Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4098
Peer-review started: June 26, 2019
First decision: November 12, 2019
Revised: November 17, 2019
Accepted: November 20, 2019
Article in press: November 20, 2019
Published online: December 6, 2019
Processing time: 169 Days and 23.3 Hours
Duchenne muscular dystrophy (DMD), which is caused by a mutation/deletion in the dystrophin gene on the X-chromosome, is the most common type of neuromuscular disorder in pediatrics. Skeletal muscle weakness progressively develops in DMD patients and usually leads to respiratory failure in the early adolescent years. Cardiac muscle is frequently affected in DMD patients, which leads to a high burden of cardiomyopathy and heart failure. In the era of improved respiratory care, cardiac deaths are becoming the major cause of mortality in DMD patients.
We report the case of a 15-year-old boy who presented to the hospital due to recurrent orthopnea for 6 mo and palpitations for 4 mo. He was diagnosed with progressive muscular dystrophy at the age of 3 years and was confined to a wheelchair at 12 years. He was prescribed diuretics and digoxin at the outpatient clinic; however, his symptoms did not resolve. Sacubitril/valsartan was added 1 mo prior to presentation, but he experienced recurrent episodes of palpitations. The electrocardiogram showed atrial tachycardia with a heart rate of 201 bpm, and he was then hospitalized. Hypotension was found following the administration of sacubitril/valsartan tablets; he could not tolerate even a small dose, always developing tachyarrhythmia. His symptoms were relieved after discontinuing sacubitril/valsartan, and his heart rate was controlled by a small dose of metoprolol tartrate and digoxin. Atrial tachycardia spontaneously converted in this patient, and his symptoms attenuated in the following 6 mo, without palpitation episodes.
Blood pressure should be closely monitored in DMD patients with advanced heart failure when taking sacubitril/valsartan.
Core tip: A 15-year-old boy with Duchenne muscular dystrophy presented to the hospital due to recurrent orthopnea for 6 mo. He was diagnosed with heart failure and was prescribed oral diuretics and digoxin at the outpatient clinic, but his symptoms did not resolve. Sacubitril/valsartan was added to his therapeutic regimen 1 mo before presentation, but resulted in recurrent hypotension and palpitation episodes until discontinuation of this medication. Although sacubitril/valsartan has been shown to be beneficial for heart failure with a reduced ejection fraction, hypotension is a common side effect of this medication, and blood pressure should be closely monitored in Duchenne muscular dystrophy patients with advanced heart failure.
- Citation: Li JM, Chen H. Recurrent hypotension induced by sacubitril/valsartan in cardiomyopathy secondary to Duchenne muscular dystrophy: A case report. World J Clin Cases 2019; 7(23): 4098-4105
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4098.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4098
Duchenne muscular dystrophy (DMD) is the most common congenital neuromuscular disorder in children. As an X-linked recessive disorder, it is caused by a lack of dystrophin, due to a mutation or deletion of one or more exons in the X-chromosome. The prevalence of DMD in newborns ranges from 1.59 to 1.95 in every 10000 live births[1]. Children suffering from DMD usually present with myasthenia, then gradually lose their motor function, and finally develop respiratory failure. In addition to respiratory complications, cardiac muscles can also be affected in DMD patients, leading to a high prevalence of cardiomyopathy and heart failure in this population. Cardiac enlargement and diverse arrhythmia (mostly supraventricular arrhythmia) are frequently found in DMD patients[2,3].
Renin-angiotensin system (RAS) inhibitors, beta-adrenergic blockers (β-blockers) and mineralocorticoid receptor antagonists (MRAs) were considered the cornerstone of heart failure treatment until recently, when their use was rivaled by angiotensin receptor-neprilysin inhibitors (ARNIs)[4]. Sacubitril/valsartan is the first available ARNI on the market, and it inhibits neprilysin, elevates natriuretic peptide levels, dilates blood vessels and promotes urinary sodium excretion. It has been proven to be a potent medication in improving the cardiac performance and prognosis of heart failure patients with reduced ejection fraction[5]. The PARADIGM-HF trial included 8442 heart failure patients with New York Heart Association (NYHA) class II-IV disease, comparing sacubitril/valsartan with the angiotensin-converting enzyme inhibitor (ACEI) enalapril and demonstrated that ARNI reduced mortality and hospitalization more than ACEI in heart failure with reduced ejection fraction (HFrEF) patients[6]. The 2016 AHA/ACC guidelines state that ARNI is recommended for patients with chronic HFrEF to reduce morbidity and mortality[6], and it is also recommended by the European Society of Cardiology with class I recommendation and level B evidence[7]. Nevertheless, the application of ARNI in NYHA class IV patients remains controversial as only 60 patients with baseline NYHA class IV were included in the PARADIGM-HF trial. Severe hypotension/cardiogenic shock was reported in a heart failure patient with NYHA class IV after initiation of ARNI[8]; however, improvements in the NYHA functional class and 6-min walking test were also found in NYHA class IV patients taking ARNI[9,10]. Here, we report a boy with DMD and NYHA class IV disease who suffered recurrent hypotension after taking sacubitril/valsartan.
A 15-year-old boy presented to the Emergency Department of our hospital complaining of recurrent orthopnea and palpitations.
The patient started to experience orthopnea 6 mo prior to presentation. The orthopnea episodes recurred with increased frequency, and he started to have palpitation episodes 4 mo prior to presentation. He was prescribed oral diuretics and digoxin at the outpatient clinic, although his symptoms did not resolve. Sacubitril/valsartan was added to his therapeutic regimen 1 mo before presentation; however, the palpitations and orthopnea episodes became even more frequent. Six hours prior to presentation, he experienced persistent palpitations and shortness of breath.
At the age of 3 years, the patient was found to have significantly elevated serum aspartate aminotransferase (AST) and creatine kinase (CK) levels when he had a check-up for an unknown fever. His peak serum CK level was > 20000 U/L, and gastrocnemius biopsy revealed swelling and dystrophy of the skeletal muscle, with partial replacement of muscle by fat and fibrotic tissue. A clinical diagnosis of muscular dystrophy was established after genetic testing of dystrophin showed a deficiency at exons 48, 49, 50 and 51. He was regularly followed at the children’s hospital and treated with nutrients and steroids; however, the medication did not alter his disease progression. He had difficulty running and developed toe walking 5 years prior to presentation and required hand assistance to push himself into an upright position when arising from the floor (termed the Gower’s sign) 4 years previously. In the past 3 years, he has been completely confined to a wheelchair. Although lasting for no more than 2 min, he experienced chest tightness several times in the past 3 years, especially when he developed a cold or became tired.
The patient’s mother gave birth to him at the age of 36 years. The patient learned to walk at 15 mo, and his language development was consistent with his peers. The patient’s mother was proven to be the carrier of the dystrophin gene defects.
The patient’s body temperature was 37.4°C, heart rate was 202 bpm, respiratory rate was 19 breaths per minute and blood pressure was 97/78 mmHg. The breath sounds were normal, and no wheeze or rales were heard in both lungs. The heart rhythm was irregular, and no obvious murmurs were auscultated. On percussion, the cardiac borders were enlarged on both sides. The muscle strength of the upper extremities was grade 4 at the distal part and grade 2 at the proximal part, while the muscle strength of the lower extremities was grade 0, with apparent gastrocnemius hypertrophy.
The patient’s laboratory findings revealed a serum CK level of 3374 U/L, CK-MB level of 75 U/L and cardiac troponin I (cTnI) level of 0.168 ng/mL. The alanine aminotransferase level was 245 U/L, the AST level was 170 U/L, and the creatinine level was 16 μmol/L.
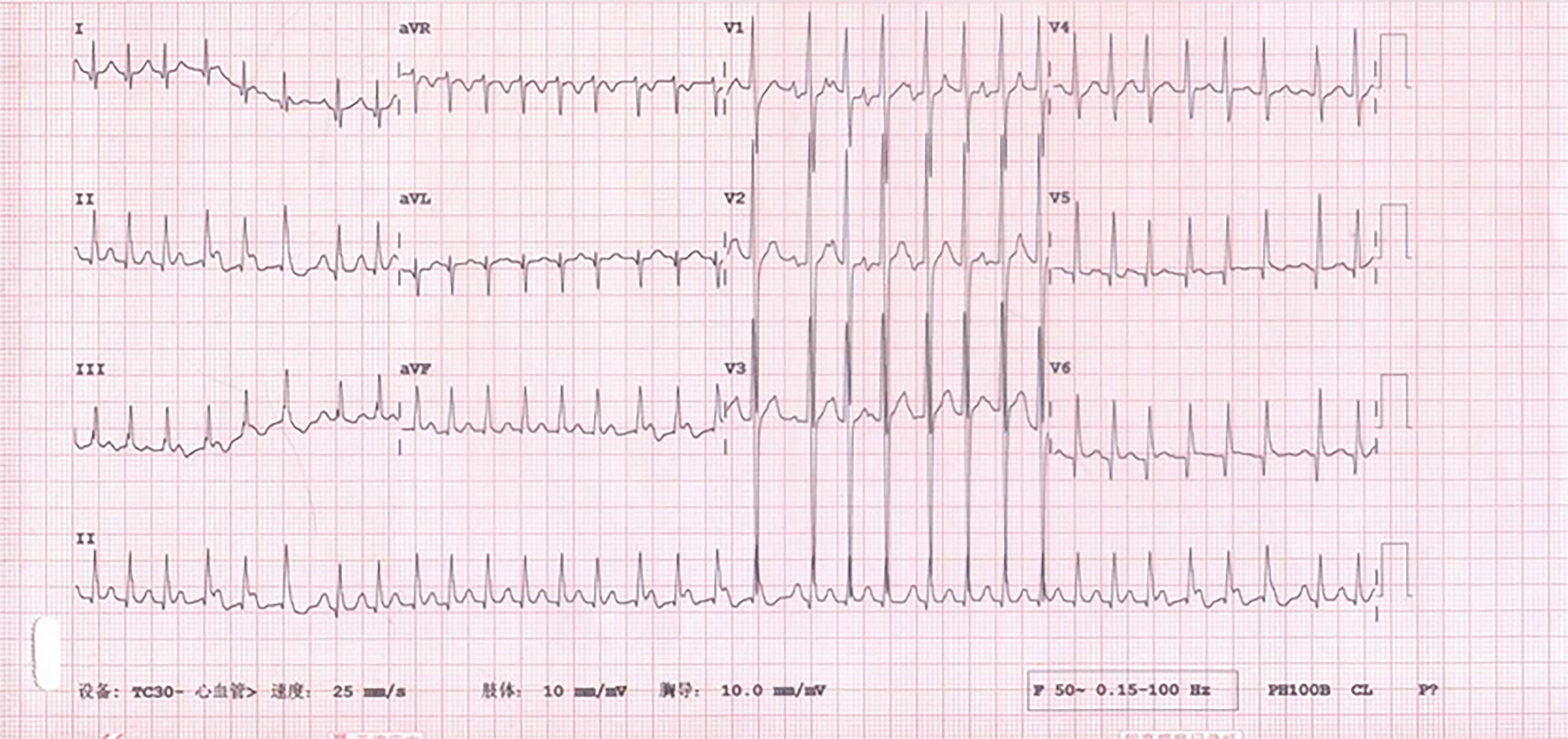
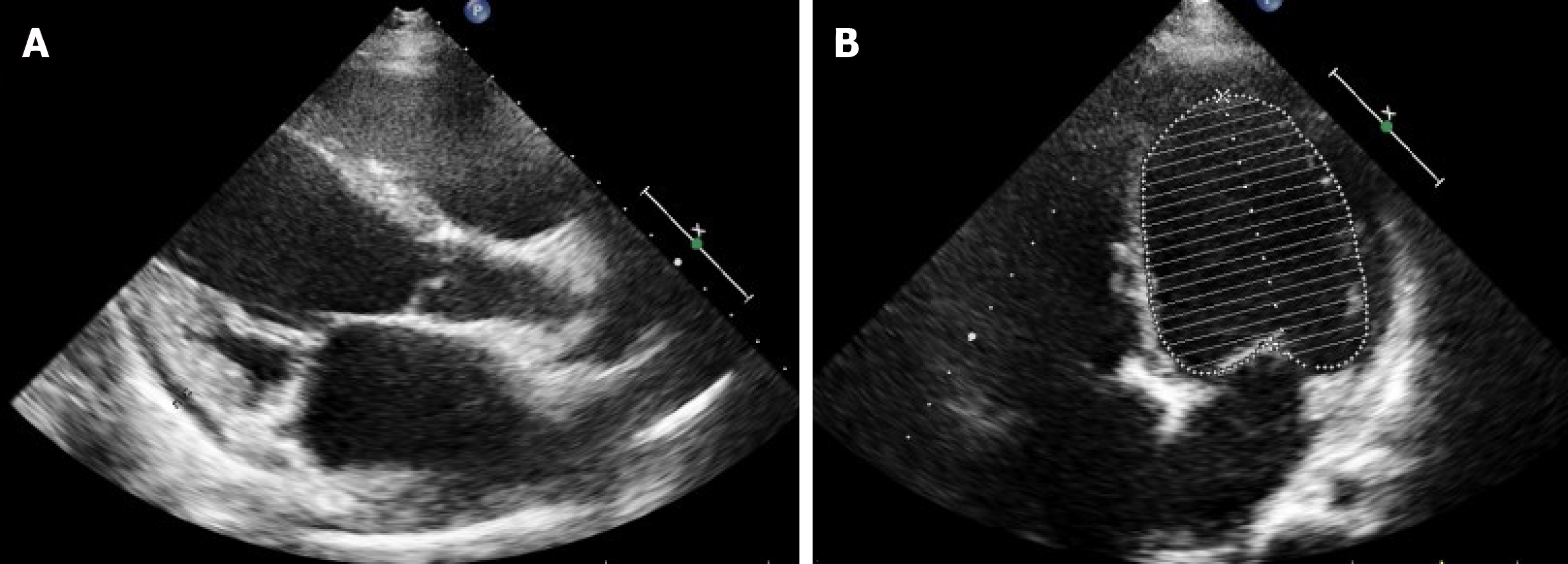
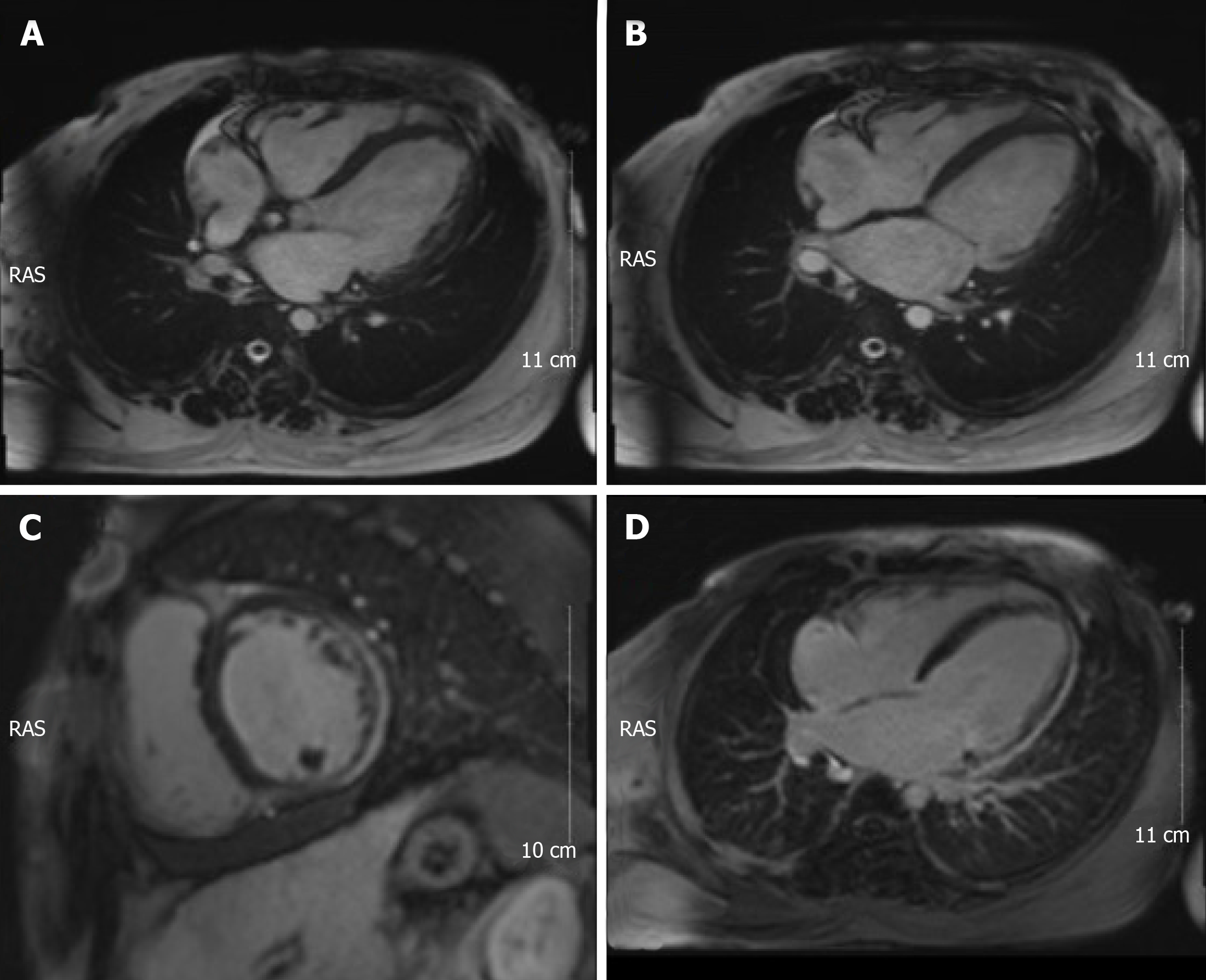
Electrocardiograms recorded in the Emergency Department revealed a fast heart rate (Figure 1) in the form of atrial tachycardia with Wenckebach block, and the patient was then hospitalized for further observation and treatment. Echocardiogram (Figure 2) and cardiac magnetic resonance images (Figure 3) are shown below.
The final diagnosis of the presented case was DMD, dilated cardiomyopathy, NYHA class IV disease, and atrial tachycardia with Wenckebach block.
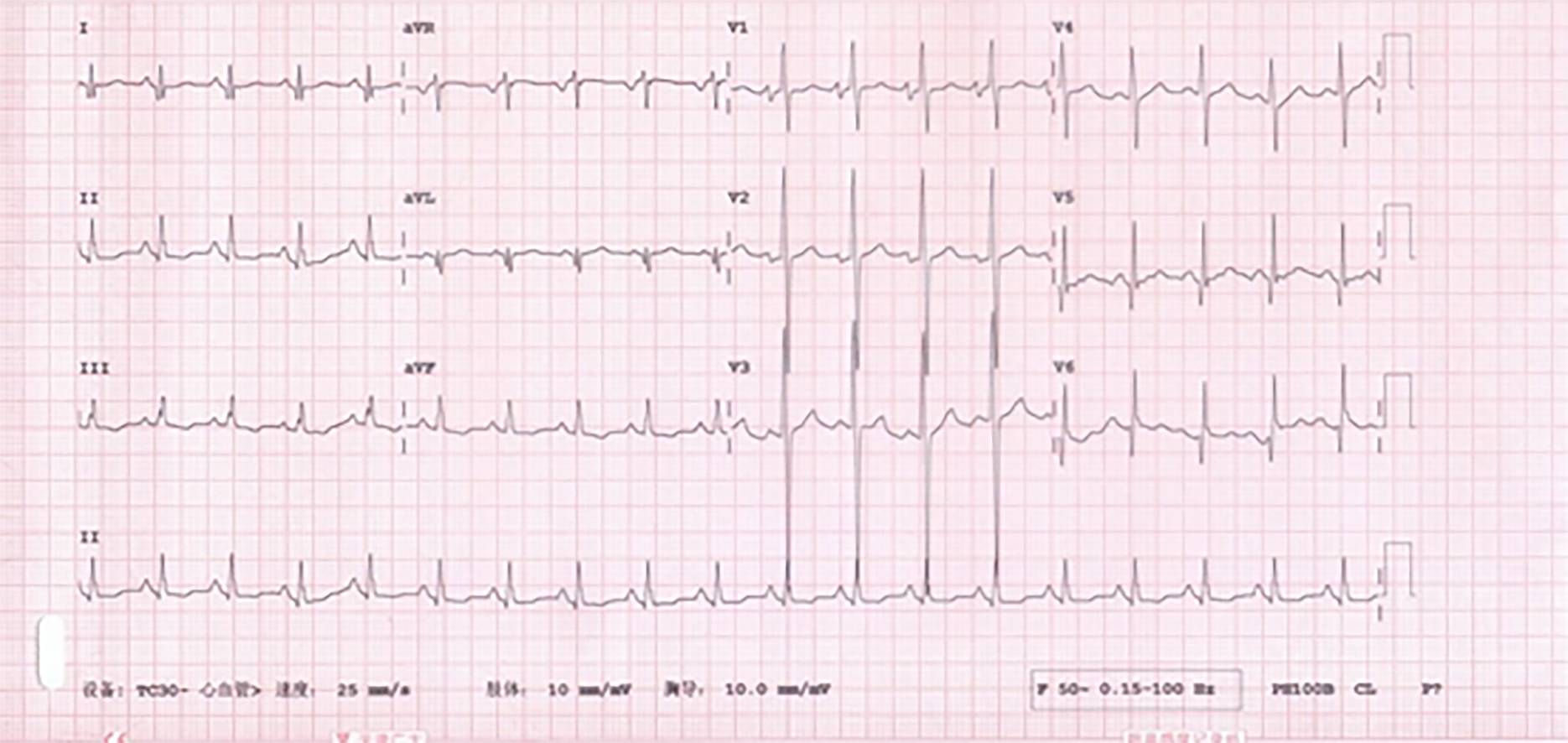
Sacubitril/valsartan was stopped due to recurrent hypotension. Intravenous cedilanid (lanatoside C) 0.2 mg and oral metoprolol tartrate 6.25 mg twice daily were introduced to control the ventricular rate. Within 2 h of admission, the patient felt relief of his symptoms, and an electrocardiogram showed that he was in sinus rhythm (Figure 4) with a heart rate of 113 bpm. The dosage of metoprolol tartrate was increased to 6.25 mg every 8 h for better rate control. However, the patient was intolerant of metoprolol tartrate due to hypotensive episodes. Oral administration of amiodarone 0.2 g once daily controlled the heart rhythm.
The patient was observed in the hospital for 5 d and maintained sinus rhythm before discharge. He was still intolerant of sacubitril/valsartan and metoprolol tartrate after several attempts, ending in hypotension. He remained in sinus rhythm and was free from orthopnea and palpitations at the 6-month follow-up, with oral administration of amiodarone, which had been reduced to 0.1 g once daily 2 mo after discharge.
Dystrophin, a protein encoded by the dystrophin gene on chromosome Xp21.1, is located in skeletal muscle and cardiac muscle cells and connects membrane elements with the cytoskeleton[11]. A deficiency of dystrophin leads to DMD and Becker muscular dystrophy, as well as X-linked dilated cardiomyopathy[12,13]. Although the distribution and regulatory mechanism of dystrophin have been extensively studied in skeletal muscle cells, the physiological role of dystrophin in cardiac muscle has not been fully elucidated. Clinical observation has revealed a high incidence of cardiac involvement in patients with muscular dystrophy. Dilated cardiomyopathy, arrhythmias and congestive heart failure are commonly found in DMD patients, and end-stage heart failure is becoming the main cause of death in the DMD population[14,15]. Early diagnosis of cardiac impairment is challenging in these patients because they usually lose their ambulatory capacity in the early adolescent years, and symptoms of cardiac insufficiency such as shortness of breath might also be mixed with symptoms of respiratory failure, which makes the early detection of cardiac dysfunction even more difficult.
RAS inhibitors, including ACEI and angiotensin receptor blockers (ARBs), β-blockers and MRAs, have been proven to reverse left ventricular remodeling and improve the outcome of patients with systolic heart failure. However, there are limited data on the effects of these medications in DMD patients with heart failure. Ogata et al[16] studied the combination of ACEI and β-blockers in a cohort of 52 DMD patients who had begun treatment for heart failure, and these medications showed beneficial effects on the long-term survival of DMD patients with left ventricular dysfunction[16]. However, Blain et al tested the combination of ACEI and β-blockers in an mdx mouse model of DMD and found that these medications did not confer any advantage in DMD cardiomyopathy. El-Aloul et al[17] summarized the studies on pharmacological therapy for the prevention and management of cardiomyopathy in DMD patients and found modest improvements in LV systolic dysfunction on either monotherapy or combination therapy with ACEI, ARB or β-blockers. However, data regarding the optimal pharmacological regimens for delaying the onset of cardiomyopathy in DMD patients and when to initiate therapy to prevent or treat heart failure are largely insufficient and lacking[17].
The ARNI sacubitril/valsartan, which has been tested in the PARADIGM-HF Trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure), is the state-of-the-art medication for heart failure treatment[18]. Sacubitril/valsartan is the only available ARNI on the market. The substantial improvement by ARNI compared to ACEI, in addition to optimal treatment, results in a class I guideline recommendation for patients with chronic HFrEF to reduce morbidity and mortality[6]. The idea of administering ARNIs in DMD patients with heart failure seems appealing, but we must consider that teenagers under 18 years and patients with symptomatic hypotension were excluded from the PARADIGM-HF Trial, which might be the usual case among the DMD population. In addition, patients with NYHA class II accounted for approximately 70% of all the participants in the PARADIGM-HF Trial, while patients with NYHA class IV accounted for less than 1% of those in the PARADIGM-HF Trial, and most of the DMD patients were not suitable for cardiac function evaluation according to the NYHA criteria based on their loss of ambulation. Although the guidelines of the United States and Europe recommend the administration of sacubitril/valsartan in NYHA class II-IV patients, analysis of real-world eligibility data suggests that only 20%-40% of HFrEF patients will be eligible for sacubitril/valsartan initiation based on current guidelines[19]. ARNIs inhibit the RAS and enhance the activity of natriuretic peptides and bradykinin, thus demonstrating an effect on lowering blood pressure[20]. As predicted, hypotension was more prevalent in patients with sacubitril/valsartan than with enalapril in the PARADIGM-HF Trial[21]. During the run-in stage of the PARADIGM-HF Trial, baseline low blood pressure, low estimated glomerular filtration rate, and higher N-terminal pro-brain natriuretic peptide levels were associated with a higher risk for noncompletion. However, it was reported that patients with lower systolic blood pressure (SBP) at randomization were at higher risk but generally tolerated sacubitril/valsartan and had the same relative benefit over enalapril as patients with higher baseline SBP[22]. It is debatable whether hypotension is beneficial or detrimental to cardiac output and organ perfusion, but it will always be important for us to monitor blood pressure during the uptitration period of sacubitril/valsartan in heart failure patients with reduced ejection fraction. Hsiao et al[23] reported a case with prolonged first-dose hypotension induced by sacubitril/valsartan. In this case, the patient experienced recurrent intolerable hypotension episodes shortly after taking sacubitril/valsartan, even with a quarter of a 50-mg (sacubitril 24 mg/valsartan 26 mg) tablet. This might be related to the poor cardiac reserve in this patient and implies a more advanced stage than we thought in this particular population with heart failure.
Cheeran et al[24] studied the predictors of death in adults with DMD-associated cardiomyopathy, and found that a lower body mass index, a lower maximum inspiratory pressure and an elevated NT-proBNP level were risk factors for a poorer outcome. Early intervention for cardiac dysfunction in DMD patients, especially at the time of preserved ejection fraction, might postpone the process of left ventricular remodeling and improve the cardiac outcomes in these patients. A recent multicenter, randomized double-blind placebo-controlled study reported the effect and safety of combined treatment with an ACEI and a β-blocker in a German cohort of DMD patients with preserved left ventricular function. Thirty-eight patients from 10 sites were centrally randomized into the intervention group (combined treatment with enalapril and metoprolol) and a placebo control group. The results showed that the administration of ACEI and β-blocker delayed the progression of intrinsic cardiomyopathy to left ventricular failure in DMD patients with preserved left ventricular function, although this did not reach statistical significance[25]. Further studies should be conducted to determine the effect of ARNIs in DMD patients with preserved left ventricular function.
It is challenging to evaluate cardiac function in heart failure patients with DMD according to the NYHA criteria, mainly due to their limited ambulatory capacity. Although clinical trials have revealed that sacubitril/valsartan greatly improve cardiac performance in patients with reduced heart failure, hypotension is a common side effect of this medication. We hereby advise close monitoring of blood pressure after sacubitril/valsartan administration in severe DMD patients with heart failure. Further studies on the administration of sacubitril/valsartan in DMD patients with reduced or preserved left ventricular ejection fraction are of crucial importance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bamoshmoosh M, Pastromas S, Ueda H S-Editor: Dou Y L-Editor: Webster JR E-Editor: Wu YXJ
| 1. | Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 2. | Perloff JK. Cardiac rhythm and conduction in Duchenne's muscular dystrophy: a prospective study of 20 patients. J Am Coll Cardiol. 1984;3:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Villa CR, Czosek RJ, Ahmed H, Khoury PR, Anderson JB, Knilans TK, Jefferies JL, Wong B, Spar DS. Ambulatory Monitoring and Arrhythmic Outcomes in Pediatric and Adolescent Patients With Duchenne Muscular Dystrophy. J Am Heart Assoc. 2015;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Docherty KF, McMurray JJV. Angiotensin receptor-neprilysin inhibitors: A new paradigm in heart failure with reduced ejection fraction. Int J Cardiol. 2019;281:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Krittanawong C, Kitai T. Pharmacogenomics of angiotensin receptor/neprilysin inhibitor and its long-term side effects. Cardiovasc Ther. 2017;35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 494] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 7. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 4921] [Article Influence: 546.8] [Reference Citation Analysis (4)] |
| 8. | Rawal HA, Kocheril AG. Sacubitril/Valsartanstive Heart Failure: Cardiogenic Shock. Case Rep Cardiol. 2018;2018:8231576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Rodil Fraile R, Malafarina V, Tiberio López G. Sacubitril-valsartan in heart failure and multimorbidity patients. ESC Heart Fail. 2018;5:956-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Beltrán P, Palau P, Domínguez E, Faraudo M, Núñez E, Guri O, Mollar A, Sanchis J, Bayés-Genís A, Núñez J. Sacubitril/valsartan and short-term changes in the 6-minute walk test: A pilot study. Int J Cardiol. 2018;252:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Fayssoil A, Abasse S, Silverston K. Cardiac Involvement Classification and Therapeutic Management in Patients with Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2017;4:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, Meng G, Müller CR, Lindlöf M, Kaariainen H, de la Chapellet A, Kiuru A, Savontaus ML, Gilgenkrantz H, Récan D, Chelly J, Kaplan JC, Covone AE, Archidiacono N, Romeo G, Liechti-Gailati S, Schneider V, Braga S, Moser H, Darras BT, Murphy P, Francke U, Chen JD, Morgan G, Denton M, Greenberg CR, Wrogemann K, Blonden LA, van Paassen MB, van Ommen GJ, Kunkel LM. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989;45:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Towbin JA, Hejtmancik JF, Brink P, Gelb B, Zhu XM, Chamberlain JS, McCabe ER, Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 306] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Kamdar F, Garry DJ. Dystrophin-Deficient Cardiomyopathy. J Am Coll Cardiol. 2016;67:2533-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 15. | D'Amario D, Amodeo A, Adorisio R, Tiziano FD, Leone AM, Perri G, Bruno P, Massetti M, Ferlini A, Pane M, Niccoli G, Porto I, D'Angelo GA, Borovac JA, Mercuri E, Crea F. A current approach to heart failure in Duchenne muscular dystrophy. Heart. 2017;103:1770-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Ogata H, Ishikawa Y, Ishikawa Y, Minami R. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol. 2009;53:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | El-Aloul B, Altamirano-Diaz L, Zapata-Aldana E, Rodrigues R, Malvankar-Mehta MS, Nguyen CT, Campbell C. Pharmacological therapy for the prevention and management of cardiomyopathy in Duchenne muscular dystrophy: A systematic review. Neuromuscul Disord. 2017;27:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4738] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 19. | Yandrapalli S, Andries G, Biswas M, Khera S. Profile of sacubitril/valsartan in the treatment of heart failure: patient selection and perspectives. Vasc Health Risk Manag. 2017;13:369-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Bruno RM, Taddei S. Sacubitril/valsartan and low blood pressure in heart failure with reduced ejection fraction. Eur Heart J. 2017;38:1144-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Vardeny O, Claggett B, Kachadourian J, Pearson SM, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Incidence, Predictors, and Outcomes Associated With Hypotensive Episodes Among Heart Failure Patients Receiving Sacubitril/Valsartan or Enalapril: The PARADIGM-HF Trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11:e004745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 22. | Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJV. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. 2017;38:1132-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Hsiao FC, Chu PH. Prolonged First-Dose Hypotension Induced by Sacubitril/Valsartan. Acta Cardiol Sin. 2018;34:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Cheeran D, Khan S, Khera R, Bhatt A, Garg S, Grodin JL, Morlend R, Araj FG, Amin AA, Thibodeau JT, Das S, Drazner MH, Mammen PPA. Predictors of Death in Adults With Duchenne Muscular Dystrophy-Associated Cardiomyopathy. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Dittrich S, Graf E, Trollmann R, Neudorf U, Schara U, Heilmann A, von der Hagen M, Stiller B, Kirschner J, Pozza RD, Müller-Felber W, Weiss K, von Au K, Khalil M, Motz R, Korenke C, Lange M, Wilichowski E, Pattathu J, Ebinger F, Wiechmann N, Schröder R; German Competence Network for Congenital Heart Defects and the Treat-NMD Neuromuscular Network Investigators list of additional local Investigators and co-workers of the German Competence Network for Congenital Heart Defects and the Treat-NMD Neuromuscular Network. Effect and safety of treatment with ACE-inhibitor Enalapril and β-blocker metoprolol on the onset of left ventricular dysfunction in Duchenne muscular dystrophy - a randomized, double-blind, placebo-controlled trial. Orphanet J Rare Dis. 2019;14:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |