Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3980
Peer-review started: August 14, 2019
First decision: September 10, 2019
Revised: October 21, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: December 6, 2019
Processing time: 114 Days and 7.8 Hours
In previous studies, celiomesenteric trunk (CMT) was narrowly defined as a hepato-gastro-spleno-mesenteric (HGSM) trunk, but other possible types were ignored. With the widespread use of multidetector computed tomography (MDCT) angiography, it is easy to collect a large sampling of data on arterial anatomy of the abdomen in daily radiological practice. A new classification system for CMT may be created based on its MDCT angiographic findings and variation patterns.
To identify the spectrum and prevalence of CMT according to a new classification based on MDCT angiographic findings, and discuss the probable embryological mechanisms to explain the CMT variants.
A retrospective study was carried out on 5580 abdominal MDCT angiography images. CMT was defined as a single common trunk arising from the aorta and its branches including the superior mesenteric artery and at least two major branches of the celiac trunk. Various types of CMT were investigated.
Of the 5580 patients, 171 (3.06%) were identified as having CMT. According to the new definitions and classification, the CMT variants included five types: I, II, III, IV and V, which were found in 96 (56.14%), 57 (33.33%), 4 (2.34%), 3 (1.75%) and 8 (4.68%) patients, respectively. The CMT variants also were classified as long type (106 patients, 61.99%) and short type (65 patients, 38.01%) based on the length of single common trunk. Further CMT classification was based on the origin of the left gastric artery: Type a (92 patients, 53.80%), type b (57 patients, 33.33%), type c (11 patients, 6.43%) and type d (8 patients, 4.68%).
We systematically classified CMT variants according to our new classification system based on MDCT angiographic findings. Dislocation interruption, incomplete interruption and persistence of the longitudinal anastomosis could all be embryological mechanisms of various types of CMT variants.
Core tip: A new definition and classification system for celiomesenteric trunk (CMT) was created in our study. CMT can be classified into various types according to its variation patterns, origins of left gastric artery and length of single common trunk on multidetector computed tomography angiographic images. CMT is not very rare in these observed patients and has wide-ranging health implications. Dislocation interruption, incomplete interruption and persistence of the longitudinal anastomosis could all be embryological mechanisms of various types of CMT variants.
- Citation: Tang W, Shi J, Kuang LQ, Tang SY, Wang Y. Celiomesenteric trunk: New classification based on multidetector computed tomography angiographic findings and probable embryological mechanisms. World J Clin Cases 2019; 7(23): 3980-3989
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/3980.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.3980
The celiac trunk is an arterial trunk originating from the aorta to the branching point of the common hepatic artery (CHA) and the splenic artery (SA), with the left gastric artery (LGA) being the first branch also stemming from the arterial trunk[1,2]. The superior mesenteric artery (SMA) originates separately from the aorta about 1.0 cm below the celiac trunk. However, there is a very close anatomical and functional connection between the celiac trunk and the SMA, and considerable variations on their branching pattern have been observed in previous studies[1-9]. Among these variations, celiomesenteric trunk (CMT) has been considered as one of the rare types, with incidence ranging from 0.4% to 2.7%[1,4,7,8,10-15].
In recent 20 years, with the widespread use of multidetector computed tomography (MDCT) and angiography, it is easy to collect a large sampling of data on the angiographic anatomy of the abdomen in daily radiological practice. Then, the variation patterns and radiological findings of CMT can be classified and evaluated in detail by MDCT angiography. The main purpose of this study was to develop a new classification for the CMT according to its variation patterns, and use MDCT angiography to identify its types and prevalence in a large study population. We also discussed their clinical implications and the probable embryological mechanisms by which the observed variations are achieved.
This study was approved by our Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. The requirement for informed patient consent was waived due to the retrospective nature of the study. A total of 6000 patients underwent abdominal multiphase enhanced MDCT and angiography from February 2008 to April 2018. Data obtained during the arterial phase were used to evaluate the anatomy of the celiac trunk, the SMA and their major branches. The exclusion criteria applied were the presence of any condition likely to affect arterial anatomy and the MDCT or any image reformation that encountered technical deficiency.
MDCT was performed on either a 64-row MDCT scanner (GE Healthcare, United States) or a 256-slice MDCT scanner (Philips Healthcare, Cleveland) with a coverage extending from the dome of the diaphragm to the inferior margin of the right kidney. The scan parameters were as follows: Collimation of 64 × 0.625 mm or 256 × 0.5 mm and table speed of 64 or 256 mm per rotation. The following parameters were applied to all scans: Pitch 0.984, matrix 512 × 512, field of view 180-240 mm, tube voltage 120 kV, and tube current 300 mA.
Multiphase enhanced MDCT scan was performed after intravenous administration of contrast agent (Ultravist; Bayer Schering Pharma, Berlin, Germany) at 370 mg of iodine per milliliter and 30 mL of sterile saline (0.9% NaCl) by using a power injector at a rate of 3-4 mL/s. The dose of the contrast agent was 2 mL/kg body weight and the upper limit of dose was set at 120 mL for every patient. Images of the hepatic arterial, portal vein and equilibrium phases were acquired at 20, 50 and 120 s, respectively.
For the purposes of this study, only the data obtained during the arterial phase were downloaded onto an off-line workstation (ADW 4.3; General Electric Healthcare, Milwaukee, United States) for image post-processing and analysis. Multiplanar reformation, volume rendering and maximum intensity projection were used to reformat and analyze the target arteries by three abdominal radiologists with 12, 15 and 23 years of experience, respectively. The radiologists independently assessed the images, and final assessment was achieved by consensus. Statistical analyses were done with the Statistical Package for the Social Sciences version 20.0 (SPSS, Chicago, IL, United States).
We analyzed the variation patterns of the CMT according to the origins for the five major arteries-the celiac trunk, SMA, LGA, CHA and SA-with adherence to our new definitions of celiac trunk and CMT. We defined the celiac trunk as an arterial trunk containing at least two of its major branches irrespective of its origin and anatomic course which was described in our previous study[1], and CMT as a single common trunk arising from the aorta and its branches including the SMA and at least two major branches of the celiac trunk
With the new definitions of the celiac trunk and CMT, various variation patterns of CMT can be objectively described. In order to comprehensively describe results of a systematic analysis of variation patterns of CMT, we developed a new classification system (Table 1). By integrating data obtained from the analysis of MDCT angiographic images, we classified these variation patterns in the CMT variants. Finally, we suggested hypothetical embryological mechanisms to explain the observed variations.
| Definition and type | Description |
| Definition | |
| CMT | We defined the CMT as a single common trunk arising from the aorta and its branches including the SMA and at least two major branches of the celiac trunk |
| Type (abbreviation) | |
| HGSM trunk (type I) | Hepato-gastro-spleno-mesenteric trunk |
| HSM trunk + LGA (type II) | Hepato-spleno-mesenteric trunk with LGA arising from the aorta |
| GSM trunk + CHA (type III) | Gastro-spleno-mesenteric trunk with CHA arising from the aorta |
| HGM trunk + SA (type IV) | Hepato-gastro-mesenteric trunk with SA arising from the aorta |
| Other (type V) | Any other variation that meets the above definition of the CMT |
A total of 420 patients were excluded from our study; 394 of them had a pathological condition affecting arterial anatomy, and 26 MDCT examinations were considered technically inadequate, including insufficient arterial contrast enhancement (4 patients), motion artifact (5 patients) and post-processing deficiency (17 patients), which caused difficulty in identifying the origins of the celiac trunk or its major branches. Eventually, a total of 5580 patients, 3774 men and 1806 women, were included once each in the study, with an age range of 12 to 89 years (mean age, 54.7 ± 14.8 years).
Of the 5580 patients, 171 (3.06%) were identified as having CMT. All of them were characterized by origination of the celiac trunk and the SMA from a single common trunk of the aorta. Their ages ranged from 14 to 86 years (mean age, 55.97 ± 14.31 years). Among them, 120 were male (3.18%; 120/3,774) and 51 were female (2.82%; 51/1806) (P = 0.471), with male and female ages ranging from 14 to 86 years (mean age, 55.3 ± 15.24 years) and 34 to 79 years (mean age, 57.55 ± 17.81 years) (P = 0.349), respectively.
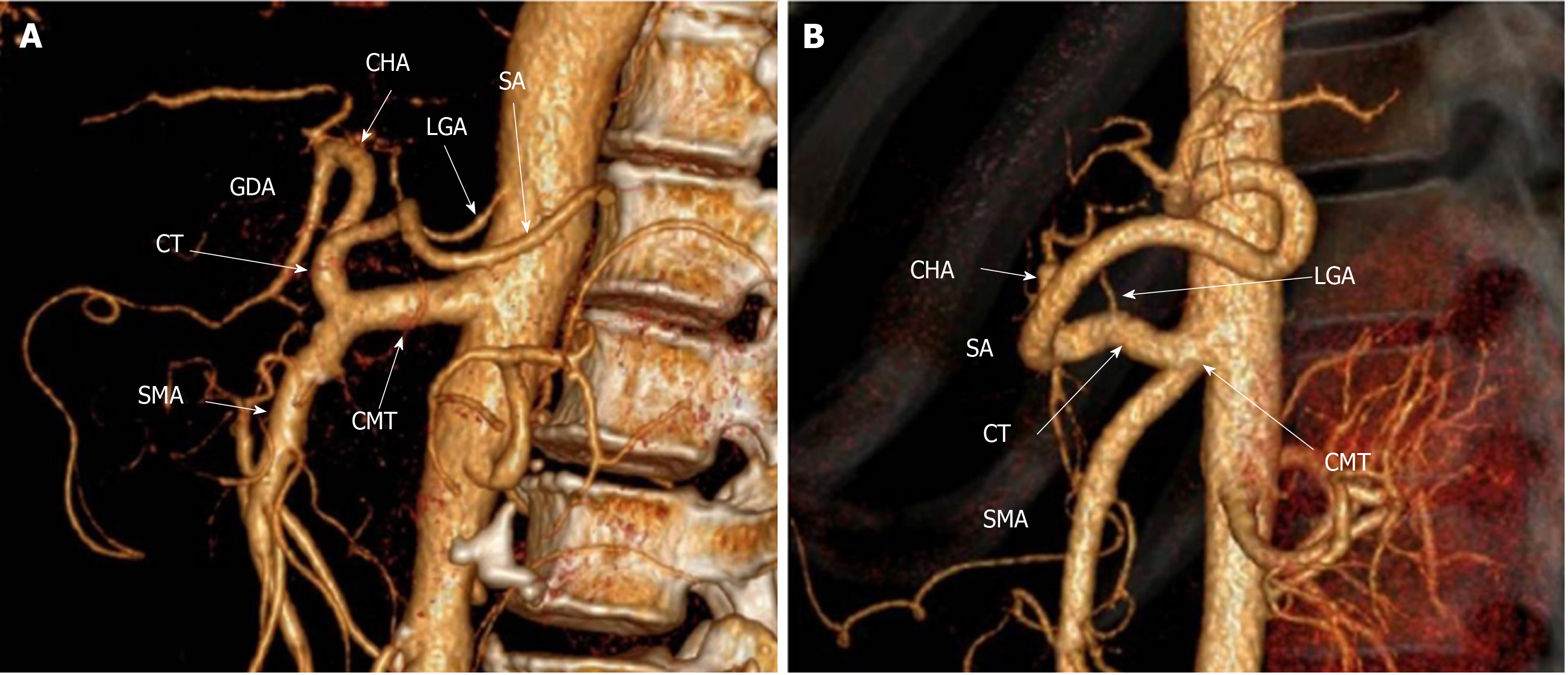
CMT can be classified into long or short type according to the length of the single common trunk. Then, the long type was defined when the single common trunk branched into the celiac trunk and the SMA a long distance after originating from the aorta, resulting in a length range from 17 to 39 mm (Figure 1A). The short type was defined when the single common trunk branched into the celiac trunk and SMA immediately or within a shorter continuation, with a resulting length range from 6 to 14 mm (Figure 1B). The long type (106 patients, 61.99%) was more common than the short type (65 patients, 38.01%) (P < 0.001, Table 2), and its single common trunk (28.1 ± 5.3 mm) was longer compared with the short type (10.5 ± 1.9 mm) (P < 0.001).
| Type | Number | Percentage (%) | P value |
| Normal anatomy | 5031 | 90.16 | |
| Anatomical variation | 549 | 9.84 | |
| CMT | 171 | 3.06 | |
| Sex | |||
| Male | 120/3774 | 3.18 | 0.471 |
| Female | 51/1806 | 2.82 | |
| Type | |||
| Length of single common trunk | |||
| Long type | 106/171 | 61.99 | 0.0001c |
| Short type | 65/171 | 38.01 | |
| Variation pattern | |||
| HGSM trunk (type I) | 96/171 | 56.14 | 0.0001c |
| HSM trunk + LGA (type II) | 57/171 | 33.33 | |
| GSM trunk + CHA (type III) | 4/171 | 2.34 | |
| HGM trunk + SA (type IV) | 3/171 | 1.75 | |
| Other (type V) | 8/171 | 4.68 | |
| Origin of LGA | |||
| Celiac trunk (type a) | 92/171 | 53.80 | 0.0001c |
| Abdominal aorta (type b) | 57/171 | 33.33 | |
| Single common trunk (type c) | 11/171 | 6.43 | |
| Other artery (type d) | 8/171 | 4.68 | |
| Failed to identify | 3/171 | 1.75 | |
| Other variation | 378 | 6.77 |
Table 2 shows the incidence and percentage for various types of CMT. According to its variation patterns, CMT can be classified into five types. Type I, an HGSM trunk, can be considered a complete CMT and was found to be the most common type, accounting for 96 (56.14%) of the patients, which can be further divided into type Ia-the LGA originating from the celiac trunk in 85 patients (Figure 2A) and type Ib-the LGA originating from the single common trunk in 11 patients (Figure 2B). Then, from type II to type V, they were all incomplete CMT. Type II, HSM trunk + LGA was the second most common type, found in 57 (33.33%) patients (Figure 2C). Type III (GSM trunk + CHA) and type IV (HGM trunk + SA) were observed in 4 (2.34%) and 3 (1.75%) patients, respectively (Figure 2D and E). The remaining variation patterns fell into type V (8 patients, 11.11%), including the LGA originating from other arteries except the abdominal aorta, celiac trunk and single common trunk in 7 patients (Figure 2F and G) and the CHA arising from the SMA in 1 patient (Figure 2H). Three patients were not included in typing analysis due to difficulty in identifying their origin of the LGA.
Of 171 patients with CMT, the origin of the LGA was found to be highly variable, but the origins of the SMA, CHA and SA were relatively constant. The SMA originated from the single common trunk in all 171 patients. The CHA and SA originated from the abdominal aorta only in 4 and 3 patients, respectively; the CHA originated unusually from the SMA in 1 patient, and from the celiac trunk in all the remaining patients. Then, according to the origin of the LGA, CMT can be classified into four types (Table 2): Type a, the LGA originates from the celiac trunk (Figure 2A, D, E and H); type b, the LGA originates from the abdominal aorta (Figure 2C); type c, the LGA originates from the single common trunk (Figure 2B); and type d, the LGA originates from any of the other arteries (including the CHA in 3 patients, the left hepatic artery in 2 patients, the proper hepatic artery in 2 patients and the SA in 1 patient) (Figure 2F and G). Similarly, three patients were not included in typing analysis due to failures in identifying their origin of the LGA. Of the remaining 168 patients, there were more examples of type a (92 patients, 53.80%) and type b (57 patients, 33.33%) than those of type c (11 patients, 6.43%) and type d (8 patients, 4.68%) (P = 0.0001, Table 2).
Six other types of variations were also observed in a total of 378 (6.77%) of these observed patients. The most common variant was hepato-mesenteric (HM) trunk, which was found in 248 (4.44%) patients, including the two subtypes of HM trunk + gastro-spleno (GS) trunk (230 patients) and HM trunk + LGA + SA (18 patients). This was followed by spleno-mesenteric (SM) trunk in 67 (1.2%) patients, which included SM trunk + hepato-gastro (HG) trunk and SM trunk + CHA + LGA in 60 patients and 7 patients, respectively. Additionally, CHA + GS trunk + SMA was observed in 30 (0.54%) patients, HS trunk + LGA + SMA observed in 25 (0.27%), CHA + LGA + SA + SMA in 11 (0.2%) and HG trunk + SA + SMA in 7 (0.13%).
Generally, the celiac trunk and the SMA arise from the aorta separately. But, this pattern varies considerably in humans[16]. CMT is a congenital variation where the celiac trunk and the SMA arise off a single common trunk from the aorta. The reported incidences were different from 0.4% to 2.7% in previous studies[1,4,7,8,10-15]. In these studies, CMT was narrowly defined as a hepato-gastro-spleno-mesenteric trunk, but other possible types were ignored. Our new classification system defined CMT as an arterial trunk containing the SMA and at least two major branches of the celiac trunk. According to its variation patterns, CMT can be theoretically divided into five types. In our study, 171 patients (3.06%) were identified as having CMT in a large sample data of 5580 patients on MDCT.
Panagouli et al[7] reported that gender and ethnicity seemed to influence the presence or absence of variations of the celiac trunk. In most studies, the celiac trunk variations had a relation with ethnicity and origin of the study population, but had no relation with gender[7,17,18]. Japanese and Korean populations presented more variations of the celiac trunk than Caucasian ones[7]. The prevalence of CMT in our study was more than that of previous studies, and we speculate that our study population may have a higher prevalence of CMT than that of other study population. Similarly, our studies also showed that the frequency of CMT had no significant difference between males and females.
Regarding the embryologic development of the celiac trunk and the SMA, Tandler[19] stated that the visceral arteries form via four splanchnic roots arising from the primitive dorsal abdominal aorta. The four arteries (namely, the LGA, the CHA, the SA and the SMA) are connected by a longitudinal ventral anastomosis. Normally, the longitudinal anastomosis is interrupted just above the SMA, resulting in a complete separation of the SMA from the celiac trunk and its branches (Figure 3A). Abnormal regression or continuous growth of parts of the longitudinal anastomosis leads to the development of various variations in the celiac trunk and the SMA.
Our new definition and classification for CMT can be used to further analyze the embryological mechanisms of its various types. Type Ia can be due to a persistence of the longitudinal anastomosis among all four roots during embryonic development (Figure 3B) and type Ib can be attributed to an incomplete interruption of the longitudinal anastomosis between roots 1 and 2 (Figure 3C), thus resulting in two complete CMT types, and both should also be long type. The short type can be due to an incomplete interruption of the longitudinal anastomosis between roots 3 and 4 (Figure 3D). Type II, with the LGA arising from the aorta, may be attributed to a dislocation interruption of the longitudinal anastomosis between roots 1 and 2 (Figure 3E). Since the origins of CHA, SA and LGA approximate at the same level, the up and down position among the three arteries may not be invariant in human embryos[1]. If the root 1 is CHA and a dislocation interruption occurs between roots 1 and 2, a type III CMT would form (Figure 3F). However, if the root 1 is the SA, the dislocation interruption would result in a type IV CMT (Figure 3G). Type V, with more variant origins in the LGA, may be due to complete regression or absence of root 1, a replaced LGA arising from other arteries (Figure 3H).
In our 171 patients with CMT, the most common variation was type I with an incidence of 56.14%, and the incidence was significantly higher than that of the other four types. To the best of our knowledge, our population is the largest series of CMT cases analyzed in the literature. Except for our previous study[1], other previous studies on CMT were a series of 30 cases from cadaver dissections reported by Higashi et al[20] and a series of 36 cases from MDCT imaging reported by Maldjian et al[6]. Higashi et al[20] classified CMT into four types based on the configuration of the arteries. Maldjian et al[6] classified CMT into two types: Type A, corresponding to Higashi’ type I, and type B, corresponding to Higashi’ type III. Type A tended to be shorter single common trunk than type B. Our type I CMT corresponds to the description of Higashi’ type I and Maldjian’ type A. Similar to our results, both Higashi et al[20] and Maldjian et al[6] reported that type I and type A were the most common type in their studies.
We found that CMT can be classified into long and short types on the basis of the length of the single common trunk, and there were significant differences in the percentages and average lengths between the two types. A short single common trunk could make diagnosis of common origin on axial images more challenging and requiring sagittal images to discriminate common origin from close proximity of two separate ostia. A short single common trunk could also lead to misdiagnosis during catheter angiography as with selective catheterization, the catheter tip could easily traverse a short single common trunk and enter the celiac trunk or SMA, leaving the single common trunk undiscovered[21].
Knowledge about the spectrum of CMT variations is important for planning surgical or interventional procedures in the upper abdomen. Identification of CMT variations may avoid vascular complications during medical procedures, such as hepatobiliary surgery, pancreatic surgery, gastrectomy and others like transcatheter arterial chemoembolization[22-25]. As we know, total gastrectomy and esophagogastic resections require ligation of the LGA in its origin, and the right gastroepiploic and gastroic arteries must be preserved to maintain adequate blood supply to the reconstructive gastric tube[7,26]. In an anatomic variant, the right gastroic artery is missing, and taking the LGA there could have been dramatic and result in irreversible ischemia to the gastric tube[3,26]. Hence, surgeons should be aware of the LGA origin in CMT variants. Our study showed that the origin of the LGA was highly variable compared with the origins of the SMA, CHA and SA in CMT variants.
A patient with CMT is at risk of mesenteric ischemia because there lack some of the protective benefits of dual-origin vessels with multiple mutually supporting anastomoses[1,3,27]. Anything that compromises the single common trunk arising from the abdominal aorta puts the entire vascular region of the major abdominal viscera at risk of ischemia. Besides, it would be plausible that the CMT variant could change the SMA angle from the aorta, thus increasing or decreasing the potential for compression of the third portion of the duodenum. Therefore, the occurrence rate of SMA syndrome in patients with a CMT variant is worth to further study.
Our study has some limitations. First, it was performed retrospectively. Second, it was conducted on the basis of image interpretations performed by means of consensus opinion. Third, we have not assessed the effect of using the proposed classification system on interventional and surgical procedures. However, the new classification system enables us to describe in detail various types of CMT variations, and discuss their probable embryological mechanisms.
In conclusion, we systematically classified CMT variants according to our new definition and classification system based on MDCT angiography images. By analyzing the MDCT findings of CMT variants, we were able to generate hypothetical embryological mechanisms to explain all observed CMT variants. Dislocation interruption, incomplete interruption and persistence of the longitudinal anastomosis could all be embryological mechanisms of various types of CMT variants.
Most of previous studies focused on celiomesenteric trunk (CMT) based on case reports or corpse dissection, and some studies defined CMT as a hepato-gastro-spleno-mesenteric trunk and ignored other possible types. Abdominal multidetector computed tomography (MDCT) angiography is widely performed in daily radiological practice, a large sampling of data regarding CMT can be obtained and analyzed, and a new classification system for CMT may be created based on its MDCT angiographic findings and variation patterns.
With the wide use of abdominal MDCT angiography, CMT variants can be systematically and comprehensively described. By analyzing MDCT findings of CMT variants, a new redefinition and classification system for CMT may be created.
The study aimed to identify the spectrum and prevalence of CMT according to a new classification based on MDCT angiographic findings, and discuss the probable embryological mechanisms of various type of CMT.
A total of 5580 patients who underwent abdominal MDCT angiography were retrospectively analyzed by three abdominal radiologists for the prevalence and classification of CMT based on the new definition. CMT was redefined as a single common trunk arising from the aorta and its branches including the SMA and at least two major branches of the celiac trunk.
According to the new definitions and classification, a total prevalence of 3.06% (171/5580) was found on MDCT for CMT variants. The CMT variants included five types: I (56.14%), II (33.33%), III (2.34%), IV (1.75%) and V (4.68%). The CMT variants also were classified as long type (61.99%) and short type (38.01%) based on the length of the single common trunk. Further CMT classification was based on the origin of the left gastric artery: Type a (53.80%), type b (33.33%), type c (6.43%) and type d (4.68%).
The study systematically and comprehensively classified various types of CMT variants according to our new classification system based on MDCT findings. Dislocation interruption, incomplete interruption and persistence of the longitudinal anastomosis could all be embryological mechanisms of various types of CMT variants.
Knowledge about the spectrum of CMT variants is important for planning surgical or interventional procedures in the upper abdomen. Future studies need to further assess the effect of using the proposed classification system on interventional and surgical procedures.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gassler N, Milone M, Poddighe D, Ricci G, Smith RC S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Wang Y, Cheng C, Wang L, Li R, Chen JH, Gong SG. Anatomical variations in the origins of the celiac axis and the superior mesenteric artery: MDCT angiographic findings and their probable embryological mechanisms. Eur Radiol. 2014;24:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, Cho BH, So YH, Park JH. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010;255:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Alakkam A, Hill RV, Saggio G. Superior mesenteric origin of the proper hepatic artery: embryological and clinical implications. Surg Radiol Anat. 2016;38:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yan J, Nagasawa Y, Nakano M, Hitomi J. Origin of the celiac and superior mesenteric arteries in a common trunk: description of a rare vessel variation of the celiacomesenteric trunk with a literature review. Okajimas Folia Anat Jpn. 2014;91:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Iezzi R, Cotroneo AR, Giancristofaro D, Santoro M, Storto ML. Multidetector-row CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat. 2008;30:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Maldjian PD, Chorney MA. Celiomesenteric and hepatosplenomesenteric trunks: characterization of two rare vascular anomalies with CT. Abdom Imaging. 2015;40:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Panagouli E, Venieratos D, Lolis E, Skandalakis P. Variations in the anatomy of the celiac trunk: A systematic review and clinical implications. Ann Anat. 2013;195:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Marco-Clement I, Martinez-Barco A, Ahumada N, Simon C, Valderrama JM, Sanudo J, Arrazola J. Anatomical variations of the celiac trunk: cadaveric and radiological study. Surg Radiol Anat. 2016;38:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Yi SQ, Terayama H, Naito M, Hayashi S, Moriyama H, Tsuchida A, Itoh M. A common celiacomesenteric trunk, and a brief review of the literature. Ann Anat. 2007;189:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Rountas Ch, Fanariotis M, Vlychou M, Arvanitis DL, Fezoulidis I, Vassiou K. Celiomesenteric trunk demonstrated by multi-detector computed tomography angiography: two cases of a rare vascular variation. Folia Morphol (Warsz). 2013;72:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Cavdar S, Sehirli U, Pekin B. Celiacomesenteric trunk. Clin Anat. 1997;10:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Katagiri H, Ichimura K, Sakai T. A case of celiacomesenteric trunk with some other arterial anomalies in a Japanese woman. Anat Sci Int. 2007;82:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kara E, Celebi B, Yildiz A, Ozturk N, Uzmansel D. An unusual case of a tortuous abdominal aorta with a common celiacomesenteric trunk: demonstrated by angiography. Clinics (Sao Paulo). 2011;66:169-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Lin J. Celiomesenteric trunk demonstrated by 3-dimensional contrast-enhanced magnetic resonance angiography. Hepatobiliary Pancreat Dis Int. 2005;4:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lawler LP, Fishman EK. Celiomesenteric anomaly demonstration by multidetector CT and volume rendering. J Comput Assist Tomogr. 2001;25:802-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | MICHELS NA. Variations in the blood supply of the supramesocolonic organs. J Int Coll Surg. 1949;12:625-627. [PubMed] |
| 17. | Chen H, Yano R, Emura S, Shoumura S. Anatomic variation of the celiac trunk with special reference to hepatic artery patterns. Ann Anat. 2009;191:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Venieratos D, Panagouli E, Lolis E, Tsaraklis A, Skandalakis P. A morphometric study of the celiac trunk and review of the literature. Clin Anat. 2013;26:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Tandler J. Über die varietäten der arteria coeliaca und deren entwickelung. Anat Embryol. 1904;25:473-500. |
| 20. | Higashi N, Sone C. [A case of celiaco-mesenteric trunk]. Kaibogaku Zasshi. 1987;62:550-556. [PubMed] |
| 21. | Petscavage JM, Maldjian P. Celiomesenteric trunk: two variants of a rare anomaly. Australas Radiol. 2007;51 Suppl:B306-B309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Matsuki M, Tanikake M, Kani H, Tatsugami F, Kanazawa S, Kanamoto T, Inada Y, Yoshikawa S, Narabayashi I, Lee SW, Nomura E, Okuda J, Tanigawa N. Dual-phase 3D CT angiography during a single breath-hold using 16-MDCT: assessment of vascular anatomy before laparoscopic gastrectomy. AJR Am J Roentgenol. 2006;186:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, Blumgart LH. CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol. 2007;189:W13-W19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Egorov VI, Yashina NI, Fedorov AV, Karmazanovsky GG, Vishnevsky VA, Shevchenko TV. Celiaco-mesenterial arterial aberrations in patients undergoing extended pancreatic resections: correlation of CT angiography with findings at surgery. JOP. 2010;11:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Ganeshan A, Upponi S, Hon LQ, Warakaulle D, Uberoi R. Hepatic arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol. 2008;19:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | D'Amico TA. Outcomes after surgery for esophageal cancer. Gastrointest Cancer Res. 2007;1:188-196. [PubMed] |
| 27. | Wang Y, Chen P, Shen N, Yang JT, Chen JH, Zhang WG. Celiomesenteric trunk with concurrent aneurysm: report of a case. Surg Today. 2010;40:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |