Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4234
Peer-review started: September 8, 2019
First decision: November 11, 2019
Revised: October 14, 2019
Accepted: November 27, 2019
Article in press: November 27, 2019
Published online: December 26, 2019
Processing time: 108 Days and 6.1 Hours
The guiding effect of prognostic stratification in multiple myeloma (MM) for treatment has been increasingly emphasized in recent years. The stratification of risk factors based on the International Staging System (ISS), Durie-Salmon (DS) staging and related indicators is affected by the renal function of patients, resulting in poor performance. This study assesses the relationship between interleukin-32 (IL-32) and related risk factors in 67 patients with MM and their clinical outcomes.
To investigate the feasibility of IL-32 in evaluating prognosis in patients with MM and the factors influencing prognosis.
This was a pragmatic, prospective observational study of patients with MM at a single center. According to IL-32 level, patients were divided into two groups. The variables under consideration included age, blood β2-microglobulin, albumin, C-reactive protein, serum calcium, serum creatinine, lactate dehydrogenase, M protein type, ISS stage, DS stage, and IL-32 levels and minimal residual disease (MRD) after induction treatment. The main outcomes were progression-free survival (PFS) and overall survival (OS).
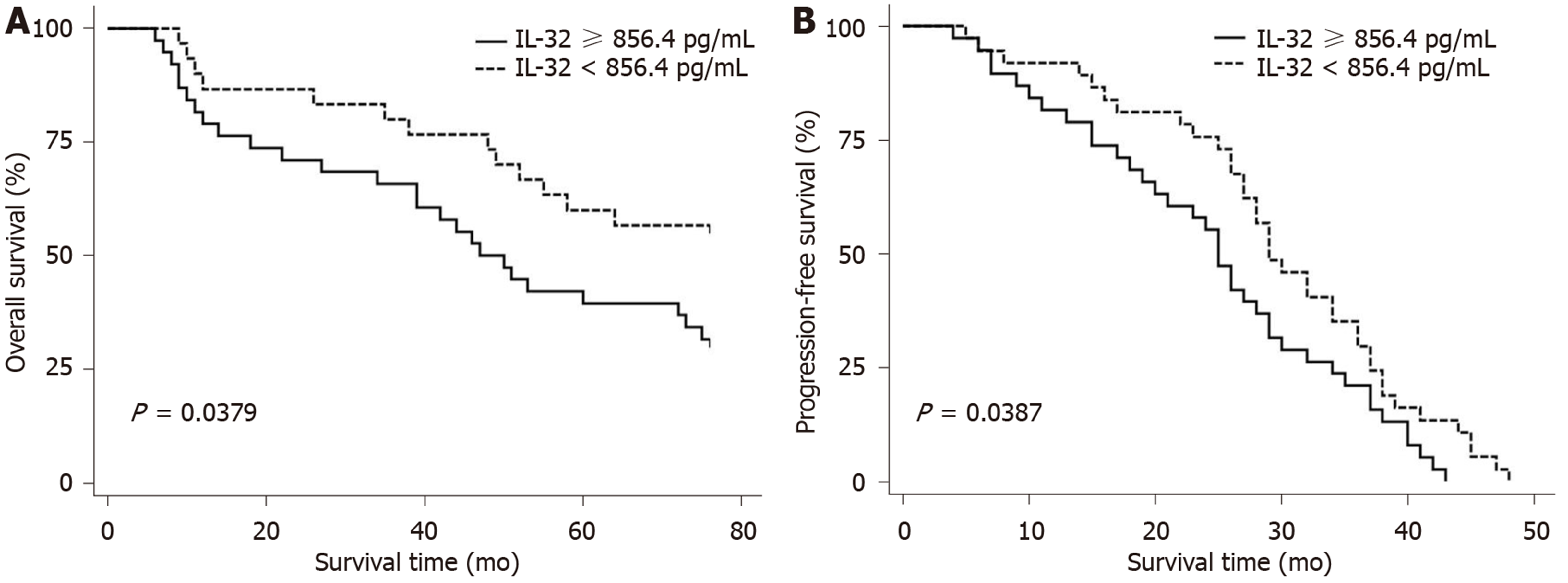
IL-32 was an important factor affecting PFS and OS in patients with MM. Compared with patients with IL-32 levels ≥ 856.4 pg/mL, patients with IL-32 levels < 856.4 pg/mL had longer PFS (P = 0.0387) and OS (P = 0.0379); Univariate analysis showed that IL-32 level and MRD were significantly associated with OS and PFS (P < 0.05). Multivariate analysis showed that IL-32 levels ≥ 856.4 pg/mL and MRD positive were still independent risk factors for OS and PFS (P < 0.05).
IL-32 is valuable for assessing the prognosis of MM patients. IL-32 level combined with MRD may be a useful routine evaluation index for MM patients after treatment.
Core tip: Multiple myeloma (MM) is a heterogeneous disease with a survival time ranging from several months to 20 years, and its clinical manifestations are complex. There are many factors affecting the prognosis. The International Staging System and Durie-Salmon staging have been established to assess prognosis of the disease, but the accuracy of β2-microglobulin and albumin is controversial. In recent years, new prognostic indicators of MM are being continuously investigated. Studies have shown that renal function in association with the degree of bone destruction, and hypercalcemia, can determine the prognosis of MM and predict overall survival (OS). Moreover, minimal residual disease can also predict OS and progression-free survival in patients with MM. We collected data from MM patients in our hospital, analyzed their clinical efficacy, follow-up results and laboratory examination indicators, which suggested that interleukin-32 can be used as an auxiliary indicator for post-treatment efficacy and prognosis assessment.
- Citation: Wang G, Ning FY, Wang JH, Yan HM, Kong HW, Zhang YT, Shen Q. Expression of interleukin-32 in bone marrow of patients with myeloma and its prognostic significance. World J Clin Cases 2019; 7(24): 4234-4244
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4234.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4234
Multiple myeloma (MM) is a monoclonal malignant tumor of plasma cells originating from B lymphocytes, with an incidence of approximately 10% of all hematopoietic tumors, after lymphoma[1,2]. MM is associated with older age, and leads to liver and kidney and other organ damage along with disease progression and even death. Due to various factors such as low immunity and poor drug tolerance, patients usually have a poor prognosis, and MM is considered to be an incurable disease[3]. Currently, the sequential application of various therapies, including lenalidomide and proteinase inhibitor, has significantly prolonged the survival time of patients, but their prognosis is still far from ideal, and their heterogeneity and early recurrence rate are high[4]. Due to advances in research, it was found that minimal residual disease (MRD) is the main cause of tumor recurrence. There are a small number of myeloma cells in patients, even in patients with a complete response (CR) to treatment, resulting in recurrence[5]. The study by Anderson et al[6] also showed that MRD plays an important role in determining the condition, evaluating prognosis and guiding subsequent therapy. However, because the MRD detection method is not standardized and the sensitivities of different instruments are different, it varies among different medical institutions[7,8]. Therefore, attention should be paid to identifying a highly sensitive and reliable indicator for the evaluation of prognosis using MRD detection technology. Long-term clinical practice has confirmed that MM is closely related to inflammation, and inflammatory mediators such as interleukin-6 (IL-6), IL-32, and toll-like receptors are key factors in tumor transformation[9]. Marcondes et al[10] found abnormal expression of IL-32 in patients with myelodysplastic syndrome and chronic myelomonocytic leukemia. In addition, our previous animal experiments revealed that IL-32 may promote the proliferation of bone marrow stromal cells by inducing IL-6 production in the bone marrow stroma. All of the above studies suggest that IL-32 has the potential to predict the progression of MM[11], but there is little research in this field, and the ability of IL-32 to evaluate the prognosis of MM patients is unclear. The objective of the current study was to analyze the expression of IL-32 in the bone marrow of MM patients, and determine the impact of IL-32 on the prognosis of MM patients.
A total of 67 patients with MM, 39 males and 28 females with a median age of 58 (40-77) years, who were diagnosed and treated in our hospital from 2012 to 2017 were enrolled in this study. All patients met the diagnostic criteria of the International Myeloma Working Group and the exclusion criteria included: (1) The survival time of patients was expected to be less than 3 mo and long-time follow up was not performed; (2) The patient was treated with radiotherapy, chemotherapy and related drugs for other diseases; and (3) Patients with other malignant tumors or serious infections. According to histopathology and laboratory examinations, the immunophenotype of patients was identified, including IgG in 32 (47.8%), IgA in 17 (25.4%), IgD in 2 (3.0%), light chain in 14 (20.9%) and nonsecretory in 2 (3.0%), respectively. Staging was mainly based on the Durie-Salmon (DS) staging standard and the International Staging System (ISS). All participants or guardians provided signed informed consent, and this study was approved by the Medical Research Ethics Committee of our hospital.
The demographic indices such as gender and age, and following laboratory tests were obtained: Blood β2-microglobulin (β2-MG), albumin (ALB), C reactive protein (CRP), serum calcium levels, serum creatinine levels, hemoglobin, bone marrow plasma cells, and lactate dehydrogenase (LDH). Hematuria light chain quantification and the white blood cell count were recorded. Routine detection of liver and kidney function, electrolytes and bone marrow were performed.
All MM patients received 4 courses of induction therapy consisting of combination chemotherapy. PCD (bortezomib 1.3 mg/m2, subcutaneous injection on days 1, 8, 15, and 22; cyclophosphamide 200 mg/m2, intravenous drip on days 1, 8, 15, and 22; dexamethasone 20 mg/d, intravenous drip on days 1, 2, 8, 9, 15, 16, 22 and 23, a course of treatment was 28 d) was the major therapy and 38 (56.7%) patients were given this treatment option. Other bortezomib regimens were as follows: Bortezomib and dexamethasone in 10 (14.9%) cases, and bortezomib, epirubicin and dexamethasone in 8 (11.9%) cases. Nine patients were treated with thalidomide-based treatment regimens, 6 (9%) of whom were treated with thalidomide and dexamethasone, and 3 (4.5%) were treated with thalidomide, cyclophosphamide and dexamethasone. Two (3.0%) cases were treated with lenalidomide and dexamethasone. Autologous stem cell transplantation or consolidation therapy was performed according to the patient's age, condition, suitability for transplantation, and efficacy was evaluated based on the Myeloma Working Group standard.
After induction treatment, the MRD was determined by Multiparameter flow cytometry (MFC) with the help of the Adicon Clinical Laboratories and the sensitivity was 10-4. According to the clinical global impression of the blood test application form, the appropriate test method was established and the antibody combination was as follows: CD138-FITC/CD117-PE/CD45-ECD/CD56-PC5/CD19-PECY7/CD38-APC/cκ-FITC/cλ-PE/CD45-ECD/CD38-PC5; the isotype negative control was: IgG1-FITC/IgG1-PE/CD45-ECD/IgG1-PC5/IgG1-PECY7/IgG1-APC/IgG1-APCCY7. The procedure for sample preparation and detection was as follows: The above membrane antibody was added to 100 μL bone marrow solution and incubated at room temperature for 10 min without light. Red blood cell lysate was added for complete lysis of blood cells, and then incubated with 2 mL BC hemolysin for 10 min in the absence of sunlight. After centrifugation for 5 min at 800 g, the precipitate was carefully collected, mixed with PBS and centrifuged again. Finally, the precipitate was dissolved in 500 μL PBS buffer and filtered for detection. Operation of the instrument was carried out in strict accordance with the document"ACEA NovoCyte Flow Cytometry Use and Maintenance Standard Operating Procedures", and the document "Laboratory Safety Management Regulations" and Biosafety Notice were strictly adhered to.
The content of IL-32 in bone marrow solution was measured by ELISA on the day of MRD determination, and the kit was purchased from the Endogen Corporation, United States. 2 mL bone marrow solution was taken and EDTA-K2 solution was used to prevent coagulation. The supernatant of the bone marrow solution was extracted by centrifugation at 800 g for 10 min, in accordance with the instructions, and placed in a pre-coated 96-well plate after dilution. 200 μL assay diluent, 50 μL standards and the sample to be tested were added, and the mixture was incubated for 2 h at room temperature. Then, 200 μL IL-32 enzyme-labeled antibody was added. The optical density values were read at 450 nm on an Epoch enzyme analyzer (BioTek, United States), and the IL-32 concentration was determined from the standard curve.
The outpatient revisit service and telephone were used for follow-up. All patients were followed up to January 2019 and the total follow-up time ranged from 1.0 to 76.0 mo, with a median follow-up time of 28.0 mo. Overall survival (OS) time was calculated from the date of diagnosis until the date of death from any cause or the last follow-up. Progression-free survival (PFS) time was calculated from the initiation of diagnosis to the date of disease progression or relapse.
Statistical analysis was performed by SPSS 19.0 software, and the measurement data were analyzed by the t test or Wilcoxon rank sum test. The categorical variables were expressed as a percentage and assessed by the χ2 test or Fisher exact probability. The receiver operating characteristic (ROC) curve was used to calculate the optimal cut-off value of IL-32. Kaplan–Meier analysis was performed to evaluate the OS and PFS of MM patients, and statistical significance was assessed with the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard model to assess potential risk factors for PFS and OS. Variables with P < 0.10 in the univariate analysis were included in the multivariate Cox proportional hazard model. All calculations were two-sided, P < 0.05 was considered statistically significant.
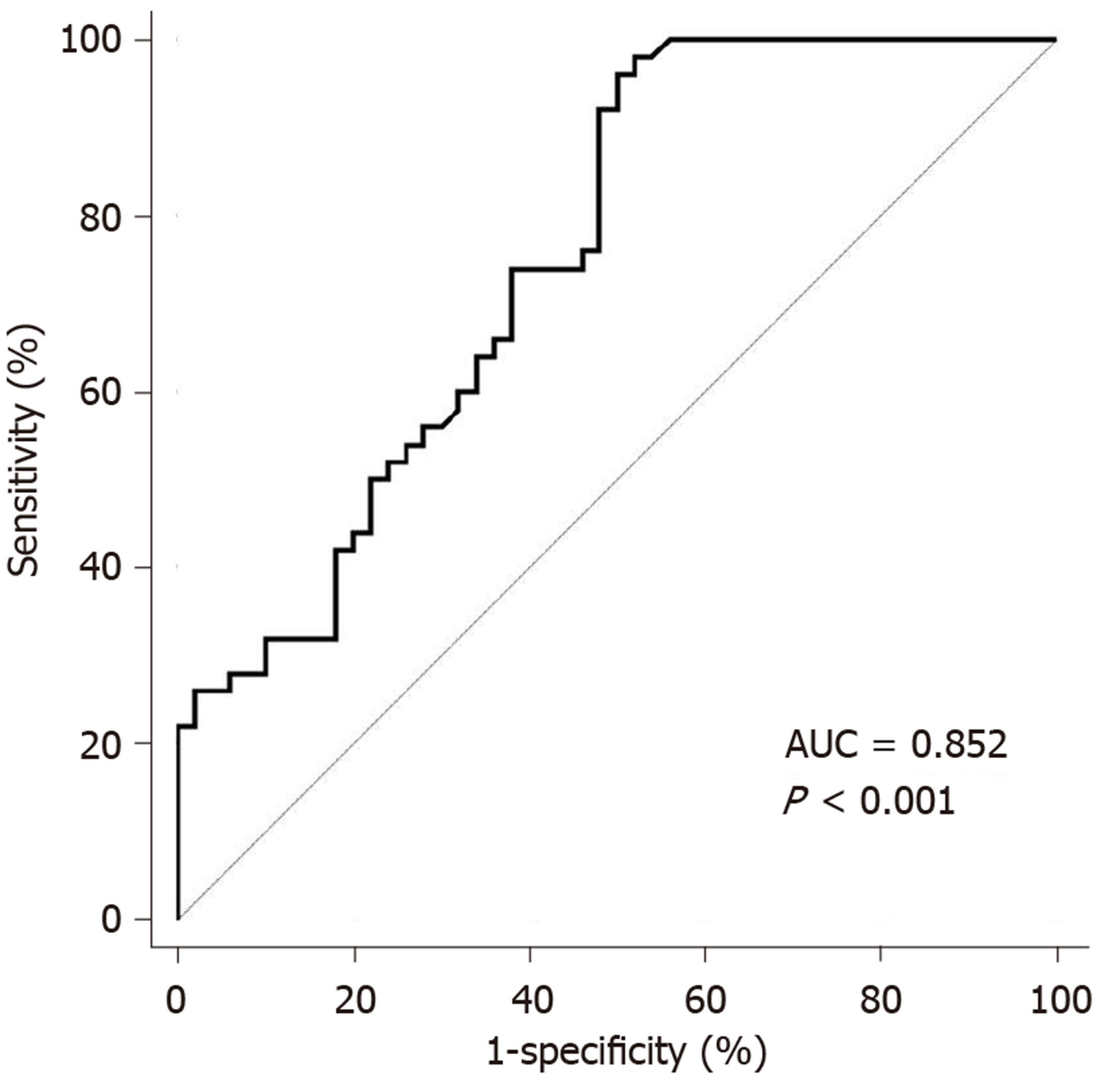
The results showed that the median IL-32 level in these patients was 798.2 (298.5-11693.5) pg/mL, which was then used as the cut-off point to analyze PFS and OS. The log-rank test showed that IL-32 level was associated with both PFS (P = 0.019) and OS (P = 0.035), and the difference was statistically significant. The ROC curve was used to analyze the relationship between the patient's survival status and IL-32 level. The results showed that the area under the curve for IL-32 was 0.752 (95%CI: 0.656-0.833), and the sensitivity and specificity for evaluating the patient's survival status were 88.17% and 67.23%, respectively. The optimal cut-off value was 856.4 pg/mL, which was close to the median value. Thus, IL-32 with a cut point of 856.4 pg/mL was chosen to distinguish the survival rate of patients (Figure 1).
The baseline data of 67 patients are shown in Table 1. MM patients tended to be older and mostly male. More than 50% of patients had high levels of β2-MG and CRP and 8 cases had a blood calcium concentration higher than 2.75 mmol/L, 42 with bone disease and 17 with autologous stem cell transplantation. DS stage was stage I in 7 cases, stage II in 13 cases, and stage III in 47 cases. ISS stage was stage I in 11 cases, stage II in 21 cases and stage III in 35 cases. A total of 23 patients were negative for initial detection of MRD. The patients were divided into two groups according to the IL-32 level, and the baseline data were compared. The results showed that there were no significant differences in gender, age, β2-MG, ALB, CRP, serum calcium, serum creatinine, hemoglobin, LDH and other factors between the two groups (P > 0.05), but the negative conversion rate of MRD was lower in patients with an IL-32 level ≥ 856.4 pg/mL (P < 0.001), as summarized in Table 2.
| Characteristics | All (n = 67) | IL-32 ≥ 856.4 pg/mL (n = 38) | IL-32 < 856.4 pg/mL (n = 29) | P value |
| Gender | 0.448 | |||
| Male | 39 (58.2) | 23 (60.5) | 16 (55.2) | |
| Female | 28 (41.8) | 15 (39.5) | 13 (44.8) | |
| Age (yr), median (Range) | 58 (40-77) | 62 (54-77) | 57 (40-75) | 0.521 |
| β2-MG (mg/L) | 0.378 | |||
| < 3.5 | 30 (44.8) | 16 (42.1) | 14 (48.3) | |
| ≥ 3.5 | 37 (55.2) | 22 (57.9) | 15 (51.7) | |
| LDH (U/L) | 0.174 | |||
| < 245 | 55 (82.1) | 30 (78.9) | 25 (86.2) | |
| ≥ 245 | 12 (17.9) | 8 (21.1) | 4 (13.8) | |
| CRP (mg/L) | 0.900 | |||
| < 8 | 28 (41.8) | 16 (42.1) | 12 (41.4) | |
| ≥ 8 | 39 (58.2) | 22 (57.9) | 17 (58.6) | |
| Serum calcium (mmol/L) | 0.524 | |||
| < 2.75 | 59 (88.1) | 33 (86.8) | 26 (89.7) | |
| ≥ 2.75 | 8 (11.9) | 5 (13.2) | 3 (10.3) | |
| Bone disease | 42 (62.7) | 25 (65.8) | 17 (58.6) | 0.294 |
| ALB (g/L) | 0.210 | |||
| < 35 | 29 (43.3) | 15 (39.5) | 14 (48.3) | |
| ≥ 35 | 38 (56.7) | 23 (60.5) | 15 (51.7) | |
| Creatinine (μmol/L) | 0.528 | |||
| < 176 | 50 (74.6) | 29 (76.3) | 21 (72.4) | |
| ≥ 176 | 17 (25.4) | 9 (23.7) | 8 (27.6) | |
| Induction therapy | 0.790 | |||
| Bortezomib | 54 (80.6) | 32 (84.2) | 24 (82.8) | |
| Thalidomide | 11 (16.4) | 6 (15.8) | 5 (17.2) | |
| Stem cell transplantation | 17 (25.4) | 11 (28.9) | 6 (20.7) | 0.179 |
| M protein type | 0.926 | |||
| Type IgG | 31 (46.3) | 18 (47.4) | 13 (44.8) | |
| Type IgA | 13 (19.4) | 6 (15.8) | 7 (24.1) | |
| Type IgD | 5 (7.5) | 3 (7.9) | 2 (6.9) | |
| Light-chain | 16 (23.9) | 10 (26.3) | 6 (20.7) | |
| Non-secretory | 2 (3.0) | 1 (2.6) | 1 (3.4) | |
| ISS | 0.710 | |||
| I | 11 (16.4) | 5 (13.2) | 6 (20.7) | |
| II | 21 (31.3) | 13 (34.3) | 8 (27.6) | |
| III | 35 (52.2) | 20 (52.6) | 15 (51.7) | |
| DS | 0.797 | |||
| I | 7 (10.4) | 3 (7.9) | 4 (13.8) | |
| II | 13 (19.4) | 8 (21.1) | 5 (17.2) | |
| III | 47 (70.1) | 27 (71.0) | 20 (69.0) |
| Characteristics | All patients (n = 67) | IL-32 ≥ 856.4 pg/mL (n = 38) | IL-32 < 856.4 pg/mL (n = 29) | P value |
| IL-32 (pg/mL), median (Range) | 798.2 (232.1-4214) | 1354.4 (856.4-4214) | 426.7 (232.1-856.4) | < 0.001 |
| MRD negative | 23 (35.9) | 9 (23.7) | 14 (48.3) | < 0.001 |
| MRD positive | 44 (65.7) | 29 (76.3) | 15 (51.7) |
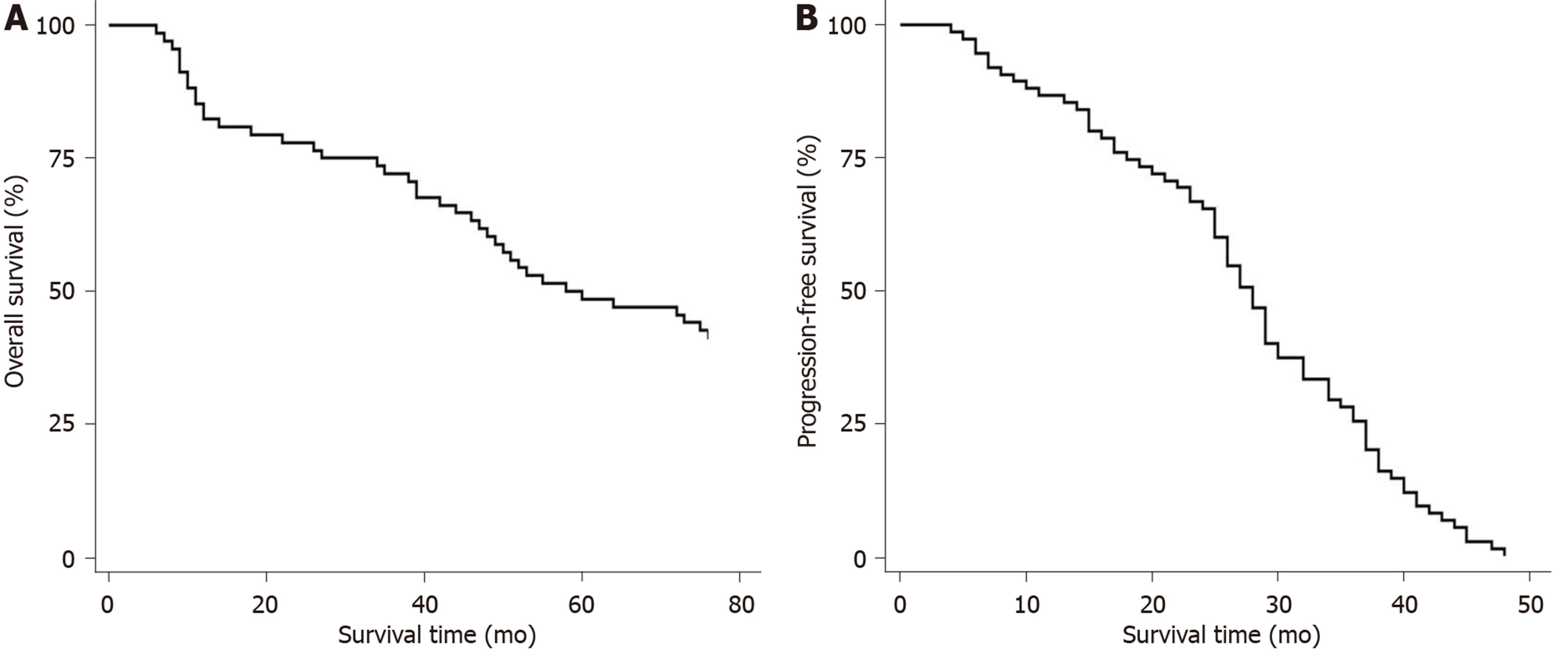
The overall response rate of 67 patients receiving combination chemotherapy was 82%. Of these 55 cases, 22 (32.8%) experienced CR, 24 (35.8%) achieved very good partial remission and 9 (13.4%) achieved partial remission. The median number of effective courses was 1.5 (1-4), and the median time was 45 (22-138) d. The median number of courses for optimal efficacy was 2.5 (1-5), and the median time was 83 (23-230) d. At the end of the follow-up period, 29 patients were alive and 38 had died. The 1-, 3- and 5-year survival rates were 82.1%, 71.6%, and 47.8%, respectively. The OS was 1.0 to 76.0 mo, and the median was 41.0 mo; while the PFS was 1.0 to 62.0 mo, and the median was 24.0 mo (Figure 2). Kaplan-Meier analysis showed that IL-32 level was an important factor affecting PFS and OS in patients with MM. Compared with patients with an IL-32 level ≥ 856.4 pg/mL, patients with an IL-32 level < 856.4 pg/mL had a longer PFS and OS, as shown in Figure 3.
Factors that may affect patients' PFS and OS, including age, gender, IL-32 level, creatinine level, ISS stage, bone disease, stem cell transplantation and MRD were entered into the Cox regression risk model. Univariate analysis showed that an IL-32 level ≥ 856.4 pg/mL, ISS and DS stage (II/III), and MRD positive were associated with a shorter OS. The factors related to shorter PFS were an IL-32 level ≥ 856.4 pg/mL, ISS stage (II/III) and MRD positive (Table 3). Multivariate analysis showed that the independent risk factors for OS and PFS were an IL-32 level ≥ 856.4 pg/mL and MRD positive, while ISS and DS staging had little effect (P > 0.05), as shown in Table 4.
| Variables | Overall survival | Progression-free survival | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age (yr) | < 65/≥ 65 | 1.34 | 0.63-2.83 | 0.442 | 1.21 | 0.42-3.55 | 0.723 |
| Sex | Male/Female | 0.96 | 0.48-.94 | 0.917 | 0.99 | 0.55-1.79 | 0.989 |
| IL-32 (pg/mL) | < 856.4/≥ 856.4 | 4.21 | 1.25-4.1 | 0.023 | 4.80 | 1.36-16.93 | 0.015 |
| Creatinine (μmol/L) | < 176/≥ 176 | 2.11 | 0.50-8.88 | 0.308 | 1.1 | 0.08-14.67 | 0.941 |
| ISS | I/II/III | 1.71 | 1.02-2.87 | 0.042 | 1.74 | 1.09-2.79 | 0.021 |
| DS | I/II/III | 4.19 | 0.83-21.21 | 0.083 | 2.03 | 0.87-4.73 | 0.102 |
| Bone disease | No/Yes | 1.31 | 0.85-2.00 | 0.228 | 1.38 | 0.66-2.87 | 0.388 |
| Stem cell transplantation | No/Yes | 1.05 | 0.75-1.48 | 0.773 | 1.08 | 0.76-1.53 | 0.674 |
| MRD | Negative/Positive | 3.64 | 1.06-12.5 | 0.044 | 3.06 | 1.13-8.30 | 0.028 |
| Variables | Overall survival | Progression-free survival | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| IL-32 (pg/mL) | < 856.4/≥ 856.4 | 1.77 | 1.05-2.99 | 0.032 | 1.67 | 1.09-2.56 | 0.023 |
| ISS | I/II/III | 1.01 | 0.94-1.10 | 0.764 | 1.33 | 0.30-5.97 | 0.711 |
| DS | I/II/III | 1.5 | 0.58-3.91 | 0.407 | 1.02 | 0.99-1.06 | 0.242 |
| MRD | Negative/Positive | 3.64 | 1.06-12.5 | 0.044 | 1.68 | 1.20-2.35 | 0.003 |
MM is a malignant plasma disease closely associated with inflammation. Bone marrow stromal cells located in the MM bone marrow microenvironment secrete IL-6 and induce proliferation of MM cells[9,12]. IL-6 is one of the essential cytokines for the production of immunoglobulin by B lymphocytes, and is also an important growth factor for human myeloma cells. During the development of MM, IL-6 together with interleukin-1β and tumor necrosis factor promote the progression of osteolytic lesions[13-15]. Kim[16] found that IL-32 was involved in the formation of IL-6 and the secretion of various inflammatory factors, and has pro-inflammatory effects, suggesting that IL-32 may play an important role in the formation of MM, with the potential to assess MM prognosis. Studies have reported the relationship between IL-32 level and cancer-related diseases, but the effect of IL-32 in hematological malignancies is still controversial. IL-32 is significantly increased in patients with myelodysplastic syndrome but decreased in patients with chronic myelomonocytic leukemia[10]. In a previous study, we examined the expression levels of IL-32 in the bone marrow of healthy individuals and MM patients, and the results showed that MM patients had higher IL-32 expression than healthy individuals[11]. In this study, our analysis revealed that patients with lower IL-32 levels had longer PFS and OS. This provides new prognostic information that can be used to further improve the current risk stratification strategy for MM patients. Kwon et al[17] reported that poor prognosis in patients with inflammatory diseases and autoimmune diseases had higher IL-32 expression. In a separate study, high expression of IL-32 was also found to be positively associated with metastasis and recurrence of renal cell carcinoma and gastric cancer[18]. Given the commonality of MM with autoimmune diseases and malignant tumors, it is speculated that the high expression of IL-32 in MM cells may contribute to the evolution of malignant plasma cells, which is consistent with our results.
Additionally, our previous studies examined the role and mechanism of IL-32 in the inflammatory cytokine network and MM cell proliferation, and showed that IL-32 derived from MM cells may increase IL-6 expression in bone marrow stromal cells by activating the nuclear factor kappa-B and STAT3 inflammatory signaling pathways to support MM cells through feedback, leading to the proliferation and growth of MM cells[11]. This explains the relationship between IL-32 and MM from a biological perspective and further confirms our results.
Drug resistance and relapse are still fundamental problems in MM treatment, and the direct cause of recurrence is the presence of MRD[19-21]. Studies of MRD have shown that it has the ability to indicate the prognosis of patients, and its application prospects in MM treatment and prognosis are gradually being investigated. Furthermore, several retrospective studies have demonstrated the value of MRD in predicting early MM recurrence and assessing patient outcomes, it is expected to guide patient follow-up[22,23]. A recent study by Gu et al[24] showed that patients in CR with persistently positive MRD have a higher early recurrence rate and significantly shorter PFS and OS, as compared with CR patients with negative MRD. Another study on MM prognosis also showed that the effect of MRD on PFS and OS in patients is irrelevant in terms of disease stage, induction treatment plan, cytogenetic risk stratification and routine CR, which is significantly better than the traditional efficacy evaluation system[25]. In this study, it was found that MRD-positive and an IL-32 level ≥ 856.4 pg/mL were risk factors for prognosis in patients, and there were significant differences in high and low IL-32 levels, suggesting that MRD and IL-32 level have certain clinical application value and can help physicians identify resistant myeloma cells in CR patients as early as possible, providing more effective and sensitive treatment.
MFC is suitable for approximately 97% of patients and is widely used in laboratory testing. Although various techniques to detect MRD have been developed, there is marked discrepancy in the results, making comparisons difficult. It is thought that the differences in sensitivity, research populations and timing of MRD detection are the main problems. Generally, the MRD conversion rate varies from 11% to 77% in different scenarios[26-28]. MRD with a sensitivity of 10-4 was used to detect MRD in our research and all patients after induction therapy were enrolled for analysis. The initial conversion rate was 35.9%, which was consistent with the results of Gupta et al[29]. It is worth mentioning that potential MM tumor stem cells expressing a naive immune phenotype may be neglected in the MFC detection, affecting the stability of results[30-32]. For the various reasons given above, its clinical application is limited. Due to the convenience and maturity of the measurement procedure, IL-32 can compensate for the disadvantage of MRD detection. Therefore, besides placing more emphasis on standardizing MRD detection, it is significant to note that IL-32 level can be used to improve the sensitivity and specificity of prediction.
The limitations of this study are the presence of certain restrictions in the study sample and research, the statistical analysis of data may be biased and these limitations require further investigation to improve the results.
In conclusion, as a useful supplement to MRD, IL-32 level has good predictive ability in evaluating the outcome of MM, with the potential to be widely used for monitoring prognosis.
Multiple myeloma (MM) is a malignant disease in which clonal plasma cells proliferate abnormally, and ranks second in common malignant tumors of the blood system. It often occurs in the elderly and is still incurable. Studies have found that the development of MM is closely related to the secretion of interleukin-6 (IL-6) by bone marrow stromal cells. As a pro-inflammatory factor, IL-32 can increase the secretion of inflammatory factors such as IL-6, IL-1β and tumor necrosis factor, thus inducing an inflammation cascade amplification effect, which suggests that it also plays an important role in MM.
MM has obvious biological and clinical heterogeneity, which results in marked differences in efficacy and prognosis. National and international scholars have proposed a series of new staging systems and related indicators, but the ability of a single factor to predict prognosis was unsatisfactory, and there are many other prognostic factors being investigated. This suggests that the prognosis analysis of MM should be updated from time to time. Several studies have shown that MM is closely associated with inflammation, and the change in IL-32 level indicates the progress of the patient's condition. Furthermore, minimal residual disease (MRD) has also been confirmed to predict prognosis. However, the relationship between the above indicators and development of the disease is not clear, and their ability to assess the prognosis and survival of patients requires further research.
We conducted a follow-up and prognostic analysis of 67 patients with MM diagnosed in our hospital from 2012 to 2017, and analyzed the relationship between both IL-32 level and MRD and prognosis of the disease, in order to identify the relevant factors and the applicable prognostic indicators.
The clinical data of 67 primary MM patients from 2012 to 2017 were collected and grouped according to the patient's IL-32 level using receiver operating characteristic curve. The baseline data of the patients were analyzed, and the follow-up outcomes and treatment effects in the two groups were compared. Cox regression was used to perform univariate and multivariate prognostic analysis, and further determine the factors affecting the prognosis of patients.
The cutoff value of IL-32 equal to 856.4 pg/mL significantly impacted the OS and PFS of patients. According to the IL-32 level, 38 patients were classified in the IL-32 ≥ 856.4 group and 29 in the IL-32 < 856.4, with a 3-year overall survival (OS) rate of 65.8% and 80%, respectively. The results showed that when the IL-32 level was < 856.4, OS and progression-free survival (PFS) were significantly better than those in patients with an IL-32 level ≥ 856.4. In multivariate analysis, IL-32 ≥ 856.4 and MRD positive were risk factors for poor prognosis.
Different cutoff levels of IL-32 may have different effects on the prognoses of patients with newly diagnosed MM, which is valuable for assessing MM prognosis. IL-32 level and MRD could better predict OS and PFS in unselected nonclinical trial myeloma patients.
In recent years, with the wide application of targeted new drugs and autologous hematopoietic stem cell transplantation, the prognosis of MM patients has greatly improved, but the prognosis and outcome varies greatly in different patients. The International Staging System has not fully met the current clinical needs during the new drug period. Therefore, this study analyzed several factors that may influence the prognosis of patients with MM, especially IL-32, a pro-inflammatory factor closely related to the development of MM. The results confirmed the clinical value of IL-32 and MRD in evaluating the prognosis of patients with MM.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dustin LB, Yuki S S-Editor: Wang JL L-Editor: Webster JR E-Editor: Liu JH
| 1. | Anderson KC. Progress and Paradigms in Multiple Myeloma. Clin Cancer Res. 2016;22:5419-5427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Rajkumar SV. Evolving diagnostic criteria for multiple myeloma. Hematology Am Soc Hematol Educ Program. 2015;2015:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Landgren O, Rajkumar SV. New Developments in Diagnosis, Prognosis, and Assessment of Response in Multiple Myeloma. Clin Cancer Res. 2016;22:5428-5433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Bailly C, Leforestier R, Jamet B, Carlier T, Bourgeois M, Guérard F, Touzeau C, Moreau P, Chérel M, Kraeber-Bodéré F, Bodet-Milin C. PET Imaging for Initial Staging and Therapy Assessment in Multiple Myeloma Patients. Int J Mol Sci. 2017;18:E445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Flores-Montero J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, Sanoja L, Puig N, Lecrevisse Q, Vidriales MB, van Dongen JJ, Orfao A. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016;90:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Anderson KC, Auclair D, Kelloff GJ, Sigman CC, Avet-Loiseau H, Farrell AT, Gormley NJ, Kumar SK, Landgren O, Munshi NC, Cavo M, Davies FE, Di Bacco A, Dickey JS, Gutman SI, Higley HR, Hussein MA, Jessup JM, Kirsch IR, Little RF, Loberg RD, Lohr JG, Mukundan L, Omel JL, Pugh TJ, Reaman GH, Robbins MD, Sasser AK, Valente N, Zamagni E. The Role of Minimal Residual Disease Testing in Myeloma Treatment Selection and Drug Development: Current Value and Future Applications. Clin Cancer Res. 2017;23:3980-3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Galtseva IV, Davydova YO, Kapranov NM, Julhakyan HL, Mendeleeva LP. Minimal residual disease in multiple myeloma: Benefits of flow cytometry. Int J Lab Hematol. 2018;40:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kusenda J, Kovarikova A. Multiple myeloma, immunotherapy and minimal residual disease. Neoplasma. 2016;63:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Li Y, Du Z, Wang X, Wang G, Li W. Association of IL-6 Promoter and Receptor Polymorphisms with Multiple Myeloma Risk: A Systematic Review and Meta-Analysis. Genet Test Mol Biomarkers. 2016;20:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105:2865-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Lin X, Yang L, Wang G, Zi F, Yan H, Guo X, Chen J, Chen Q, Huang X, Li Y, Zhang E, Wu W, Yang Y, He D, He J, Cai Z. Interleukin-32α promotes the proliferation of multiple myeloma cells by inducing production of IL-6 in bone marrow stromal cells. Oncotarget. 2017;8:92841-92854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Victor M, Evgeniy H, Gergana T, Julia P, Vasil V, Borislav M, Ivo B, Zlatina G, Kamen T. Serum Hepcidin Levels in Multiple Myeloma. Clin Lab. 2017;63:1273-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Chakraborty B, Vishnoi G, Gowda SH, Goswami B. Interleukin-6 gene-174 G/C promoter polymorphism and its association with clinical profile of patients with multiple myeloma. Asia Pac J Clin Oncol. 2017;13:e402-e407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Bakkus MH, Brakel-Van Peer KM, Adriaansen HJ, Van Den Akker TW, Benner R. Detection of Interleukin-1β and Interleukin-6 in Human Multiple Myeloma by Fluorescent in situ Hybridization. Leuk Lymphoma. 1991;4:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Sanacora S, Chang TP, Vancurova I. Chromatin immunoprecipitation analysis of bortezomib-mediated inhibition of NFκB recruitment to IL-1β and TNFα gene promoters in human macrophages. Methods Mol Biol. 2014;1172:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kim S. Interleukin-32 in inflammatory autoimmune diseases. Immune Netw. 2014;14:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Kwon OC, Kim S, Hong S, Lee CK, Yoo B, Chang EJ, Kim YG. Role of IL-32 Gamma on Bone Metabolism in Autoimmune Arthritis. Immune Netw. 2018;18:e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Khawar MB, Abbasi MH, Sheikh N. IL-32: A Novel Pluripotent Inflammatory Interleukin, towards Gastric Inflammation, Gastric Cancer, and Chronic Rhino Sinusitis. Mediators Inflamm. 2016;2016:8413768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, Sherrington P, Samur MK, Georgieva A, Anderson KC, Gregory WM. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017;3:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 405] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 20. | Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2013;4:2186-2207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Hari P. Recent advances in understanding multiple myeloma. Hematol Oncol Stem Cell Ther. 2017;10:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Nishihori T, Song J, Shain KH. Minimal Residual Disease Assessment in the Context of Multiple Myeloma Treatment. Curr Hematol Malig Rep. 2016;11:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Takamatsu H, Takezako N, Zheng J, Moorhead M, Carlton VEH, Kong KA, Murata R, Ito S, Miyamoto T, Yokoyama K, Matsue K, Sato T, Kurokawa T, Yagi H, Terasaki Y, Ohata K, Matsumoto M, Yoshida T, Faham M, Nakao S. Prognostic value of sequencing-based minimal residual disease detection in patients with multiple myeloma who underwent autologous stem-cell transplantation. Ann Oncol. 2017;28:2503-2510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Gu J, Liu J, Chen M, Huang B, Li J. Longitudinal Flow Cytometry Identified "Minimal Residual Disease" (MRD) Evolution Patterns for Predicting the Prognosis of Patients with Transplant-Eligible Multiple Myeloma. Biol Blood Marrow Transplant. 2018;24:2568-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Berger N, Kim-Schulze S, Parekh S. Minimal Residual Disease in Multiple Myeloma: Impact on Response Assessment, Prognosis and Tumor Heterogeneity. Adv Exp Med Biol. 2018;1100:141-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Takamatsu H. Comparison of Minimal Residual Disease Detection by Multiparameter Flow Cytometry, ASO-qPCR, Droplet Digital PCR, and Deep Sequencing in Patients with Multiple Myeloma Who Underwent Autologous Stem Cell Transplantation. J Clin Med. 2017;6:E91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, García-Sánchez O, Böttcher S, van der Velden VHJ, Pérez-Morán JJ, Vidriales MB, García-Sanz R, Jimenez C, González M, Martínez-López J, Corral-Mateos A, Grigore GE, Fluxá R, Pontes R, Caetano J, Sedek L, Del Cañizo MC, Bladé J, Lahuerta JJ, Aguilar C, Bárez A, García-Mateo A, Labrador J, Leoz P, Aguilera-Sanz C, San-Miguel J, Mateos MV, Durie B, van Dongen JJM, Orfao A. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094-2103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 474] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 28. | Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 29. | Gupta R, Kumar L, Dahiya M, Mathur N, Harish P, Sharma A, Sharma OD, Shekhar V. Minimal residual disease evaluation in autologous stem cell transplantation recipients with multiple myeloma. Leuk Lymphoma. 2017;58:1234-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Rawstron AC, Gregory WM, de Tute RM, Davies FE, Bell SE, Drayson MT, Cook G, Jackson GH, Morgan GJ, Child JA, Owen RG. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125:1932-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 31. | Sasaki M. Current treatment of refractory and relapsed multiple myeloma. Rinsho Ketsueki. 2016;57:2084-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Guo J, Zhao Y, Fei C, Zhao S, Zheng Q, Su J, Wu D, Li X, Chang C. Dicer1 downregulation by multiple myeloma cells promotes the senescence and tumor-supporting capacity and decreases the differentiation potential of mesenchymal stem cells. Cell Death Dis. 2018;9:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |