Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4277
Peer-review started: September 28, 2019
First decision: November 11, 2019
Revised: November 22, 2019
Accepted: November 27, 2019
Article in press: November 27, 2019
Published online: December 26, 2019
Processing time: 81 Days and 23 Hours
Asplenia, the lack of a spleen, can be congenital and increases susceptibility to severe infections caused by encapsulated bacteria, such as Streptococcus pneumoniae (S. pneumoniae). We report two cases of severe pneumococcal infection in two asplenic family members living in the same household.
Patient 1, a 38-year-old man with a history of congenital hepatitis B infection and hypospadias, was brought to our emergency department with complaints of cyanosis, cough, and edema of his limbs. He was clinically diagnosed as hyposplenic with overwhelming pneumococcal sepsis. He was admitted to the intensive care unit and was administered antibiotics and catecholaminergic therapy but died 2 h after admission. Patient 2, a 63-year-old woman with a history of type 2 diabetes, was brought to our emergency department one month after admission of Patient 1. She was diagnosed as asplenic with overwhelming pneumococcal sepsis. History-taking revealed that she was the mother of Patient 1 and the two had lived in the same household. She was admitted to the intensive care unit and was rapidly provided antibiotics and catecholaminergic intervention but died one day after admission.
Pneumococcal bacteremia caused by virulent S. pneumoniae may be transmitted within households. All residents of households where individuals with pneumococcal bacteremia are living should be educated about the risk of transmissibility. Family members of patients with congenital asplenia/hyposplenia, all family members should be examined to assess their splenic function.
Core tip: We present two cases of severe pneumococcal infection transmitted between family members with congenital asplenia. Streptococcus pneumoniae infection may be transmitted within a household, so all household members should be warned when there is an infection in the house. In addition, asplenia/hyposplenia is sometimes congenital, so family members should be screened for these conditions.
- Citation: Shibata J, Hiramatsu K, Kenzaka T, Kato T. Pneumococcal infection transmission between family members with congenital asplenia: A case report. World J Clin Cases 2019; 7(24): 4277-4284
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4277.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4277
The spleen is a lymphoid organ housing splenic macrophages that eliminate infected cells and bacteria from the body via phagocytosis[1]. It is also a major site of early immunoglobulin M production, which helps to clear pathogens from the bloodstream[2]. Asplenia or hyposplenia may occur as a congenital anomaly or as a result of splenectomy following hematologic disease or trauma[3]. Asplenic individuals are at increased risk of invasive infections caused by encapsulated organisms, such as Streptococcus pneumoniae (S. pneumoniae), which can progress to fulminant sepsis within a short period. Since these infections have high mortality rates even with aggressive treatment, preventive education and vaccination of asplenic individuals are of the utmost importance[4].
Pneumococcal pneumonia infections can be transmitted among people living in close contact with each other, such as those within a single household[5]. Here we report two cases of fulminant S. pneumoniae infection transmitted between two asplenic family members living in a single household. Through this report we aim to make readers aware of the familial nature of some asplenism and of the need to screen family members of individuals with congenital asplenism.
Case 1: Patient 1, a 38-year-old male with a medical history of congenital hepatitis B infection and hypospadias was brought to our emergency department complaining of cyanosis, cough, and edema of his limbs.
Case 2: Patient 2, a 63-year-old female with a history of type 2 diabetes, was brought to our emergency department one month after admission of Patient 1. Her chief complaint was vomiting and general malaise that had lasted for four hours. Although she had been well earlier that morning, she was in a lethargic state by noon.
Case 1: The patient had a medical history of congenital hepatitis B infection and hypospadias.
Case 2: The female with a history of type 2 diabetes.
Case 1: His family history contained nothing of note; specifically, there was no family history of any immunodeficiency disorder or other congenital anomalies.
Case 2: History-taking revealed that she was the mother of Patient 1 and the two had lived in the same household.
Case 1: His vital signs were markedly abnormal with a respiratory rate of 40 /min, body temperature of 35.9 °C, a heart rate of 144 beats/min, and oxygen saturation of 82% with oxygen 5 L/min. His blood pressure was 52/38 mmHg. He was oriented in time, place and person. On physical examination he was found to have cyanosis of the limbs.
Case 2: Her vital signs were markedly abnormal with a respiratory rate of 30 /min, body temperature of 40.1 °C, heart rate of 135 beats/min, oxygen saturation of 96% in ambient air, and blood pressure of 137/84 mmHg; her Glasgow coma scale score was 10 (E2V3M5) points. Nothing abnormal was detected upon physical examination.
Case 1: Laboratory test results revealed a neutrophilia with normal white blood cell count (6400 cells/μL, with 90.2% neutrophils), and a platelet count of 34000 cells/μL. Liver and kidney function test results were grossly abnormal, and coagulation times were severely prolonged. The results of the blood test taken at this stage are presented in Table 1. A urinary sample could not be obtained because the patient had anuria.
| Parameter | Recorded value | Standard value |
| White blood cell count | 6400 /µL | 3590-9640 /µL |
| Neutrophils | 90.2% | 41.2%-74.7% |
| Lymphocytes | 8.4% | 21.2%-51.0% |
| Hemoglobin | 16.1 g/dL | 13.2-17.2 g/dL |
| Hematocrit | 47.3% | 40.4%-51.1% |
| Platelets | 3.4 × 104 /µL | (14.8-33.9) × 104 /µL |
| C-reactive protein | 18.2 mg/dL | ≤ 0.30 mg/dL |
| Total protein | 5.5 g/dL | 6.7-8.3 g/dL |
| Albumin | 2.9 g/dL | 3.9-4.9 g/dL |
| Aspartate aminotransferase | 232 U/L | 13-33 U/L |
| Alanine aminotransferase | 154 U/L | 6-30 U/L |
| Lactate dehydrogenase | 700 U/L | 119-229 U/L |
| Creatine phosphokinase | 356 U/L | 62-287 U/L |
| Blood nitrogen urea | 35 mg/dL | 8-22 mg/dL |
| Creatinine | 4.72 mg/dL | 0.60-1.10 mg/dL |
| Sodium | 136 mEq/L | 138-146 mEq/L |
| Potassium | 4.4 mEq/L | 3.6-4.9 mEq/L |
| Glucose | 118 mg/dL | 60-160 mg/dL |
| Hemoglobin A1c | 6.7% | 4.6%-6.2% |
| Prothrombin time | 24.8 s | 9.4-12.5 s |
| Activated partial thromboplastin time | 97.8 s | 25.1-36.5 s |
| Fibrinogen | 131 mg/dL | 155-415 mg/dL |
| Fibrin/Fibrinogen degradation products | 68.6 µg/mL | 0-4.9 µg/mL |
Case 2: The results of the blood test taken at this stage are presented in Table 2.
| Parameter | Recorded value | Standard value |
| White blood cell count | 2260 /µL | 3040-8540 /µL |
| Neutrophils | 61.1% | 38.3%-71.1% |
| Lymphocytes | 37.5% | 21.3%-50.2% |
| Hemoglobin | 10.9 g/dL | 10.8-14.9 g/dL |
| Hematocrit | 32.8% | 35.6%-45.4% |
| Platelets | 15.6 × 104/µL | (15.0-36.1) × 104/µL |
| C-reactive protein | 0.34 mg/dL | ≤ 0.30 mg/dL |
| Total protein | 6.5 g/dL | 6.7-8.3 g/dL |
| Albumin | 3.9 g/dL | 3.9-4.9 g/dL |
| Aspartate aminotransferase | 23 U/L | 13-33 U/L |
| Alanine aminotransferase | 11 U/L | 6-27 U/L |
| Lactate dehydrogenase | 276 U/L | 119-229 U/L |
| Creatine phosphokinase | 200 U/L | 45-163 U/L |
| Blood nitrogen urea | 20 mg/dL | 8-22 mg/dL |
| Creatinine | 1.32 mg/dL | 0.40-0.70 mg/dL |
| Sodium | 144 mEq/L | 138-146 mEq/L |
| Potassium | 3.4 mEq/L | 3.6-4.9 mEq/L |
| Glucose | 191 mg/dL | 60-160 mg/dL |
| Hemoglobin A1c | 6.8% | 4.6%-6.2% |
| Prothrombin time | 12.8 s | 9.4-12.5 s |
| Activated partial thromboplastin time | 22.1 s | 25.1-36.5 s |
| Fibrinogen | 131 mg/dL | 155-415 mg/dL |
| Fibrin/Fibrinogen degradation products | 24.6 µg/mL | 0-4.9µg/mL |
Case 1: His chest X-ray was unremarkable.
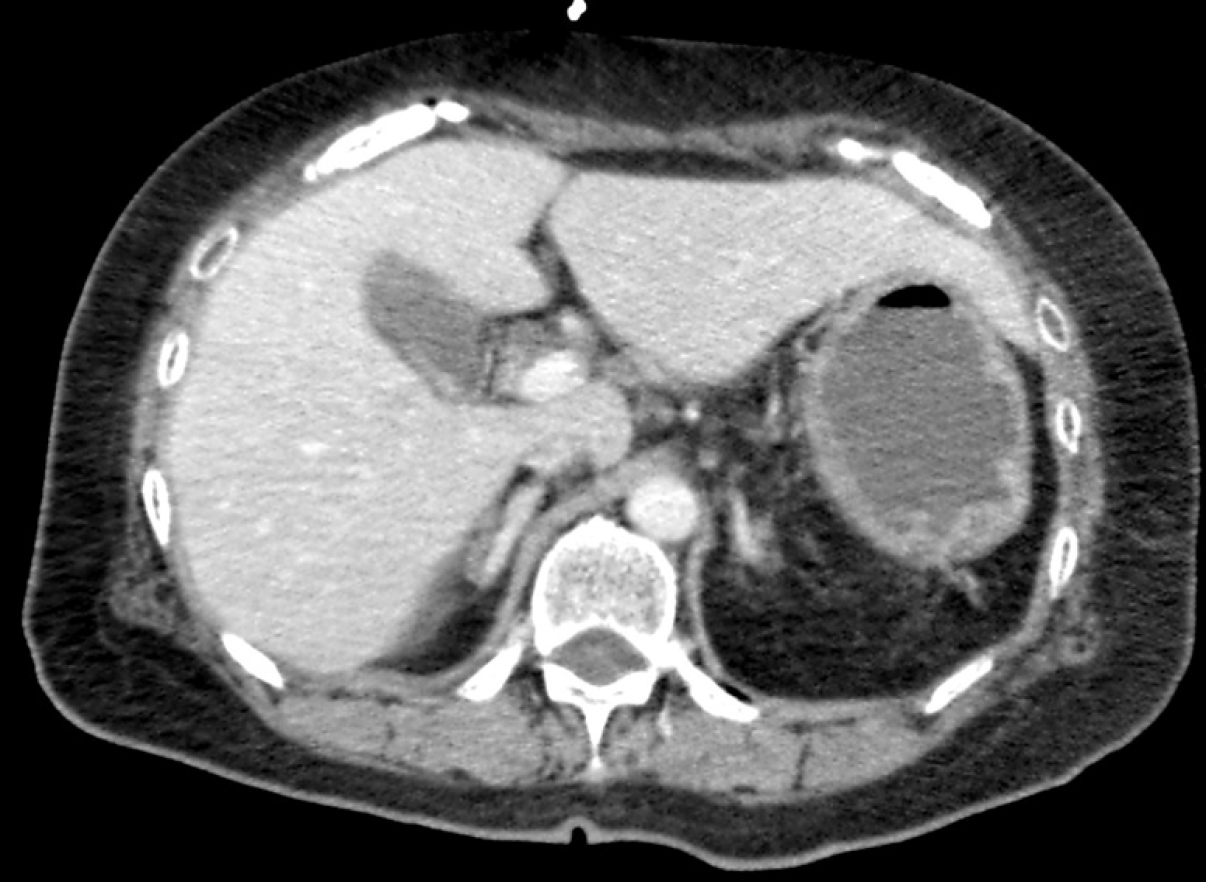
Case 2: A computed tomography scan revealed that she had no spleen (Figure 1) and a urinary rapid test for S. pneumoniae-antigen was positive.
He was clinically diagnosed as having overwhelming pneumococcal sepsis secondary to hyposplenia.
She was diagnosed as having overwhelming pneumococcal sepsis secondary to asplenia.
In spite of rapid initiation of antimicrobial therapy, catecholaminergic therapy, fluid resuscitation, and intubation, the patient’s organ function deteriorated and he died two hours after admission.
Intravenous antibiotics, comprising 2 g ceftriaxone daily, 0.75 g vancomycin 12-hourly, and 2 g ampicillin 6-hourly, were initiated two hours after her arrival. She was transferred to the ICU where, despite the antimicrobial therapy, catecholaminergic therapy and intubation, her condition deteriorated and she died within 24 h.
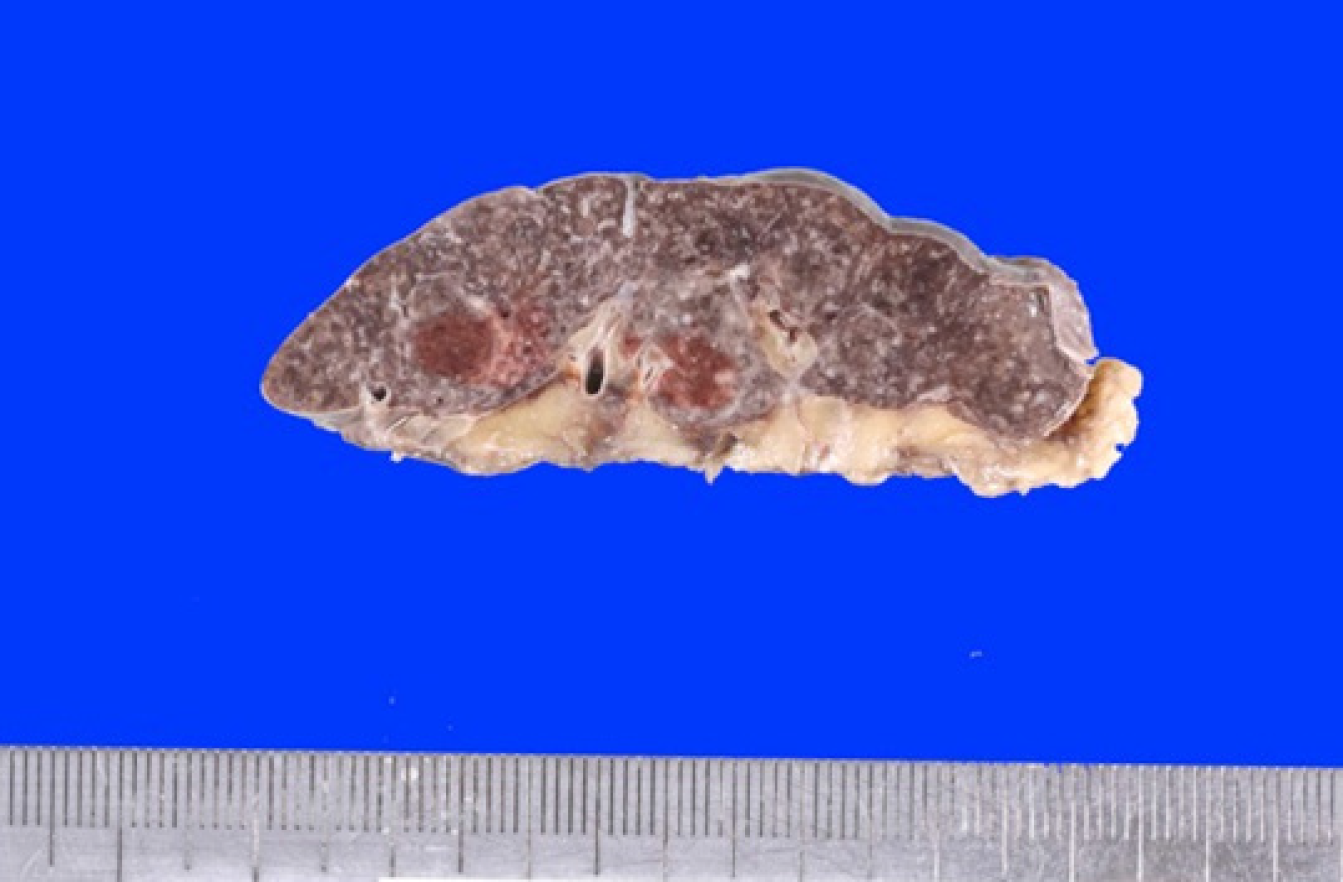
An autopsy revealed that he had an extremely small spleen (Figure 2), and S. pneumoniae was isolated from his blood, muscles, lungs, and spleen on culture.
After her death, S. pneumoniae bacteria were obtained from her blood culture.
Although bacterial strain analysis did not match with the samples from both patients, antibiotic sensitivity tests revealed that both sets of isolates had a similar antibiotic sensitivity profile (Table 3).
| Strain | PCG | ABPC | CTRX | CFPM | CCL | MEPM | CAM | AZM | CLDM | VCM | LVFX |
| Patient 1 | ≤ 0.06 | ≤ 0.12 | ≤ 0.06 | ≤ 0.06 | ≤ 0.5 | ≤ 0.015 | 1 | > 4 | 0.12 | 0.25 | 1 |
| Patient 2 | ≤ 0.06 | ≤ 0.12 | ≤ 0.06 | ≤ 0.06 | ≤ 0.5 | ≤ 0.015 | 1 | > 4 | 0.5 | 0.25 | 1 |
After encountering these two familial cases, we examined the child of the Patient 1 to assess whether she had any immune system abnormalities. According to the echocardiogram, abdominal ultrasound including splenic morphology, and blood examination with immunoglobulin and complement, the child had a normal spleen and no evidence of any immunodeficiency disorder. Since she had received only the heptavalent pneumococcal vaccine, we gave her an additional 13-valent vaccine to prevent recurrence of the infection identified in Patients 1 and 2.
We encountered two first-degree relatives with septic shock caused by S. pneumoniae. To the best of our knowledge this is the first report of possible household transmission of S. pneumoniae infection among family members with congenital hyposplenia/asplenia. Moreover, there has only been one previous report of S. pneumoniae bacteremia acquired by household transmission[6]. The two cases presented in our report provide the following lessons: (1) S. pneumoniae infection may be transmitted within a household, so other household members should be warned if another person in the household develops a S. pneumoniae infection; and (2) Asplenia/hyposplenia is sometimes congenital, so the family members of patients found to have asplenia/hyposplenia should be screened for these conditions.
The most common cause of anatomic asplenia is surgical splenectomy due to trauma or for therapeutic intervention; congenital asplenia is rare. By contrast, hyposplenism refers to the partial loss of splenic function and is often caused by medical disorders that lead to atrophy, infarction, engorgement, or infiltration of the spleen. These disorders include thalassemia, chronic liver disease, human immunodeficiency virus infection, immune disorders, and malignancies[7].
Our two patients had no evidence of acquired asplenia or hyposplenism, and are likely to have had congenital asplenia/hyposplenia. Encapsulated bacteria are particularly resistant to phagocytosis and frequently cause severe infection in patients with impaired splenic function. The most important pathogen for such individuals is S. pneumoniae, which accounts for 40%-60% of cases of overwhelming sepsis in splenectomized patients[8,9]. Encapsulated organisms proliferate in the absence of opsonization and phagocytosis by splenic macrophages. In a prospective cohort study evaluating invasive pneumococcal infection, asplenic patients were found to have a higher risk of pneumococcal meningitis, and more frequently required intensive care unit admission and intubation[10]. The clinical symptoms of S. pneumoniae infection in patients with impaired splenic function can be nonspecific, but purpura fulminans, as occurred in Patient 1 in the current report, appears to be more common in asplenic patients with pneumococcal infections[9]. Immediate empiric antibiotic administration and aggressive supportive care are essential for the management of sepsis in patients with impaired splenic function because progression to septic shock can be rapid[7]. When S. pneumoniae infection is suspected in an individual with asplenia or hyposplenia, a course of empiric intravenous broad-spectrum antibiotics should be immediately initiated. Administration of antibiotics should not be delayed even if necessary diagnostic studies, including lumbar puncture, have not been performed.
S. pneumoniae is frequently found in the nasopharynx of infants and can cause pneumonia, sepsis, and meningitis in susceptible individuals[11]. Pneumococcal infection is known to be transmitted by adults in communal and family settings[12,13]. Also, pneumococcal pneumonia infection may occur within a family[5] or, more rarely, among nursing home residents[11]. In our case, it is likely that the bacteria were transmitted from Patient 1 to Patient 2 and that she became a carrier before developing symptoms because of her asplenia. Patient 2 may also have become infected via a child, who was a carrier and was living with the family.
In cases of intrafamilial household transmission, a diagnosis of a congenital asplenic syndrome should be considered. Various syndromes are associated with asplenia, including autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome, isolated congenital asplenia (ICA), Ivemark syndrome, and Stormorken syndrome[7,14,15]. Given that our two patients had no other abnormalities, ICA, a rare condition in which affected individuals are missing their spleen but have no other developmental abnormalities, is the most likely diagnosis. The incidence of ICA is unclear because no comprehensive surveys have been performed; to our knowledge, the only such report estimated the frequency of ICA in the French population to be 0.51 per million births[16]. Although most individuals with ICA have no spleen at all, some people have a very small, nonfunctional spleen, such as that of Patient 1. ICA differs from asplenia that is associated with other complex visceral defects[16], as it is caused by a gene mutation that affects the production of ribosomal protein SA (RPSA). The mutation occurs on the short arm of chromosome 3 at position 22.1[17]. RPSA gene mutations are thought to reduce the amount of functional RPSA and expression of key spleen patterning genes[18]. The RPSA gene is inherited in an autosomal dominant pattern. However, since some of the mutations have incomplete penetrance[19], we were unable to determine whether the children of Patient 1 had not inherited the faulty gene, or had inherited the gene, but were unaffected due to incomplete penetrance. We assume the difference of splenic expression between Patients 1 and 2 was due to variable penetrance.
Since the progression of S. pneumoniae infection in individuals with asplenia or impaired splenic function is so rapid and may be overwhelming, prevention of infection is essential, and may be life-saving. Individuals with nonsurgical asplenia or hyposplenism, should be vaccinated with the pneumococcal vaccine as soon as impaired splenic function is recognized. In addition, educating these individuals about their lifelong increased risk of infection and strategies to minimize the risk is critical. Individuals who are knowledgeable about their susceptibility to infection and strategies to minimize infection risk have lower rates of severe infection compared to those without such knowledge[4]. Hence, the most important messages to stress to individuals with asplenia or hyposplenia are that they have an increased risk of infection, and that there are steps they can take to minimize their risk of infections, including vaccination, use of prophylactic antibiotics, and knowing when to seek medical care.
Of two patients in this report, we should have suspected that Patient 1 had congenital asplenia after discovering that he had hyposplenia on biopsy. Other members of his family should have been screened members and educated about their possible vulnerability to pneumococcal infections.
In conclusion, we encountered a case of septic shock syndrome associated with pneumococcal bacteremia in a man with hyposplenia and a similar case in his mother who had asplenia. Pneumococcal bacteremia caused by virulent S. pneumoniae may be transmitted within a household, and educating family members is essential. When individuals are diagnosed as having congenital asplenia/hyposplenia, other family members should be screened.
The authors are grateful to Matsuyoshi Maeda for explaining the pathology data.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anis S, Jun C S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
| 1. | Borges da Silva H, Fonseca R, Pereira RM, Cassado Ados A, Álvarez JM, D'Império Lima MR. Splenic Macrophage Subsets and Their Function during Blood-Borne Infections. Front Immunol. 2015;6:480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Sawmiller CJ, Dudrick SJ, Hamzi M. Postsplenectomy Capnocytophaga canimorsus sepsis presenting as an acute abdomen. Arch Surg. 1998;133:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Pathol. 2001;54:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | El-Alfy MS, El-Sayed MH. Overwhelming postsplenectomy infection: is quality of patient knowledge enough for prevention? Hematol J. 2004;5:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 5. | Kellner JD, Gibb AP, Zhang J, Rabin HR. Household transmission of Streptococcus pneumoniae, Alberta, Canada. Emerg Infect Dis. 1999;5:154-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Goda K, Kenzaka T, Chang B, Akita H. Two cases of pneumococcal spondylitis in the same household: a case report. BMC Infect Dis. 2018;18:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 438] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 8. | Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. 1991;78:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 318] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Theilacker C, Ludewig K, Serr A, Schimpf J, Held J, Bögelein M, Bahr V, Rusch S, Pohl A, Kogelmann K, Frieseke S, Bogdanski R, Brunkhorst FM, Kern WV. Overwhelming Postsplenectomy Infection: A Prospective Multicenter Cohort Study. Clin Infect Dis. 2016;62:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 10. | Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Asplenic patients and invasive pneumococcal disease-how bad is it these days? Int J Infect Dis. 2016;51:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Nuorti JP, Butler JC, Crutcher JM, Guevara R, Welch D, Holder P, Elliott JA. An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 1998;338:1861-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Regev-Yochay G, Raz M, Dagan R, Porat N, Shainberg B, Pinco E, Keller N, Rubinstein E. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis. 2004;38:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, Pebody R, George R, Soininen A, Edmunds J, Gay N, Kayhty H, Miller E. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Kirkineska L, Perifanis V, Vasiliadis T. Functional hyposplenism. Hippokratia. 2014;18:7-11. [PubMed] |
| 15. | Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med. 2014;371:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Mahlaoui N, Minard-Colin V, Picard C, Bolze A, Ku CL, Tournilhac O, Gilbert-Dussardier B, Pautard B, Durand P, Devictor D, Lachassinne E, Guillois B, Morin M, Gouraud F, Valensi F, Fischer A, Puel A, Abel L, Bonnet D, Casanova JL. Isolated congenital asplenia: a French nationwide retrospective survey of 20 cases. J Pediatr. 2011;158:142-148, 148.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | HUGO Gene Nomenclature Committee. Symbol report for RPSA; 18 Jul 2019. United Kingdom: Cambridge. Available from: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/6502. |
| 18. | Griffin JN, Sondalle SB, Robson A, Mis EK, Griffin G, Kulkarni SS, Deniz E, Baserga SJ, Khokha MK. RPSA, a candidate gene for isolated congenital asplenia, is required for pre-rRNA processing and spleen formation in Xenopus. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Bolze A, Boisson B, Bosch B, Antipenko A, Bouaziz M, Sackstein P, Chaker-Margot M, Barlogis V, Briggs T, Colino E, Elmore AC, Fischer A, Genel F, Hewlett A, Jedidi M, Kelecic J, Krüger R, Ku CL, Kumararatne D, Lefevre-Utile A, Loughlin S, Mahlaoui N, Markus S, Garcia JM, Nizon M, Oleastro M, Pac M, Picard C, Pollard AJ, Rodriguez-Gallego C, Thomas C, Von Bernuth H, Worth A, Meyts I, Risolino M, Selleri L, Puel A, Klinge S, Abel L, Casanova JL. Incomplete penetrance for isolated congenital asplenia in humans with mutations in translated and untranslated RPSA exons. Proc Natl Acad Sci USA. 2018;115:E8007-E8016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |