Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4314
Peer-review started: September 15, 2019
First decision: October 14, 2019
Revised: November 11, 2019
Accepted: November 20, 2019
Article in press: November 20, 2019
Published online: December 26, 2019
Processing time: 100 Days and 21 Hours
This article introduces the surgical method and early experience in performing totally laparoscopic radical gastrectomy with transrectal specimen extraction for gastric cancer, and we evaluate the short-term effects and feasibility of this new procedure for gastric cancer in a 64-year-old male patient. This approach may provide new possibilities for gastric natural orifice specimen extraction (NOSE) surgery. In addition, we believe that this new procedure may further relieve pain, speed up recovery, and cause minimal physiological and psychological impact.
We performed NOSE gastrectomy in a male patient. Tumor resection, digestive tract reconstruction, and lymph node dissection were performed totally laparoscopically; the specimen was extracted from the natural orifice of the rectum-anus. This procedure reduced damage to the abdominal wall and decreased postoperative pain. We successfully performed radical gastrectomy without conversion and complications. Total operative time and blood loss were 176 min and 50 mL, respectively. The patient resumed normal activities of daily living on day 1 without pain, and passed flatus within 48 h. Postoperative hospital stay was 10 d. The number of resected lymph nodes was 0/43. During the follow-up, no stricture or anastomotic leakage was detected. Three months postoperatively, colonoscopy showed full recovery of the rectum without stricture or scar contracture. Computed tomography and laboratory test results showed no signs of tumor recurrence. There was no visible scar on the abdominal wall.
It is safe and reliable to perform totally laparoscopic radical gastrectomy with transrectal specimen extraction for distal gastric cancer patients.
Core tip: We successfully performed transrectal specimen extraction gastrectomy in a male patient without conversion and complications. This procedure significantly reduced damage to the abdominal wall and decreased postoperative pain. There was no visible scar on the abdominal wall. Total operative time and blood loss were 176 min and 50 mL, respectively. The patient resumed normal activities on day 1 without pain, and passed flatus within 48 h. Postoperative hospital stay was 10 d. The number of resected lymph nodes was 0/43. During the follow-up, no complications were detected, and computed tomography and laboratory test results showed no signs of tumor recurrence.
- Citation: Sun P, Wang XS, Liu Q, Luan YS, Tian YT. Natural orifice specimen extraction with laparoscopic radical gastrectomy for distal gastric cancer: A case report. World J Clin Cases 2019; 7(24): 4314-4320
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4314.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4314
Gastric cancer is a high-incidence tumor, and surgery is the common treatment[1]. Scarring and pain were traditionally thought to be the inevitable outcome of stomach cancer surgery for the past century. However, with the development of technology and the concept of minimally invasive surgery, the natural orifice specimen extraction (NOSE) surgery concept has been widely recognized because it has good application in the field of colorectal cancer, and it could be gradually applied in the treatment of stomach, urological, and gynecological tumors[2,3].
The use of transvaginal NOSE gastrectomy has been reported frequently[4,5], but cases of transrectal specimen extraction are rare. Transrectal specimen extraction surgery is one kind of NOSE surgery that is completely performed laparoscopically, and the specimen is removed from the natural lumen without an extra abdominal incision. In this case, the specimen was removed from the anus through the anterior wall of the rectum. This approach is particularly suitable for gastric cancer patients with small tumors. Details of laparoscopic radical gastrectomy with transrectal specimen extraction in the male patient are described as follows.
A 64-year-old male patient was admitted to the hospital because of upper abdominal pain lasting for 1 year.
The patient had upper abdominal distension and discomfort for more than 1 year, accompanied by poor appetite. After eating, the patient felt upper abdominal fullness and painful discomfort, which could be relieved slightly by resting or taking acid-inhibiting drugs orally. The symptoms were slightly aggravated 3 mo ago, accompanied by weight loss.
The patient denied any history of hypertension, diabetes, and coronary heart disease; hepatitis, tuberculosis, typhoid fever, malaria, and other infectious diseases; and major trauma, surgery, and drug or food allergies. Vaccination history is unknown. He had a history of smoking and drinking for more than 40 years.
No significant family history.
The patient’s weight was 56 kg and his height was 170 cm, with a calculated body mass index of 19.3 kg/cm2. His Karnofsky Performance Scale score was 100. No enlarged superficial lymph nodes were palpable. His abdomen was flat, and there was no gastrointestinal or peristaltic waves. There was mild tenderness in the upper abdomen, but there was no rebound pain. The shifting dullness was negative, and the frequency of borborygmus sounds was 4 times/min.
Tumor marker levels [carcinoembryonic antigen, cancer antigen (CA)-199, and CA125] and routine laboratory blood test results were within normal ranges.
Computer tomography examination showed thickening of the gastric angle. Gastroscopic examination was performed, and an ulcer at the gastric horn was found. Then pathological biopsy findings confirmed that the ulcer was a gastric adenocarcinoma (clinical stage: cT1bN0M0).
Distal gastric adenocarcinoma.
Laparoscopic radical gastrectomy for distal gastric cancer was performed, and the surgical procedures are described as follows.
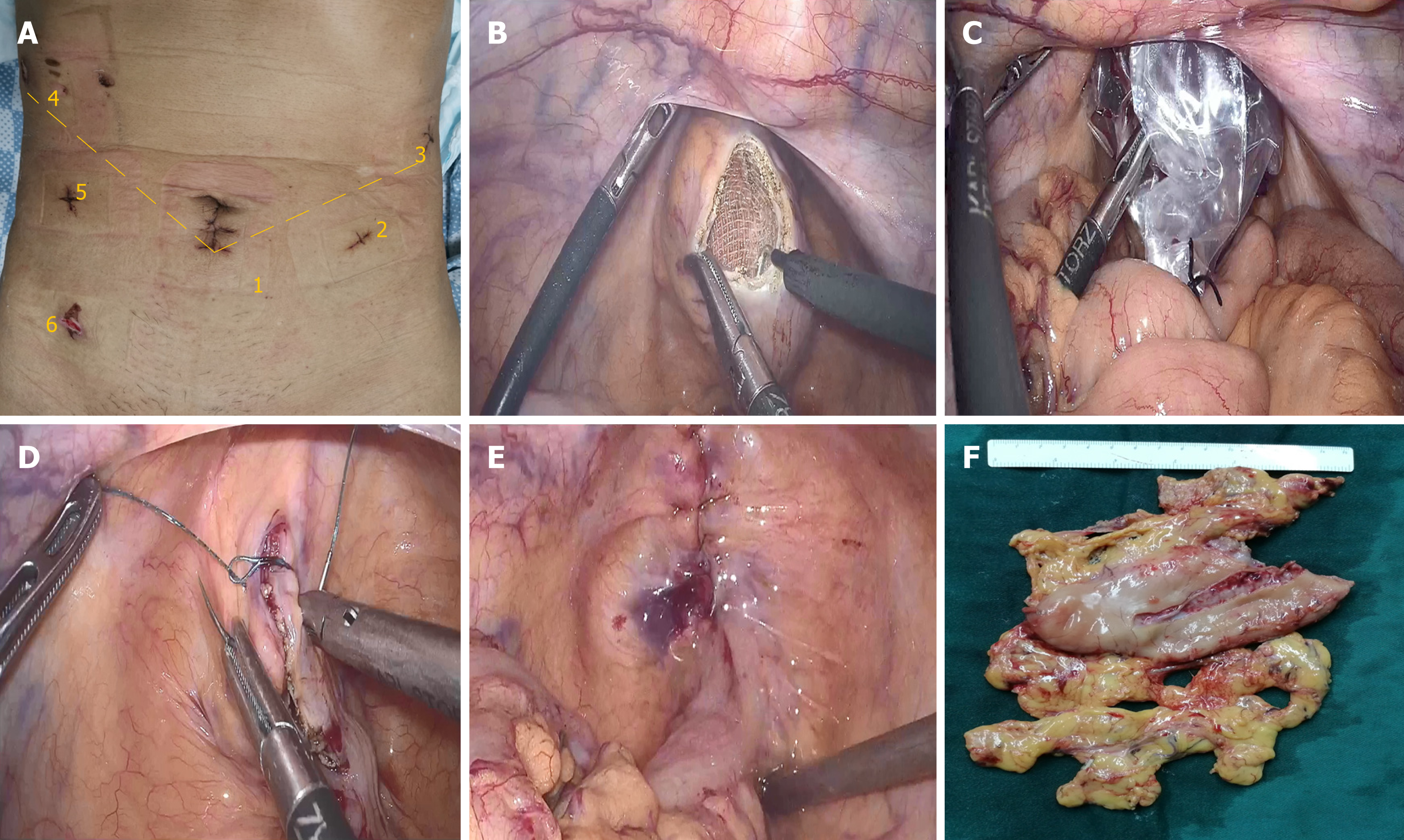
After general anesthesia was induced, the patient was placed in the functional lithotomy position. After routine disinfection was performed, the V glyph 5-port method was used to set the trocars (Figure 1A). A 12-mm trocar was inserted into the abdomen through the subumbilical area as the observation port (Figure 1A-1), the surgeon’s main operating port was located in the patient’s hypochondriac region (Figure 1A-3), and the intersection of the left mid-clavicular line and umbilical horizontal line was the location of the auxiliary operating port (Figure 1A-2). The operating ports of the assistant were located in the midline of the right clavicle and the right hypochondriac region (Figure 1A-4 and 1A-5).
First, the abdominal cavity was inspected for tumor seeding or metastasis. No ascites was detected, no metastasis was observed on the liver surface, and no implant nodule was observed in the omentum, abdominal cavity, or pelvic cavity.
Then the gastric colon ligament was incised, and the anterior transverse mesenteric and pancreatic capsules were exfoliated. At the first portion of the duodenum, the right gastroepiploic vein was double-ligated with hemolock clips and divided. After resecting the sixth group of lymph nodes along the pylorus, the duodenal bulb was dissociated from the pancreatic head.
The nonvascular area of the lesser omentum was incised with an ultrasonic scalpel. Then the serosal layer of the hepatoduodenal ligament on the right side was sharply cut, and the twelfth group of lymph nodes was dissected. Next, the right gastric artery was ligated with hemolock clips and divided from the root, and the fifth group of lymph nodes was dissected.
The stomach was turned up. The truncus coeliacus, common hepatic artery, proper hepatic artery, and splenic artery were all isolated in the upper margin of the pancreas, respectively. The seventh, eighth, and ninth groups of lymph nodes were dissected, and the left gastric artery was ligated and divided from the root.
Subsequently, we opened the posterior leaf of the lesser omentum to the esophageal hiatus, the right ligament tissue of the cardia was divided with an ultrasonic scalpel, and the first group of lymph nodes was dissected. Along the direction of the lesser gastric curvature to the pylorus, the adipose lymphoid tissue was divided with the ultrasonic scalpel, and the lymph nodes of the third group were dissected until the scheduled pre-cut point on the lesser gastric curvature.
The splenic colonic ligament was divided, and the left gastric omentum blood vessel was ligated with a hemolock clip. The duodenal bulb was cut and closed with a linear stapler. Then the distal stomach was cut at the gastric body. The specimen was completely removed. We transferred the specimen into a specimen bag and moved the bag to the pelvic cavity for extraction later.
The ultrasonic scalpel was used to cut small 1-cm holes in the large curved side of the proximal stomach and duodenal stump, respectively. The two arms of the linear stapler were separately inserted into the holes, and anastomosis was performed on the posterior wall of the stomach and the posterior wall of the duodenum. We closed the joint opening with another stapler to complete the modified triangular anastomosis.
Another trocar was placed into the right lower abdomen to assist with specimen extraction (Figure 1). The anus was sufficiently dilated, the rectum was repeatedly rinsed with iodine water, and a roll of iodophor gauze was placed into the rectum to support the intestinal wall. Electrocautery was used to cut the anterior wall of the upper rectum by about 4 cm in length (Figure 1B). An oval clamp was inserted through the anus-rectum, and the specimen bag was grasped and slowly pulled out of the patient’s body (Figure 1C). The rectal incision was repeatedly disinfected with iodophor gauze, and then barbed wire sutures were used for full-thickness closure of the incision (Figure 1D). The water injection and air inflation methods were used to check if the rectal incision was completely closed (Figure 1E). Trocar ports were then closed, and the operation was completed (Figure 1F).
The total operative time was 176 min, intraoperative bleeding was 50 mL, and the patient’s vital signs were stable during the surgery. On the first day after the operation, the patient resumed normal activities of daily living (e.g., ambulation and independent transfer from bed) without pain and passed flatus within 48 h. The Numeric Rating Scale score[6,7] was merely 3 on the first day after surgery and 1 on the third day. A liquid diet was restarted on the third day. The pelvic drainage tube was removed on the fourth day, subhepatic drainage was removed on the ninth day, and the patient was discharged on the tenth day.
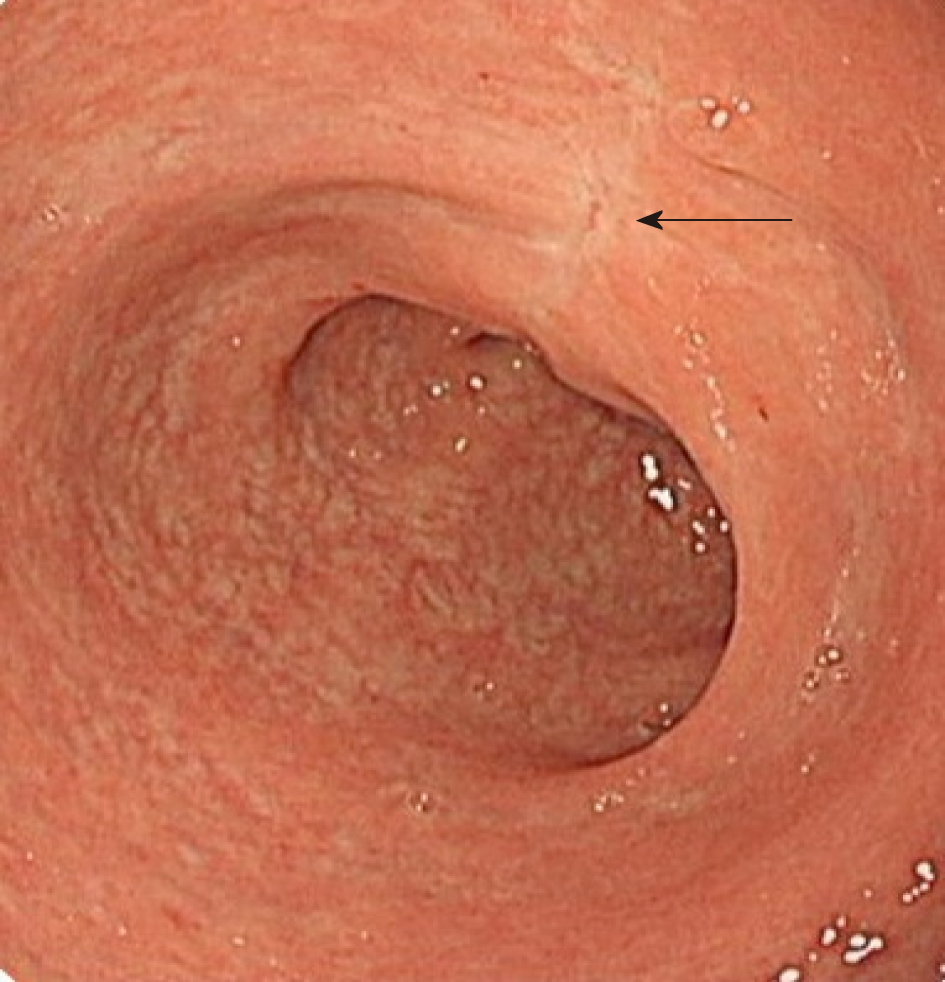
The patient recovered well after surgery, with normal flatus, normal anal sensation, and no tumor recurrence. Colonoscopy was performed 3 mo later, and the colonoscope passed through the rectum smoothly. Only streaks of mucosal hyperemia and edema were observed; no stenosis or scar contracture was present in the intestinal cavity (Figure 2).
Gastric cancer is the third most common tumor, and its mortality rate remains high, which seriously threatens the health and lifetime of patients[8]. With the popularity of screening evaluations such as gastroscopy and the continuous advancement of technology, the early diagnosis rate of gastric cancer has improved. Data show that early gastric cancer accounts for a large proportion of the total number of patients[9]. Even in patients with early gastric cancer, traditional open surgery always require a longitudinal incision from the xiphoid to the periumbilical, which causes significant injury to the abdominal wall. Although open surgery may achieve the purpose of radical cure, patients often experience tremendous trauma and pain[10].
In 1994, Kitano et al[11] reported the first case of laparoscopic-assisted distal gastrectomy. Additionally, in 2002, Kanaya et al[12] proposed complete intracorporeal gastroduodenostomy for distal gastric cancer by using triangular anastomosis technology, and created a new era of totally laparoscopic radical gastrectomy. Then Kanaya et al[13] reviewed 100 patients with early gastric cancer and compared totally laparoscopic radical gastrectomy with open surgery. The clinical data showed that totally laparoscopic gastrectomy is conducive to early eating after surgery, and it promoted rapid recovery after surgery. Kim et al[14] also compared totally laparoscopy distal gastrectomy with laparoscopy-assisted distal gastrectomy, and the short-term effects showed results similar to those of Dr. Kanaya. Although totally laparoscopic surgery has achieved better short-term results, a mini-laparotomy is still required to remove the specimen. The postoperative scar not only means that the integrity and aesthetics of the abdominal wall are destroyed, but it may also result in long-term dysfunction and activity-related pain, which may affect patients’ normal work and life in severe cases.
Recently, NOSE surgery has been considered to represent the next revolution in surgery[15], and it was developed as an extension of natural orifice transluminal endoscopic surgery. It was further improved based on totally laparoscopic techniques. In NOSE surgery, complete laparoscopic resections are performed through only five-12-mm trocar ports in the abdominal wall without any visible incisions or extensions. The key technology of NOSE surgery can be divided into two parts: Intracorporeal anastomosis and transluminal specimen extraction.
There are four potential natural orifices for specimen extraction: The mouth, urethra, rectum/anus, and vagina[16]. It has been reported that surgical specimens of early gastric cancer can removed transorally, but the specimens need to be segmented into pieces instead of whole, which disturbs the integrity of the tumor pathology[17]. Considering the malleability of the natural passage and the size of specimen, the rectal and vaginal methods are adopted. Several studies[18,19] have reported transvaginal specimen extraction in gastric cancer surgery, but transrectal surgery was barely mentioned. Especially for male patients, who lack the natural orifice of the vagina, the extraction lumen is very limited, and transrectal removal should be considered. The abdominal wall is innervated by somatic nerves and sensitive to painful stimuli; however, internal organs, such as the vagina and rectum, are not.
The operation described herein was performed by the cooperation of a gastric specialist team and colorectal specialist team. Before this operation, we have accumulated considerable experience in transrectal specimen extraction surgery. According to evaluation, our new procedure showed inspiring results compared with traditional laparoscopic surgery. In a previous randomized controlled trial of 1200 patients[20], the mean surgical time of the conventional laparoscopic group was 217.3 min, mean number of retrieved lymph nodes was 36.1, mean length of the incision was 8.0 cm, mean time to ambulation was 50 h, mean time to first flatus was 3.5 d, mean time to first liquid intake was 5.5 d, and mean duration of the postoperative hospital stay was 10.8 d. Our data (176 min, 43, 1 cm, 12 h, 2 d, 3 d, and 10 d, respectively) are superior to data of the conventional laparoscopic group. In our patient, there was no additional abdominal extension, no increase in the operative time, although there was a rectal incision, and no extra recovery time. During the operation, we demonstrated that the rectum-anus has the similar ductility as the vagina. The process of specimen extraction was smooth without any complications and lasted less than 3 min. The function of the rectum and anus was completely restored postoperatively without stenosis or dysfunction.
In previous studies, transrectal specimen extraction was more common in patients with rectal cancer and sigmoid colon cancer[21,22]. Transrectal specimen extraction in gastric cancer is particularly suitable for male patients or female patients who have undergone vaginal surgery or have vaginal stenosis. We summarize the advantages are as follows: (1) NOSE surgery causes mild postoperative pain, improves surgical experience, and avoids excessive use of analgesic drugs; (2) It shortens the patient’s postoperative activity and flatus time; (3) The procedure reduces the psychological burden on patients and increases the patient’s postoperative confidence, which is conducive to better outcomes[23,24]; (4) It has a good cosmetic result for the abdominal wall, which is important for specific occupations and particular patients (e.g., actors, dancers, models, and unmarried people); and (5) Finally, the procedure causes less dysfunction of the abdominal wall, so patients’ normal work and life are not affected. Improving the psychosocial state of patients is conducive to returning to normal social life.
This innovation in transrectal specimen extraction provides patients with early gastric cancer with a new NOSE approach, and it is stable and reliable. To make a more accurate evaluation about the outcomes of this new technique for early gastric cancer, a randomized comparison study and long-term follow-up are still necessary.
Transrectal specimen extraction with laparoscopic radical gastrectomy requires no additional abdominal incision, and therefore, results in less trauma, better cosmetic appearance, and quick postoperative recovery. It also conforms to the concept of minimal invasiveness and rapid recovery. This innovative technique provides a new approach to the treatment of gastric cancer by the laparoscopic NOSE method.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Espinel J, Muguruma N S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 2. | Hüscher CG, Lirici MM, Ponzano C. NOSE laparoscopic gastrectomies for early gastric cancer may reduce morbidity and hospital stay: early results from a prospective nonrandomized study. Minim Invasive Ther Allied Technol. 2017;26:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Guan X, Liu Z, Longo A, Cai JC, Tzu-Liang Chen W, Chen LC, Chun HK, Manuel da Costa Pereira J, Efetov S, Escalante R, He QS, Hu JH, Kayaalp C, Kim SH, Khan JS, Kuo LJ, Nishimura A, Nogueira F, Okuda J, Saklani A, Shafik AA, Shen MY, Son JT, Song JM, Sun DH, Uehara K, Wang GY, Wei Y, Xiong ZG, Yao HL, Yu G, Yu SJ, Zhou HT, Lee SH, Tsarkov PV, Fu CG, Wang XS, International Alliance of NOSES. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf). 2019;7:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Zhang S, Jiang ZW, Wang G, Feng XB, Liu J, Zhao J, Li JS. Robotic gastrectomy with transvaginal specimen extraction for female gastric cancer patients. World J Gastroenterol. 2015;21:13332-13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kayaalp C, Yagci MA. Laparoscopic Right Colon Resection With Transvaginal Extraction: A Systematic Review of 90 Cases. Surg Laparosc Endosc Percutan Tech. 2015;25:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003;3:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 476] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Degiuli M, De Manzoni G, Di Leo A, D'Ugo D, Galasso E, Marrelli D, Petrioli R, Polom K, Roviello F, Santullo F, Morino M. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol. 2016;22:2875-2893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 9. | Chen XZ, Li YY, Hu JK, Yang K, Liu J, Zhang B, Chen ZX, Chen JP, Zhou ZG. Spread and development of laparoscopic surgery for gastric tumors in mainland China: initial experiences. Hepatogastroenterology. 2012;59:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Son T, Hyung WJ. Laparoscopic gastric cancer surgery: Current evidence and future perspectives. World J Gastroenterol. 2016;22:727-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Asakuma M, Cahill RA, Lee SW, Nomura E, Tanigawa N. NOTES: The question for minimal resection and sentinel node in early gastric cancer. World J Gastrointest Surg. 2010;2:203-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Swain P. Nephrectomy and natural orifice translumenal endoscopy (NOTES): transvaginal, transgastric, transrectal, and transvesical approaches. J Endourol. 2008;22:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Dostalik J, Gunkova P, Gunka I, Mazur M, Mrazek T. Laparoscopic gastric resection with natural orifice specimen extraction for postulcer pyloric stenosis. Wideochir Inne Tech Maloinwazyjne. 2014;9:282-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Sumer F, Kayaalp C, Karagul S. Laparoscopic Gastrectomy and Transvaginal Specimen Extraction in a Morbidly Obese Patient with Gastric Cancer. J Gastric Cancer. 2016;16:51-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Jeong SH, Lee YJ, Choi WJ, Paik WY, Jeong CY, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT, Ha WS. Trans-vaginal specimen extraction following totally laparoscopic subtotal gastrectomy in early gastric cancer. Gastric Cancer. 2011;14:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 530] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 21. | Liu Z, Efetov S, Guan X, Zhou H, Tulina I, Wang G, Tsarkov P, Wang X; International Alliance of Natural Orifice Specimen Extraction Surgery (IANOSES). A Multicenter Study Evaluating Natural Orifice Specimen Extraction Surgery for Rectal Cancer. J Surg Res. 2019;243:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Efetov SK, Tulina IA, Kim VD, Kitsenko Y, Picciariello A, Tsarkov PV. Natural orifice specimen extraction (NOSE) surgery with rectal eversion and total extra-abdominal resection. Tech Coloproctol. 2019;23:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ. 2012;345:e4933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 24. | Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |