Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4091
Peer-review started: September 21, 2019
First decision: October 24, 2019
Revised: November 12, 2019
Accepted: November 15, 2019
Article in press: November 15, 2019
Published online: December 6, 2019
Processing time: 75 Days and 20.2 Hours
Hydroxyurea (HU) is a non-alkylating antineoplastic agent that is active in the S-phase of the cell cycle and inhibits the enzyme ribonucleoside reductase. HU is currently used to treat leukemia, sickle cell anemia, psoriasis, and chronic myeloproliferative disorders. Although HU is easy to use and effective and has high tolerance, there have been numerous reports of cutaneous complications during long-term therapy with HU.
We report a 67-year-old woman on long-term HU therapy for primary myelofibrosis who developed concurrent skin lesions during treatment. The first skin lesion appeared on the dorsum of her right hand in 2015. Despite continuous use of HU, her cutaneous changes were neglected. Approximately 3 years ago, she had multiple nodular and keratotic lesions on both hands with sharp margins, branny desquamation, and dotted hyperpigmentation. Furthermore, she developed acutely numerous ulcerative lesions on her hands and legs. Topical wound therapy with dressing changes and parenteral antibiotics was applied for management of the lesions. Most of the wounds healed after HU withdrawal. Lesions on both hands were replaced by scabs. Nevertheless, the wound on her left ankle reached 9 cm × 7 cm in size in January 2018. Pathology confirmed well-differentiated squamous cell carcinoma at the ulcer area. In addition, her left foot was severely affected and radical surgery with a below-the-knee amputation was suggested followed by preventive right groin nodal dissection.
In patients receiving continuous HU therapy, close dermatologic follow-up is critical for the early diagnosis and selection of appropriate treatment for cutaneous lesions.
Core tip: Long-term hydroxyurea (HU) therapy is a rare cause of cutaneous squamous cell carcinoma (cSCC). To our knowledge, fewer than 20 cases of HU-related cSCC have been reported. Recognizing patterns of HU-associated cSCC is important for surgeons and dermatologists. Moreover, early diagnosis and evaluation are critical for determining optimal treatment regimens. We review the reported cases of cSCC and discuss the pathogenic mechanisms.
- Citation: Xu Y, Liu J. Hydroxyurea-induced cutaneous squamous cell carcinoma: A case report. World J Clin Cases 2019; 7(23): 4091-4097
- URL: https://www.wjgnet.com/2307-8960 /full/v7/i23/4091.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4091
Hydroxyurea (HU) is a non-alkylating antineoplastic agent first synthesized in 1869 by Dressler and Stein. It is currently used to treat leukemia, sickle cell anemia, psoriasis, and chronic myeloproliferative disorders[1]. Following a large-scale drug screen, it has also been used as an anti-tumor agent for the management of head and neck cancers, malignant melanoma, and brain tumors. Moreover, HU is listed as an “essential medicine” by the World Health Organization[2].
HU is a well-established inhibitor of DNA synthesis. It is mainly active in the S-phase of the cell cycle by suppressing ribonucleoside reductase, which plays a vital role in catalyzing the synthesis of deoxyribonucleotides from ribonucleotides[1]. Inactivation of ribonucleoside reductase can slow the movement of DNA polymerase at replication forks and decreases deoxyribonucleotide triphosphate levels[3]. Hence, HU depletes intracellular deoxynucleotide pools, which leads to impairment of DNA synthesis and repair.
There have been numerous reports of cutaneous complications during long-term maintenance therapy with HU. Common cutaneous side effects include hyperpigmentation, xerosis, alopecia, atrophy of the skin, nail changes, facial and acral erythema, palmar or plantar keratoderma, and leg ulcers[4]. However, to date, fewer than 20 cases of HU-related cutaneous squamous cell carcinoma (cSCC) have been reported. Early diagnosis and evaluation are critical for determining optimal treatment regimens. Herein, we describe an additional case of cSCC associated with long-term HU therapy.
A 67-year-old Asian woman with a history of primary myelofibrosis presented with a painful non-healing chronic ulcer on her left ankle of 2 years duration.
The patient had primary myelofibrosis for 20 years and was initially treated with HU at a dose of 1 g/d.
Appendectomy was performed 10 years ago.
The patient had no significant family history.
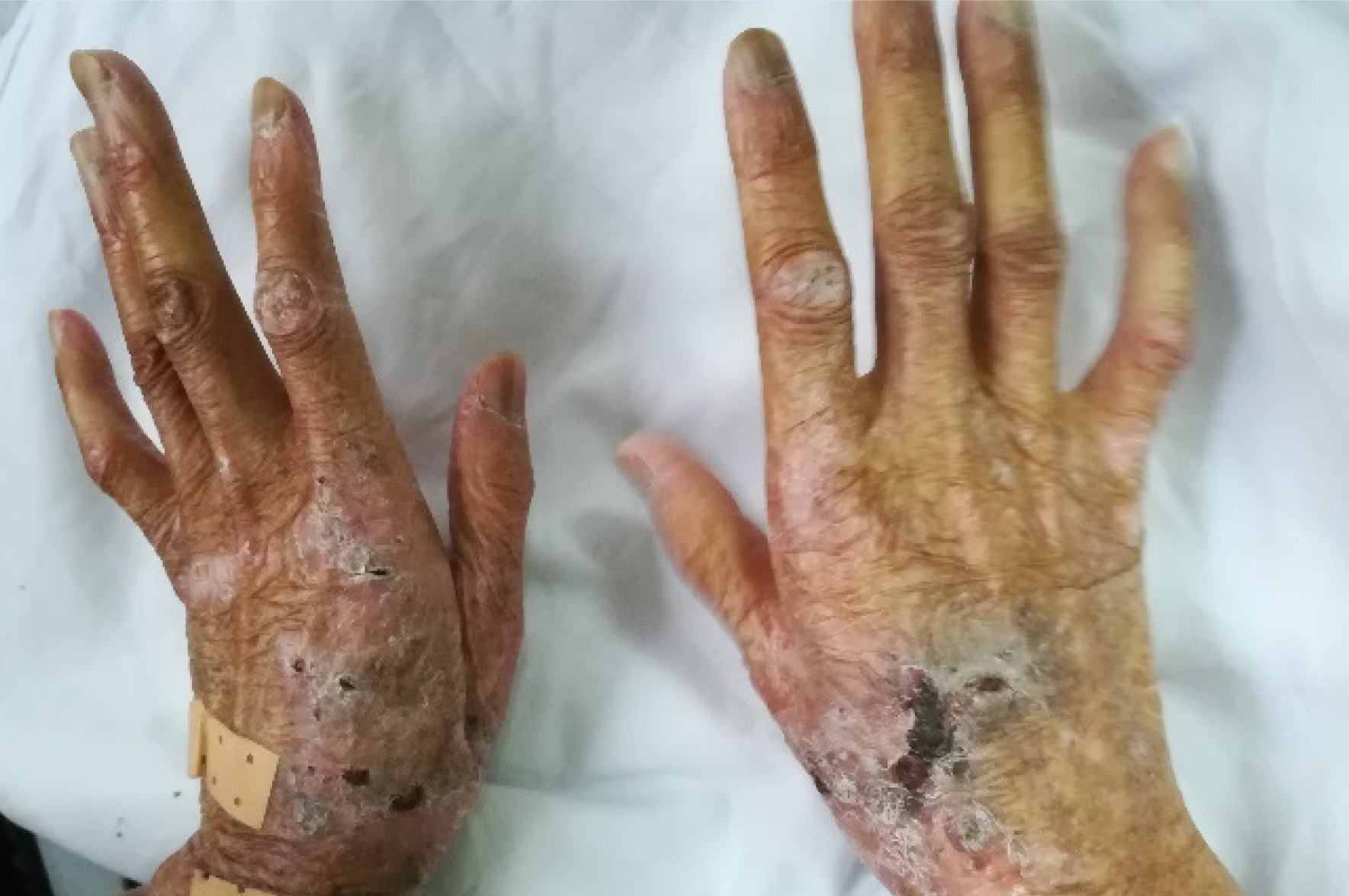
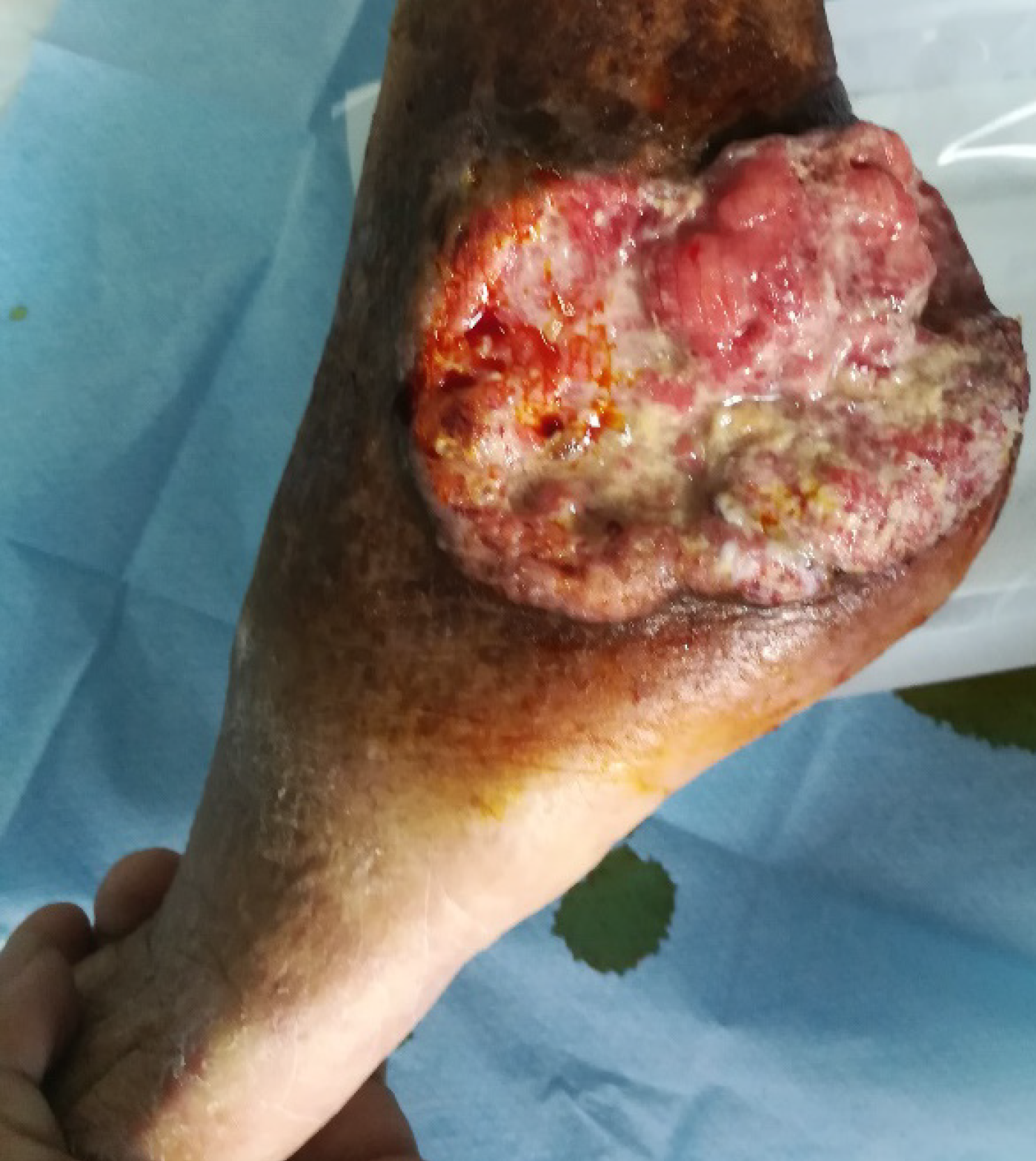
There was extensive photodamage and atrophy on the dorsal hands. The lesions on the hands consisted of multiple scabs, the largest scab on the right hand measured 1 cm × 2 cm (Figure 1). The irregularly shaped ulcer on the left ankle was 9 cm × 7 cm in size with an ill-defined margin (Figure 2).
Laboratory investigations showed that the patient had marked leukocytosis (84.5 × 109/L), a raised neutrophil count (60.8 × 109/L) and platelet count (488 × 109/L), and a low erythrocyte count (2.1 × 1012/L).
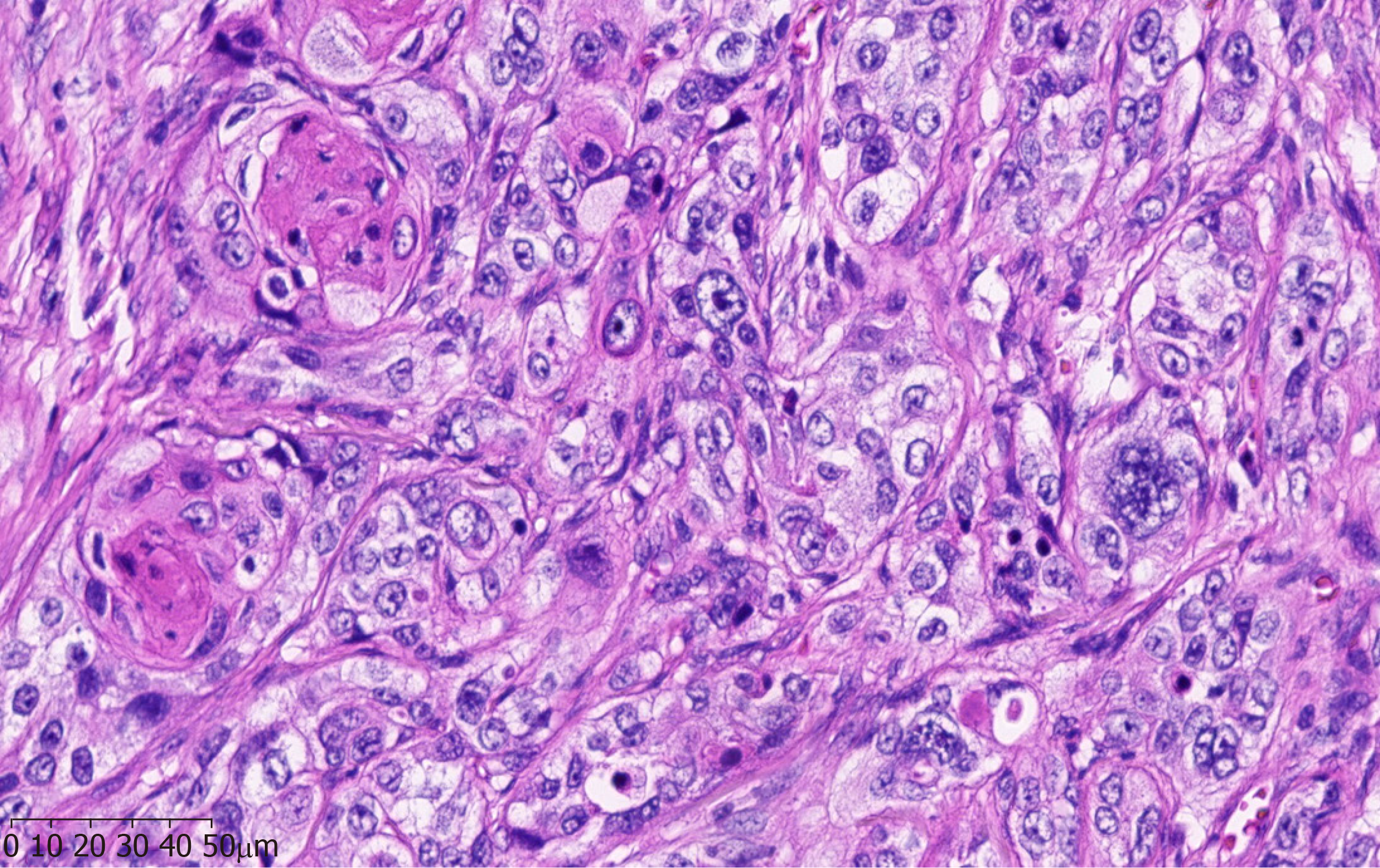
A pathological examination of the surgical specimen revealed a well-differentiated squamous cell carcinoma with evidence of abundant cytoplasmic keratin pearls (Figure 3).
Although surgeons considered performing resection of cSCC on her left foot and coverage with skin grafts, the operation was deemed too risky due to the high likelihood of failure and incomplete excision. Thus, the patient underwent a successful below-the-knee amputation followed by preventive right groin nodal dissection.
The postoperative course was uneventful with no recurrence after 12 mo of follow-up. Dermatologic changes on both hands secondary to HU therapy were extensively treated.
Long-term HU therapy is a rare cause of cSCC. In total, only 18 cases, including our patient, have been reported in the literature (Table 1). Disdier et al[5] first reported a patient with cSCC after HU treatment in 1991. In these reports, four primary diseases (primary myelofibrosis, polycythemia vera, essential thrombocythemia, and chronic myelocytic leukemia) and a wide age range (from 45 to 81 years) were presented. There was no significant sex difference in these cases (10 of 18 cases were men). Lesions were commonly located on the scalp, face, and extremities. Most patients had no history of precursor lesions or skin cancers, which rendered HU as the most likely culprit. Patients with HU-related cSCC typically showed symptoms after a latency period of approximately 2 to 13 years. HU treatment withdrawal, Mohs surgery, multiple debridements, and ablation were usually necessary to heal leg ulcers, but in five cases, the above therapies did not result in wound closure. It is suggested that the skin toxicity of HU is a long-term cumulative process and will persist after drug withdrawal, which could be defined as late toxicity. However, the mechanisms underlying the occurrence of HU-related cSCC are mostly unknown, but two pathogenic mechanisms have been proposed to explain this association.
| Ref. | Year | Age | Sex | Primary disease | HU dose | Latentpe-riod | Pathological distribution | Treatment | Prognosis |
| Saraceno et al[11] | 2008 | 81 | Male | PMF | 1 g/d | 6 mo | Face and extremity | Imiquimod cream 5% HU was not discontinued | CR of cSCC new skin lesions |
| Wen et al[12] | 2014 | 55 | Female | PMF | 1 g/d | 8 yr | Extremity | Imiquimod cream 5% Surgical excision and discontinuation of HU | / |
| Stone et al[9] | 2012 | 62 | Female | PV | 1 g/d | 10 yr | Left heel | Surgical excision and discontinuation of HU | Complete healing of the wound after multidisciplinary treatment |
| García-Martínez et al[13] | 2012 | 67 | Male | PV | 2 g/d | 12 yr | Beside the right ear | Surgical excision and discontinuation of HU | CR of cSCC |
| Hoff et al[14] | 2009 | 68 | Female | PV | / | 8 yr | Lower legs | Surgical excision and discontinuation of HU | CR of cSCC |
| Best et al[15] | 1998 | 59 | Female | ET | / | 8 yr | face and right hand | Surgical excision and discontinuation of HU | Improvement after HU discontinuation |
| Zaccaria et al[16] | 2006 | 73 | Male | ET | 1 g/d | 12 yr | face | Surgical excision and discontinuation of HU, Busulfan 8 mg/d | CR of cSCC |
| Schleußinger et al[17] | 2011 | 80 | Female | ET | 0.75 g/d | 13 yr | face | Surgical excision | / |
| Disdier et al[5] | 1991 | 65 | Male | CML | 1 g/d | 2 yr | face, scalp and both hands | Surgical excision and discontinuation of HU, radiotherapy | Continual progress |
| Best et al[15] | 1998 | 50 | Female | CML | 2.5 g/d | 5 yr | face and both hands | Surgical excision and discontinuation of HU | New skin lesions; Died |
| Stasi et al[18] | 1992 | 70 | Male | CML | 5.5 g/d | 4 yr | face, both hands and elbows | Imiquimod cream 5%; Surgical excision and discontinuation of HU | CR of cSCC; New skin lesions |
| Papi et al[19] | 1993 | 70 | Male | CML | 1 g/d | 3 yr | Face | Surgical excision and discontinuation of HU | Continual progress |
| Angeli-Besson et al[20] | 1995 | 67 | Male | CML | 1 g/d | 4 yr | Scalp | Localized electron beam therapy | Died |
| Aste et al[21] | 2001 | 60 | Male | CML | 0.05-0.1 g/d | / | Face, scalp and back of hands | Surgical excision and discontinuation of HU | Continual progress |
| Pamuk et al[22] | 2003 | 73 | Male | CML | 10 g/wk | 3 yr | Left ear | Surgical excision and discontinuation of HU, radiotherapy | Died |
| Hao et al[23] | 2013 | 45 | Male | CML | 0.4-0.8 g/d | 11 yr | Both hands | Surgical excision and discontinuation of HU | Continual progress |
| Wang et al[24] | 2013 | 66 | Female | CML | 2-2.5 g/d | 5 yr | Left hand | Radiation treatment and discontinuation of HU | PR of cSCC |
Ultraviolet (UV) irradiation is most likely the primary factor leading to the development of cSCC. In vitro, HU inhibits natural DNA repair in UV-irradiated human skin fibroblasts and directly induces chromosome damage, which further interferes with cell replication in the basal layer of the epidermis[6,7]. In vivo, HU transforms free radical nitrogen oxides that may produce high oxidative stress in patients’ epithelial tissues and cooperates with UV in DNA damage, peroxidation of membrane lipids, and signal transduction pathway changes[8]. The synergistic effect of HU and UV radiation provides an ideal environment for the formation of cSCC. In addition, HU can markedly elevate p53 levels in basal layer keratinocytes, which increases the risk of skin cancers[9]. This pathogenic mechanism could explain the occurrence of cSCC in photoexposed skin.
A chronic wound environment characterized by prolonged inflammation undoubtedly plays another critical role in HU-associated cSCC[9]. Lower limb ulceration is a significant adverse reaction caused by long-term administration of HU. Several pathogenetic hypotheses (DNA inhibition theory, tarombokinesis, and microcirculation rheology theory) could explain the formation of lower limb ulcers. It is well-known that chronic skin damage, including ulcers, burn sites, and scars, is associated with an increased incidence of skin cancer[10]. In addition to the case reported here, 11 articles refer to the presence of cSCC on extremities, which are different from the light exposure area. Hence, chronic inflammation may be another main cause of cSCC in these patients.
Taken together, these findings indicate that long-term HU administration can lead to the development of cSCC, probably through synergistic effects with UV or chronic inflammation. Hence, patients should be informed of the potential cutaneous toxicities of HU treatment in advance. Closer dermatologic follow-up is advisable, particularly in patients with continuous HU therapy and a long history of sun exposure. Moreover, regular protection against UV rays should be emphasized. When cSCC is identified, HU treatment withdrawal is necessary, and we recommend prompt replacement therapy as required. The skin toxicity of HU is considered late toxicity, and patients should be monitored for many years after HU discontinuation.
The authors thank all the treatment groups of the Department of Surgical Oncology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang AHC, Nofal A S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu MY
| 1. | Madaan K, Kaushik D, Verma T. Hydroxyurea: a key player in cancer chemotherapy. Expert Rev Anticancer Ther. 2012;12:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Singh A, Xu YJ. The Cell Killing Mechanisms of Hydroxyurea. Genes (Basel). 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Xu YJ, Singh A, Alter GM. Hydroxyurea Induces Cytokinesis Arrest in Cells Expressing a Mutated Sterol-14α-Demethylase in the Ergosterol Biosynthesis Pathway. Genetics. 2016;204:959-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Simeonovski V, Breshkovska H, Duma S, Dohcheva-Karajovanov I, Damevska K, Nikolovska S. Hydroxyurea Associated Cutaneous Lesions: A Case Report. Open Access Maced J Med Sci. 2018;6:1458-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Disdier P, Harle JR, Grob JJ, Weiller-Merli C, Magalon G, Weiller PJ. Rapid development of multiple squamous-cell carcinomas during chronic granulocytic leukemia. Dermatologica. 1991;183:47-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Francis AA, Blevins RD, Carrier WL, Smith DP, Regan JD. Inhibition of DNA repair in ultraviolet-irradiated human cells by hydroxyurea. Biochim Biophys Acta. 1979;563:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Palladino MA, Grebenau MD, Thorbecke GJ. Requirements for induction of delay hypersensitivity in the chicken. Dev Comp Immunol. 1978;2:121-132. [PubMed] [DOI] [Full Text] |
| 8. | Sanchez-Palacios C, Guitart J. Hydroxyurea-associated squamous dysplasia. J Am Acad Dermatol. 2004;51:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Stone T, Berger A, Blumberg S, O'Neill D, Ross F, McMeeking A, Chen W, Pastar I. A multidisciplinary team approach to hydroxyurea-associated chronic wound with squamous cell carcinoma. Int Wound J. 2012;9:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Potenza C, Bernardini N, Balduzzi V, Losco L, Mambrin A, Marchesiello A, Tolino E, Zuber S, Skroza N, Proietti I. A Review of the Literature of Surgical and Nonsurgical Treatments of Invasive Squamous Cells Carcinoma. Biomed Res Int. 2018;2018:9489163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Saraceno R, Teoli M, Chimenti S. Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject. Clin Ther. 2008;30:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wen LY, Feng DM, Ji CP. Hydroxyurea in the treatment of multiple cutaneous squamous cell carcinoma (SCC) during myelofibrosis: A case report. Zhongguo Pifuxing Bingxue Zazhi. 2014;3:316-317. |
| 13. | García-Martínez FJ, García-Gavín J, Alvarez-Pérez A, Alonso-González J, Ginarte M, Toribio J. Scleroderma-like syndrome due to hydroxyurea. Clin Exp Dermatol. 2012;37:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hoff NP, Akanay-Diesel S, Pippirs U, Schulte KW, Hanneken S. [Cutaneous side effects of hydroxyurea treatment for polycythemia vera]. Hautarzt. 2009;60:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Best PJ, Petitt RM. Multiple skin cancers associated with hydroxyurea therapy. Mayo Clin Proc. 1998;73:961-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zaccaria E, Cozzani E, Parodi A. Secondary cutaneous effects of hydroxyurea: possible pathogenetic mechanisms. J Dermatolog Treat. 2006;17:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Schleußinger TM, Dyall-Smith D, Field LM. Hydroxyurea-associated squamous dysplasia in a monozygotic twin. J Am Acad Dermatol. 2011;65:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Stasi R, Cantonetti M, Abruzzese E, Papi M, Didona B, Cavalieri R, Papa G. Multiple skin tumors in long-term treatment with hydroxyurea. Eur J Haematol. 1992;48:121-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Papi M, Didona B, DePità O, Abruzzese E, Stasi R, Papa G, Cavalieri R. Multiple skin tumors on light-exposed areas during long-term treatment with hydroxyurea. J Am Acad Dermatol. 1993;28:485-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Angeli-Besson C, Koeppel MC, Jacquet P, Andrac L, Sayag J. Multiple squamous-cell carcinomas of the scalp and chronic myeloid leukemia. Dermatology. 1995;191:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Aste N, Fumo G, Biggio P. Multiple squamous epitheliomas during long-term treatment with hydroxyurea. J Eur Acad Dermatol Venereol. 2001;15:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Pamuk GE, Turgut B, Vural O, Demir M, Tek M, Altaner S. Metastatic squamous cell carcinoma of the skin in chronic myeloid leukaemia: complication of hydroxyurea therapy. Clin Lab Haematol. 2003;25:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Hao LP, Liu LF. Chronic myelogenous leukemia complicated with cutaneous squamous cell carcinoma: A case report. Zhongliu Fangzhi Yanjiu Zazhi. 2013;40:627. [DOI] [Full Text] |
| 24. | Wang DM, Gao XJ, Zhang QQ, Hu JY. Chronic myeloid leukemia complicated with cutaneous squamous cell carcinoma after 5 years of hydroxyurea therapy: A case report. Shiyong Yixue Zazhi. 2013;29:4115. [DOI] [Full Text] |