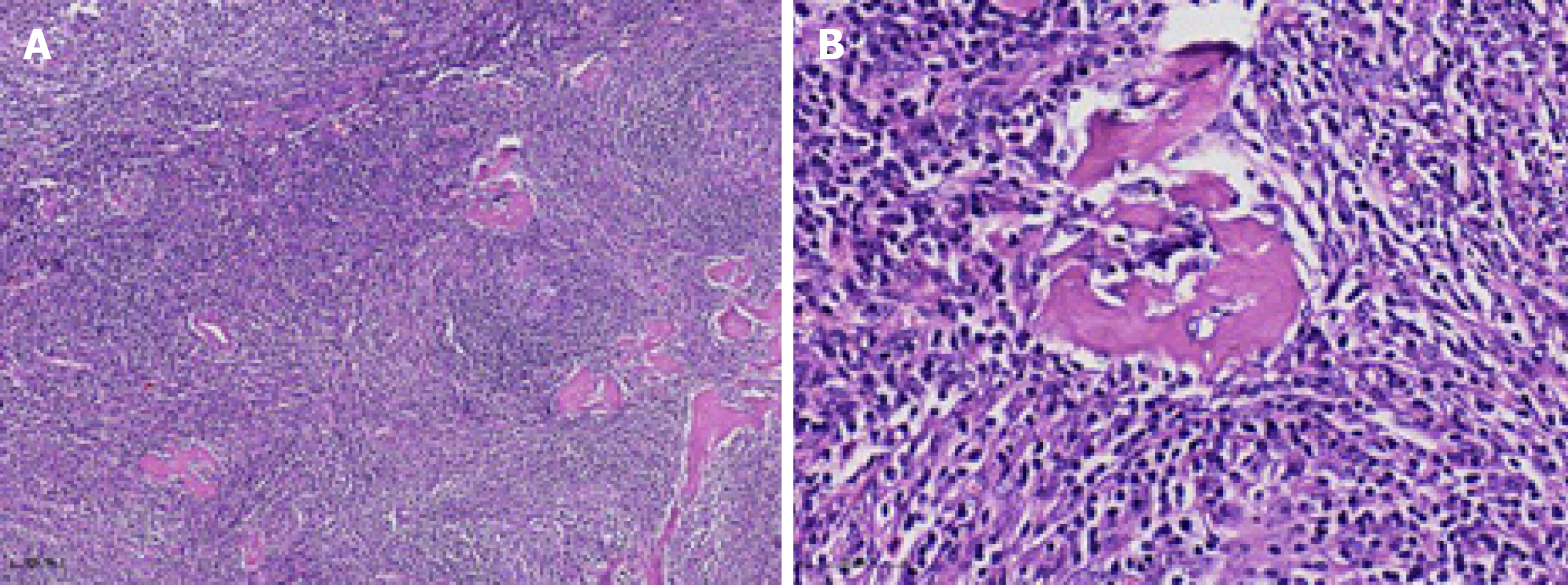
After sufficient preoperative evaluation, resection of the hepatic-gastric space mass and proximal stomach was performed. Surgical resection pathology revealed a 9 cm × 5 cm × 4.5 cm mass outside the serosa of the gastric wall, and the cut surface was gray and crisp (Figure 6). Based on immunohistochemistry, in situ hybridization and genetic testing, postoperative diagnosis was atypical hyperplasia of lymphoid tissue, consistent with CD (hyaline vascular type, Figure 7).
The general condition of the patient was acceptable after surgery. A review CT scan will be performed once a year.
Abdominal CD is a rare pathological change that is often misdiagnosed as other tumors before surgery. Enhanced CT scan usually shows a solid mass with clear boundaries and uniform density. Progressive enhancement and continuous enhancement in enhanced CT scans have certain guiding significance for CD diagnosis[9]. In magnetic resonance imaging scans, signals on T1W1 are mostly uniform and low, and signals on T2W1 are relatively variable with equal, slightly higher or higher signal, indicating that magnetic resonance imaging scans can show fiber and collagen components in the center of the lesion better than CT scans[10]. Although enhanced CT and magnetic resonance imaging scans have some characteristic manifestations, they still lack specificity. A gold standard for the pathological diagnosis is still required. EUS-FNA can extract internal tissues of the tumor for histological and cytological examinations and provide accurate diagnosis as much as possible before surgery, thereby playing a crucial role in the development of tumor treatment schemes[11-16].
Localized mass type CD should be distinguished from the following diseases in terms of pathology. First, lymphoma should be excluded. Abdominal localized tumors are mostly non-Hodgkin lymphoma in which the lymph node structure is almost completely destroyed and dysplasia of lymphocytes can be observed. The diagnosis should be confirmed using immunohistochemistry, in situ hybridization and genetic testing. Second, gastrointestinal stromal tumor should be excluded. Immunohistochemistry will show positive results for CD34, CD117 and DOG1. Third, schwannomas should be excluded. Immunohistochemistry will show 100% positive rate for S-100, which is diffuse strong positive. Fourth, ectopic pheochromocytoma should be excluded. Immunohistochemistry will show positive results for chromogranin, neuronspecific enolase and synaptophysin. Finally, intra-abdominal invasive fibromatosis should be excluded. Immunohistochemistry will show a positive result for β-catenin.
In this case, Diff-Quik staining was used for ROSE. Zhao et al[17] believed that generally samples should be obtained using 22G-27G needles for cell morphology analysis. In this case, the lesion was located in the hepatic-gastric space. The EUS was placed on the side of lesser gastric curvature, inferior to the cardia, to scan the lesion and guide the puncture. The EUS was not bent, the needle passage was straight and the lesion had no obvious blood flow signal. In order to obtain more tissue and reduce the number of punctures, COOK-ECHO 19G was used for puncturing. Regarding the value of ROSE for specimens obtained by EUS-FNA, scholars had different opinions in recent years. Koul et al[18] believed that EUS-FNA with ROSE significantly improves the sufficiency of the sample and is associated with high diagnostic accuracy. In FNA guided by EUS for pancreatic masses, the use of ROSE can improve the diagnostic rate.
In this case, according to the Diff-Quik staining and hematoxylin eosin staining results of preoperative EUS-FNA puncture smears as well as the immunohistochemistry results, CD was highly suspected. A sufficient preoperative evaluation was made and a precise surgical plan was developed. Postoperative pathology confirmed CD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Campanale M, Sogabe I S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Castleman B, Lverson L, Menendez VP. Localizde mediastinal lymph node hyperplasia resembling thymoma. Cancer. 1956;9:822-830. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Mitsos S, Stamatopoulos A, Patrini D, George RS, Lawrence DR, Panagiotopoulos N. The role of surgical resection in Unicentric Castleman's disease: a systematic review. Adv Respir Med. 2018;86:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Soumerai JD, Sohani AR, Abramson JS. Diagnosis and management of Castleman disease. Cancer Control. 2014;21:266-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Kligerman SJ, Auerbach A, Franks TJ, Galvin JR. Castleman Disease of the Thorax: Clinical, Radiologic, and Pathologic Correlation: From the Radiologic Pathology Archives. Radiographics. 2016;36:1309-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Jiang XH, Song HM, Liu QY, Cao Y, Li GH, Zhang WD. Castleman disease of the neck: CT and MR imaging findings. Eur J Radiol. 2014;83:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Dong A, Dong H, Zuo C. Castleman disease of the porta hepatis mimicking exophytic hepatocellular carcinoma on CT, MRI, and FDG PET/CT. Clin Nucl Med. 2014;39:e69-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Luo JM, Li S, Huang H, Cao J, Xu K, Bi YL, Feng RE, Huang C, Qin YZ, Xu ZJ, Xiao Y. Clinical spectrum of intrathoracic Castleman disease: a retrospective analysis of 48 cases in a single Chinese hospital. BMC Pulm Med. 2015;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Wang C, Zhou J, Ma Z. CT and MRI findings and pathological features of retroperitoneal focal Castleman disease. Zhonghua Zhongliu Zazhi. 2014;36:193-197. |
| 9. | Chen WP, Chen L, Wang H. Enhanced CT findings and clinicopathological features of Castleman's disease in different parts of the body. J Med Imaging. 2019;29:445-447. |
| 10. | Yang ZF, Zhong JP, Zhang SJ. CT and MRI findings of abdominal giant lymph node hyperplasia. Zhongguo Yixue Jisuanji Chengxiang Zazhi. 2018;24:490-494. |
| 11. | Barresi L, Tacelli M, Tarantino I, Cipolletta F, Granata A, Traina M. Improving the yield of EUS-guided histology. Endosc Ultrasound. 2018;7:301-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Vilmann P, Bhutani MS. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc Ultrasound. 2018;7:141-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Dietrich CF, Bibby E, Jenssen C, Saftoiu A, Iglesias-Garcia J, Havre RF. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Gibiino G, Larghi A. EUS-guided fine-needle biopsy for histological examination: Is it time to change our sampling technique? Endosc Ultrasound. 2018;7:71-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sahai AV. EUS is trending! Endosc Ultrasound. 2018;7:353-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sun S, Wang C, Wang S. Remember, interventional EUS is performed using an elevator-containing scope as well. Endosc Ultrasound. 2018;7:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zhao CQ, Pantanowitz L, Yang M. Fine needle aspiration cytopathology. Beijing: Beijing Science and Technology Publishing Co., Ltd., 2014. |
| 18. | Koul A, Baxi AC, Shang R, Meng X, Li L, Keilin SA, Willingham FF, Cai Q. The efficacy of rapid on-site evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Gastroenterol Rep (Oxf). 2018;6:45-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |