Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3945
Peer-review started: September 5, 2019
First decision: September 23, 2019
Revised: October 14, 2019
Accepted: October 30, 2019
Article in press: October 30, 2019
Published online: December 6, 2019
Processing time: 92 Days and 9.1 Hours
Diabetic kidney disease (DKD) is a common complication of diabetes. The patient’s prognosis is poor once DKD progresses to advanced stage. Accurate diagnosis and timely treatment of early DKD are important for improving patient’s prognosis and reducing mortality.
To explore the value of elastography point quantification (ElastPQ) in improving the accuracy of early DKD diagnosis.
A total of 69 patients with type 2 diabetes were recruited from Naval Military Medical University Affiliated Gongli Hospital. Patients were divided into early DKD group and medium DKD group according to pathological results and urinary albumin excretion rate (UAER). Another 40 patients with simple diabetes were included as the diabetes group. The baseline data, laboratory diagnostic indicators, and ultrasound indicators for each patient were recorded. The differences of the indicators in the three groups were compared. Multivariate logistic regression was used to analyze the influencing factors of the development from simple diabetes into early DKD and from early DKD into medium DKD. Receiver operating characteristic analyses of potential indicators in identifying early DKD and medium DKD, and early DKD and simple diabetes were established.
Multivariate logistic regression analysis showed that UAER (P < 0.001), renocortical Young's Modulus (YM) (P < 0.001), and renal parenchymal thickness (P = 0.013) were the independent influencing factors of the development from early DKD into medium DKD. Diabetes duration (P = 0.041), UAER (P = 0.034), and renocortical YM (P = 0.017) were the independent influencing factors of the development from simple diabetes into early DKD. Receiver operating characteristic analysis indicated that UAER, renocortical YM, and renal parenchymal thickness were accurate in identifying early DKD and medium DKD [all area under curve (AUC) > 0.9]. The accuracy of UAER (AUC = 0.744), diabetes duration (AUC = 0.757), and renocortical YM (AUC = 0.782) for the diagnosis of early DKD and simple diabetes were limited. However, the combined diagnosis of UAER, diabetes duration, and renocortical YM was accurate in identifying early DKD and simple diabetes (AUC = 0.906), which was significantly higher than any of the three indicators (all P < 0.05).
ElastPQ is of great value in the diagnosis of early DKD. When combined with the diabetes duration and UAER, it is expected to diagnose accurately early DKD.
Core tips: Early diabetic kidney disease (DKD) can be controlled or even reversed, but kidney function will be severely damaged once it develops into the advanced stage. Currently, staging of DKD relies mainly on urinary albumin excretion rate and clinical symptoms, but it is difficult to determine accurately early DKD patients. This study used elastography point quantification to measure changes in kidney stiffness in patients with different stages of DKD. It revealed that elastography point quantification can make accurate differential diagnosis for early and medium DKD and, when combined with diabetes duration and urinary albumin excretion rate, it can significantly improve the accuracy of early diagnosis of DKD.
- Citation: Liu QY, Duan Q, Fu XH, Fu LQ, Xia HW, Wan YL. Value of elastography point quantification in improving the diagnostic accuracy of early diabetic kidney disease. World J Clin Cases 2019; 7(23): 3945-3956
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/3945.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.3945
Diabetic kidney disease (DKD) is a common chronic microvascular complication of diabetes and a common cause of end-stage renal disease (ESRD)[1-3]. Early kidney damage in DKD can be controlled or even reversed, but renal function will be seriously damaged once it develops into advanced DKD, and there is currently no effective treatment[4]. Therefore, early detection, early diagnosis, and early treatment of DKD are of great significance. Currently DKD is diagnosed by urinary albumin excretion rate (UAER), serum creatinine (Scr), blood urea nitrogen (BUN), etc. Needle biopsy of kidney is the gold standard for diagnosing DKD at different stages[5,6].
DKD is usually divided into five stages based on Mogensen stage[7]. Stage I and II are early stage, and changes in the indicators at this stage are not easy to detect. Stage III is mid-term stage, and persistent microalbuminuria can be found in patients. Stage IV and V are advanced stage. The glomerular filtration rate is significantly reduced, and a large amount of proteinuria will be found at this stage. In the early stage of DKD, due to the strong compensatory capacity of the kidney, the results of multiple renal indicators can still be in the normal range if the kidney damage is not serious. However, the damage to the kidney is already present[5]. If not intervened in time, the treatment efficacy in the advanced stage is limited. The research of Grenier et al[8] found that the renal parenchyma elasticity of DKD patients at different stages was changed in ultrasound elastography. As the course of DKD progresses, kidney elasticity gradually decreases and its hardness gradually increases[9,10]. However, the changes of renal elasticity in patients with early DKD are not obvious, although ultrasound elastography can detect differences in patients with advanced DKD[11]. The elastography point quantification (ElastPQ) used in this study is a new shear wave elastography technique that can detect the Young's Modulus (YM) of the region of interest[12]. In this study, the ElastPQ technique was used to measure the changes of renal hardness in patients with different stages of DKD, and the improvement in the accuracy for diagnosing early DKD was analyzed after combining ElastPQ with UAER, Scr, and BUN, etc.
Patients diagnosed with type 2 diabetes at Naval Military Medical University Affiliated Gongli Hospital from January 2015 to May 2019 were recruited as subjects. Inclusion criteria were as follows: (1) Meeting the diagnostic criteria for diabetes[13]; (2) DKD diagnosed by a kidney physician based on retinal changes and other diagnostic indicators; (3) Patients who meet the Mogensen stage criteria I-III; and (4) Aged 18- to 80-years-old. Exclusion criteria were as follows: (1) Combined with other primary kidney disease; (2) Pregnant women, lactating women, or severely mental disease; and (3) Patients with malignant tumors that are not cured or require chemotherapy or radiation therapy. According to the UAER changes, the patients were divided into the early group (Mogensen I, II, UAER < 30 mg/24 h) and the middle group (Mogensen III, 30 mg/24 h ≤ UAER < 300 mg/24 h). Another 40 patients with simple diabetes were included as the diabetes group. All patients were fully informed of the study and signed informed consent. The study was approved by the Ethics Committee of Naval Military Medical University Affiliated Gongli Hospital.
The medical histories of all subjects were collected in detail, and the patients’ age, gender, blood pressure, and diabetes duration were recorded.
After fasting for 12 h, fasting venous blood samples were obtained for examination. The subjects were tested for Scr, BUN, blood glucose, glycosylated hemoglobin A1C (HBA1C), Cystatin C (CysC), triglyceride (TG), high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), urine routine, UAER, and creatinine clearance (Ccr) by LABOSPECT008 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
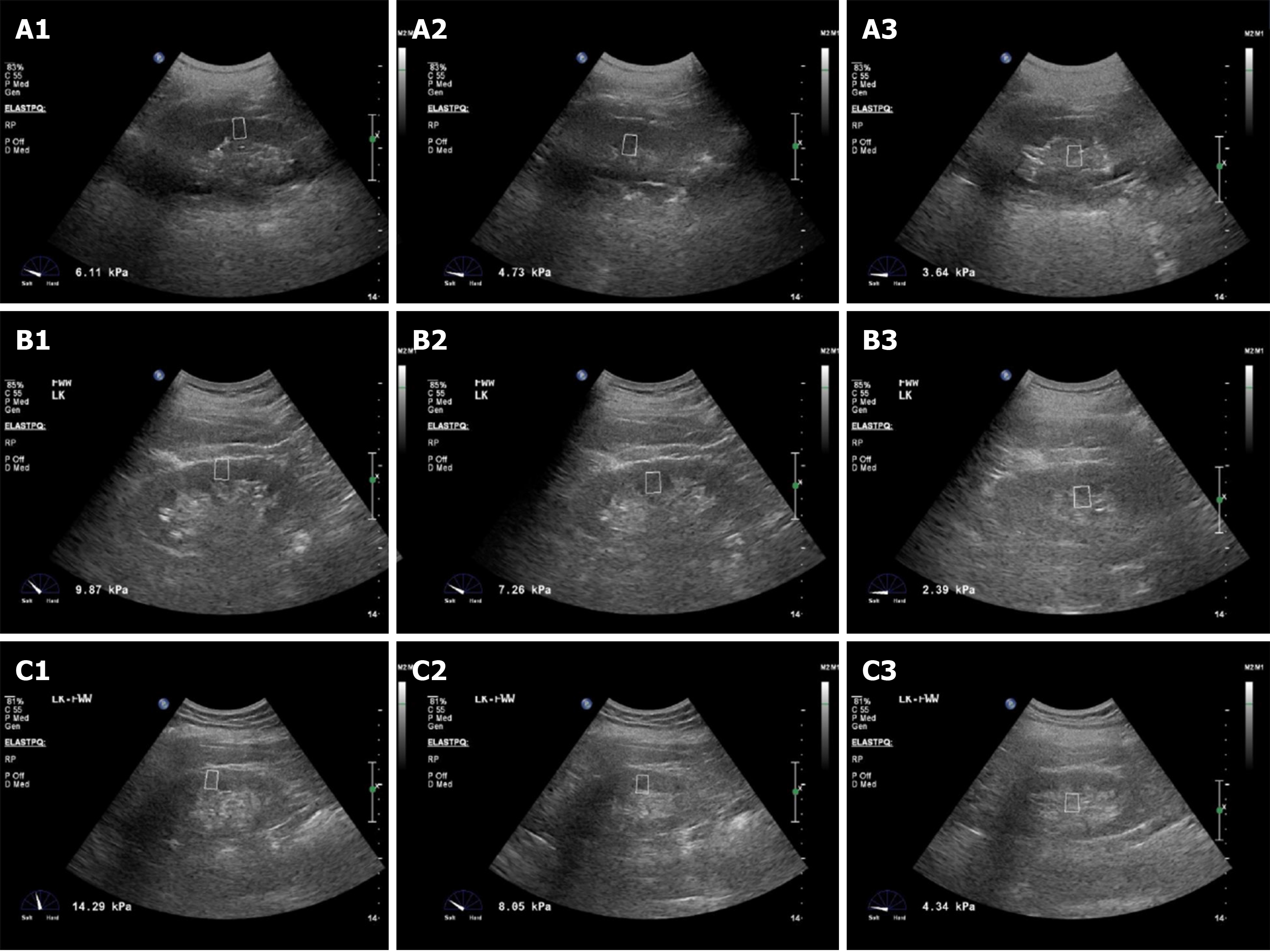
An IU ELITE Ultrasound imaging system (Philips, Eindhoven, Netherlands) with a C5-1 convex array probe was used in this study. The patient was placed in the lateral position, and a two-dimensional ultrasound examination was performed to observe the shape, size, and renal parenchymal thickness of the kidney. Renal hemodynamics was observed by color Doppler, and the blood flow resistance index (RI) of the renal artery trunk was measured. Subsequently, after displaying the ultrasound image of long axis of kidney, the virtual touch tissue quantification mode of the ElastPQ imaging software was turned on. The patient was requested to hold their breath and after the image was stable, the YMs of patient's kidney cortex, medulla, and renal sinus were measured. At least three effective measurements were repeated, and the mean value was calculated. The kidney with a higher side of YM was selected as the target organ. Two experienced sonographers were invited to perform ultrasound examinations of all patients, and the repeatability between different observers was observed.
The statistical analysis was performed using SPSS (Armonk, NY, United States) software (version 11.0) and Medcalc software (version 11.0, Ostend, Belgium). The numerical data were expressed as mean ± standard deviation, and the comparison in the three groups was performed using one way analysis of variance and Student-Newman-Keuls was used for pairwise comparison. The categorical variables were expressed as number and percentage, and the comparison between the two groups was performed by the Chi-Square test. The YM values of the renal cortex, medulla, and renal sinus measured by two sonographers were tested by Bland-Altman analysis and intraclass correlation coefficient (ICC). ICC > 0.75 indicates good repeatability, 0.4 < ICC ≤ 0.75 indicates repetitiveness, and ICC ≤ 0.4 indicates poor repeatability. Multivariate logistic regression was used to analyze the influencing factors of the development from simple diabetes into early DKD and from early DKD into medium DKD. Receiver operating characteristic (ROC) analyses of potential indicators in identifying early DKD and medium DKD, and early DKD and simple diabetes were established. Logistic regression model was used to establish a combined diagnosis, and the accuracy of the combined diagnosis to identify simple diabetes and early DKD was explored. Statistical significance was defined as 2-tailed P < 0.05 for all tests.
There were 40 patients in the diabetes group, including 21 males and 19 females in this study. In total, 36 patients were in the early DKD group, including 20 males and 16 females, and 33 patients were in the medium DKD group, including 17 males and 16 females. The differences in the performance of ElastPQ in each group of kidneys are shown in Figure 1. It showed that in any group of patients, the YM of different kidney locations was: Cortex > medulla > renal sinus. The kidney hardness increased gradually with the severity of DKD. The hardness of renal cortex, medulla, and renal sinus in patients with medium DKD was greater than those of early DKD. The hardness in patients with early DKD was larger than those in patients with simple diabetes.
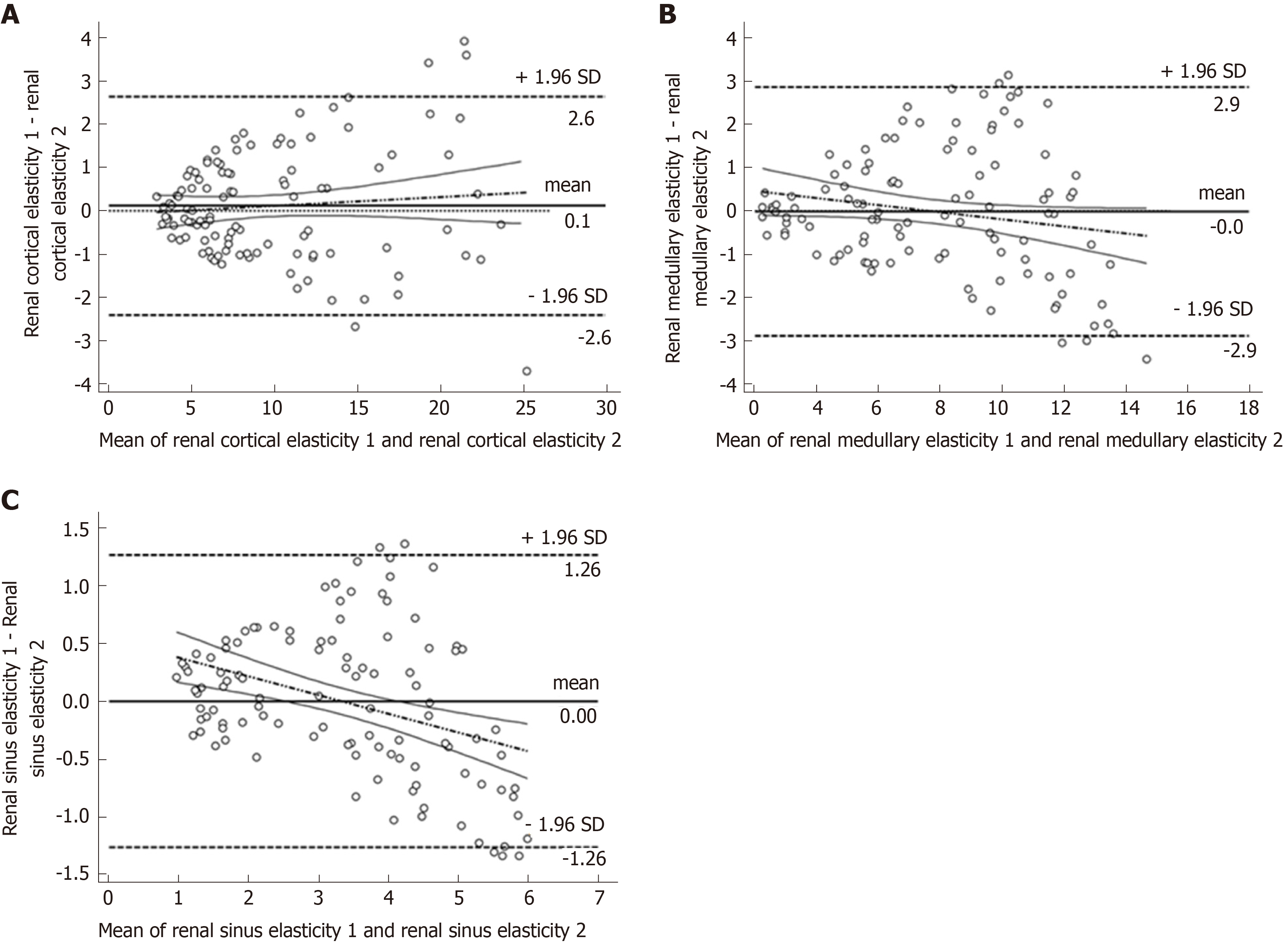
This study used Bland-Altman for consistency testing to assess the error of two sonographers in detecting the YM in renal cortex, medulla, and renal sinus. The results revealed that the consistency of YM in renal cortex, medulla, and renal sinus was excellent. The mean difference of renocortical YM was 0.1 kPa, 95% consistency limit ranged in (-2.4, 2.6) kPa (Figure 2A), and the ICC result was 0.973. The mean difference of the renal medulla YM was 0 kPa, the 95% consistency limit ranged in (-2.9, 2.9) kPa (Figure 2B), and the ICC result was 0.904. The mean difference of renal sinus YM was 0 kPa, the 95% consistency limit ranged in (-1.26, 1.26) kPa (Figure 2C), and the ICC result was 0.909.
The clinical data in the diabetes group, early DKD group, and medium DKD group found that the diabetes duration had an increasing trend in the three groups. The difference was statistically significant (F = 4.931, P = 0.003). The diabetes duration in the early DKD group and medium DKD group was larger than that in the diabetes group (both P < 0.05). The remaining clinical data, including age, gender, systolic blood pressure, and diastolic blood pressure, were not significantly different in the three groups (all P > 0.05). The comparison of the laboratory indicators in the three groups revealed that the UAER, Scr, BUN, and CysC showed an increasing trend in the three groups, and Ccr showed a decreasing trend. The pairwise comparison showed that the UAER, Scr, BUN, CysC, and Ccr in the medium DKD group were statistically different from those in the diabetes group (all P < 0.05). It is worth noting that the UAER in the early DKD group was also statistically different from the medium DKD group and the diabetes group (P < 0.05). The other laboratory tests, including blood glucose, HBA1C, UA, TG, HDL, and LDL, were not significantly different in the three groups (all P > 0.05). The comparison of the ultrasound indicators showed that the renocortical YM and renal artery RI showed an increasing trend in the three groups, and the renal parenchymal thickness showed a decreasing trend. The differences were statistically significant (P < 0.05). The pairwise comparison showed that the renocortical YM, renal artery RI, and renal parenchymal thickness in the medium DKD group were statistically different from those in the diabetes group (P < 0.05). Notably, renocortical YM in the early DKD group was statistically different from that in the medium DKD group and diabetes group. The renal parenchymal thickness of the early DKD group was statistically different from that of the medium DKD group (P < 0.05). The remaining ultrasound indicators, including renal medullary YM, renal sinus YM, and renal volume, were similar among the three groups (all P > 0.05) (Table 1).
| Diabetes group (n = 40) | Early DKD group (n = 36) | Medium DKD group (n = 33) | F/X2 value | P value | |
| Gender (Male/Female) | 21/19 | 20/16 | 17/16 | 0.126 | 0.939 |
| Age (yr) | 56.61 ± 10.11 | 55.21 ± 10.56 | 57.53 ± 11.46 | 0.973 | 0.412 |
| Diabetes duration (yr) | 3.55 ± 1.65 | 5.44 ± 1.70a | 6.73 ± 5.69a | 4.931 | 0.003 |
| Systolic blood pressure (mmHg) | 128.2 ± 19.60 | 130.0 ± 18.40 | 133.5 ± 22.10 | 1.735 | 0.162 |
| Diastolic blood pressure (mmHg) | 76.6 ± 16.50 | 80.1 ± 15.40 | 82.5 ± 16.20 | 1.836 | 0.146 |
| Blood glucose (mmol/L) | 4.9 ± 0.6 | 5.5 ± 1.1 | 5.9 ± 1.3 | 1.022 | 0.388 |
| HBA1C (%) | 6.80 ± 1.25 | 7.22 ± 1.66 | 7.54 ± 1.38 | 2.391 | 0.073 |
| UAER (mg/24 h) | 13.94 ± 1.85 | 15.35 ± 8.71a | 61.74 ± 23.72ab | 5.822 | < 0.001 |
| BUN (mmol/L) | 4.91 ± 2.04 | 5.73 ± 1.23 | 6.59 ± 2.45a | 3.702 | 0.014 |
| Scr (μmol/L) | 55.83 ± 14.12 | 61.75 ± 11.20 | 69.23 ± 13.56a | 4.523 | 0.005 |
| Ccr (ml/min) | 105.12 ± 15.1 | 98.23 ± 17.1 | 83.57 ± 23.7a | 3.821 | 0.012 |
| CysC (mg/L) | 0.73 ± 0.49 | 1.06 ± 0.67 | 1.79 ± 0.88a | 3.044 | 0.032 |
| UA (μmol/L) | 249.31 ± 60.45 | 256.22 ± 58.24 | 266.84 ± 54.26 | 2.251 | 0.087 |
| TG (mmol/L) | 1.85 ± 1.36 | 1.92 ± 1.55 | 2.03 ± 1.42 | 2.362 | 0.075 |
| HDL (mmol/L) | 1.25 ± 0.35 | 1.09 ± 0.23 | 1.13 ± 0.35 | 1.703 | 0.172 |
| LDL (mmol/L) | 2.66 ± 0.55 | 2.85 ± 0.57 | 2.82 ± 0.55 | 2.022 | 0.115 |
| YM (kPa) | |||||
| Renal cortex | 5.51 ± 1.53 | 7.93 ± 2.66a | 16.68 ± 4.48ab | 0.383 | 0.021 |
| Renal medullary | 4.29 ± 1.62 | 6.67 ± 3.22 | 8.32 ± 3.56 | 2.571 | 0.058 |
| Renal sinus | 2.89 ± 1.32 | 3.46 ± 1.24 | 3.81 ± 1.05 | 2.493 | 0.064 |
| Renal artery RI | 0.64 ± 0.06 | 0.69 ± 0.08 | 0.76 ± 0.09a | 3.182 | 0.027 |
| Renal volume (mL) | 105 ± 16.91 | 116 ± 17.10 | 112 ± 16.35 | 2.044 | 0.113 |
| Renal parenchymal thickness (mm) | 13.89 ± 2.66 | 14.57 ± 1.87 | 10.99 ± 1.83ab | 3.181 | 0.015 |
Table 1 revealed that the diabetes duration, UAER, Scr, BUN, Ccr, CysC, renocortical YM, renal artery RI, and renal parenchymal thickness were different between the early DKD group and the medium DKD group. The subsequent logistic regression found that only UAER (odds ratio (OR) = 1.725, P < 0.001), renocortical YM (OR = 4.782, P < 0.001), and renal parenchymal thickness (OR = 2.174, P = 0.013) were the independent influencing factors (Table 2).
| B | SE | Wald | P value | OR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| Diabetes duration | 0.233 | 0.414 | 1.236 | 0.083 | 1.263 | 0.561 | 2.843 |
| UAER | 0.545 | 0.124 | 7.161 | < 0.001 | 1.725 | 1.353 | 2.200 |
| BUN | 1.076 | 0.794 | 0.883 | 0.183 | 2.934 | 0.619 | 13.910 |
| Scr | 0.177 | 0.372 | 0.368 | 0.294 | 1.194 | 0.576 | 2.475 |
| Ccr | -0.134 | 0.126 | 0.802 | 0.195 | 0.875 | 0.684 | 1.120 |
| CysC | 0.989 | 0.583 | 1.094 | 0.116 | 2.688 | 0.857 | 8.427 |
| Renocortical YM | 1.565 | 0.391 | 5.294 | < 0.001 | 4.782 | 2.222 | 10.290 |
| Renal artery RI | 0.318 | 0.218 | 1.584 | 0.068 | 1.375 | 0.897 | 2.108 |
| Renal parenchymal thickness | 0.777 | 0.268 | 2.947 | 0.013 | 2.174 | 1.286 | 3.676 |
The clinical data, laboratory indicators, and ultrasound indicators of the early DKD group were similar to those of the diabetes group. The pairwise comparison in Table 1 revealed that only diabetes duration, UAER, and renocortical YM were different. Subsequent logistic regression found that the diabetes duration (OR = 1.384, P = 0.041), UAER (OR = 1.638, P = 0.034), and renocortical YM (OR = 3.792, P = 0.017) were the independent factors (Table 3).
| B | SE | Wald | P value | OR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| Diabetes duration | 0.325 | 0.084 | 3.087 | 0.041 | 1.384 | 1.174 | 1.632 |
| UAER | 0.493 | 0.182 | 3.295 | 0.034 | 1.638 | 1.147 | 2.340 |
| BUN | 1.027 | 0.649 | 0.683 | 0.207 | 2.794 | 0.783 | 9.969 |
| Scr | 0.185 | 0.482 | 0.446 | 0.278 | 1.203 | 0.468 | 3.094 |
| Ccr | -0.087 | 0.183 | 0.808 | 0.201 | 0.917 | 0.641 | 1.313 |
| CysC | 0.953 | 0.575 | 0.732 | 0.205 | 2.593 | 0.840 | 8.003 |
| Renocortical YM | 1.333 | 0.264 | 3.742 | 0.017 | 3.792 | 2.260 | 6.362 |
| Renal artery RI | 0.459 | 0.482 | 0.963 | 0.193 | 1.583 | 0.615 | 4.072 |
| Renal parenchymal thickness | 0.911 | 0.637 | 2.859 | 0.083 | 2.486 | 0.713 | 8.664 |
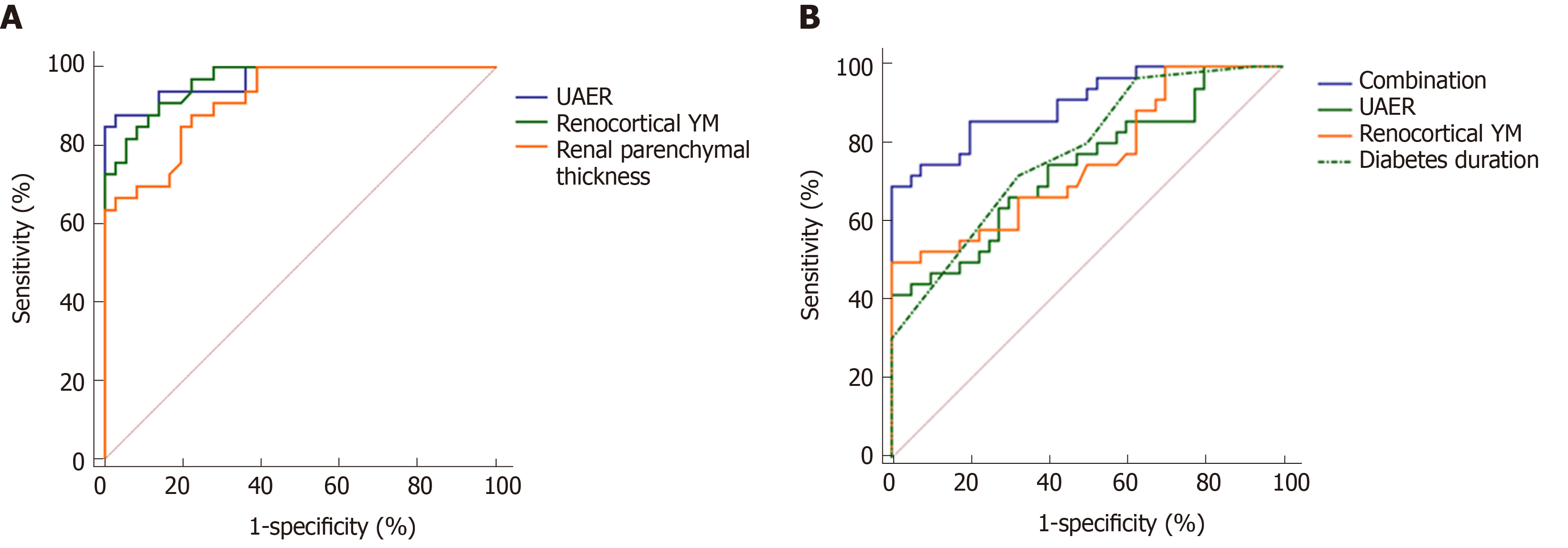
The ROC was used to analyze further the accuracy of UAER, renocortical YM, and renal parenchymal thickness in identifying early and medium DKD (Figure 3A). The identified accuracy of UAER was the highest [area under curve (AUC) = 0.969, 95% confidence interval (CI): 0.896-0.996; Sensitivity = 87.9%, Specificity = 97.2%], but the identified accuracy of renocortical YM (AUC = 0.964, 95%CI: 0.889-0.994; Sensitivity = 90.9%, Specificity = 86.1%) and renal parenchymal thickness (AUC = 0.919, 95%CI: 0.828-0.971; Sensitivity = 87.9%, Specificity = 77.8%) were similar to that of UAER. The differences were not statistically significant (UAER vs renocortical YM: z = 0.186, P = 0.852; UAER vs renal parenchymal thickness: z = 1.502, P = 0.133).
The ROC was established to analyze further the accuracy of UAER, diabetes duration, and renocortical YM in identifying early DKD and simple diabetes (Figure 3B). It revealed that the accuracies of UAER, diabetes duration, and renocortical YM were not high (UAER: AUC = 0.744, 95%CI: 0.631-0.838; Sensitivity = 41.7%, Specificity = 100%; diabetes duration: AUC = 0.757, 95%CI: 0.645-8.48; Sensitivity = 72.2%, Specificity = 67.5%; renocortical YM: AUC = 0.782, 95%CI: 0.673-0.869; Sensitivity = 50.0%, Specificity = 100%). None of them can be used independently to identify early DKD and simple diabetes. Therefore, this study combined UAER, diabetes duration, and renocortical YM based on logistic regression model. It found that the combination can accurately identify early DKD and simple diabetes (AUC = 0.906, 95%CI: 0.816-0.961; Sensitivity = 50.0%, Specificity = 100%), which was significantly greater than the AUC of any indicator (Combination vs UAER: z = 3.131, P = 0.002, Combination vs diabetes duration: z = 2.572, P = 0.010, Combination vs renocortical YM: z = 3.278, P = 0.001).
DKD is one of the main causes of ESRD and even death[14]. Once DKD develops into Mogensen stage IV or V, kidney will undergo irreversible damage and be unresponsive to treatment. Therefore, timely and accurate diagnosis of kidney damage in DKD patients is of great significance for effective control of disease progression. Renal biopsy is the gold standard for diagnosing different stages of DKD, but it is an invasive method that cannot be used as a screening tool for early DKD. In the non-invasive diagnosis of DKD, it is often diagnosed by biochemical indicators such as UAER, Scr, and BUN. However, due to the strong compensatory capacity, these examination methods often fail to diagnose accurately early DKD. It is now known that pathological damage to the kidneys already exists before these laboratory tests show significant abnormalities. The elastic changes of early DKD (especially the renal cortex) may appear earlier than the abnormalities of UAER, Scr, and BUN. The latest research uses ultrasound elastography to study the pathological and structural changes in DKD kidneys[15,16]. A study by Hassan et al[17] found that ultrasound elastography can evaluate chronic pathological changes in advanced DKD. Bob et al[18] used elastography to evaluate patients with DKD and found that the elasticity of the renal cortex was significantly reduced as the degree of impaired renal function worsened. However, studies on the changes in kidney elasticity of early DKD are still rarely reported. Therefore, this study utilized ElastPQ to evaluate quantitatively renal damage in early and medium DKD and combined UAER, Scr, BUN, and other indicators to diagnose differentially early and medium DKD and identify early DKD and simple diabetes in order to improve the accuracy of early DKD diagnosis.
ElastPQ is a new shear wave elastography technology that displays the elasticity of kidney tissue and quantifies tissue elasticity through YM. The larger the value, the greater the hardness of the tissue. The consistency analysis of this study proved that ElastPQ had very high consistency between different observers. The consistency of YMs in renal cortex, medulla, and renal sinus were excellent and can be widely used in clinic. In this study, the ElastPQ technique was used to compare the elastic differences in renal cortex, medulla, and renal sinus among the patients with simple diabetes, early DKD, and medium DKD, so as to explore the elastic changes of different kidneys in different patients. Due to differences in compositional structures, there is a difference in elasticity between different locations of the same kidney. The hardness of the kidney showed a progressive relationship: renal cortex > renal medulla > renal sinus. In ElastPQ, the YM of the renal cortex was significantly greater than that of the renal medulla and renal sinus. In addition, as the DKD progresses, kidney elasticity will change. After DKD occurs, the glomerulus appears to be hardened, the renal tubules atrophy, and the basement membrane thickens, causing interstitial fibrosis. All of the above changes lead to fibrosis of the renal parenchyma and increased hardness[10]. Therefore, the results of ElastPQ in this study revealed that kidney YM would gradually increase with the increase of DKD stage.
In this study, after one-way analysis of variance and pairwise comparisons in patients with simple diabetes, early DKD, and medium DKD, nine indicators were screened in different DKD states. They were diabetes duration, UAER, BUN, Scr, CysC, Ccr, renocortical YM, renal artery RI, and renal parenchymal thickness. The diabetes duration was the only clinical data related to the progression of DKD. The longer the course of the disease, the easier the kidney was to undergo microvascular changes and the greater the likelihood of developing DKD. Scr, BUN, CysC, and Ccr were all common indicators used to evaluate glomerular filtration function. It was found that with the progress of DKD, Scr, BUN, and CysC increased and Ccr decreased, and the difference of Scr was the largest, while the difference of CysC was the smallest. This is because although Scr, BUN, and CysC are all filtered through the glomerulus, under normal conditions, most of Scr and BUN are excreted by the kidney and CysC is almost completely reabsorbed by the renal tubule. Therefore, when glomerular filtration function is severely impaired, the concentration of Scr and BUN in the blood changes more significantly, and when the filtration function is less impaired, the change of CysC is more sensitive[19,20].
Ccr is different from the traditional Scr test, 24 h urine needs to be collected for calculation. Its reduction can more accurately indicate the decrease of glomerular filtration rate compared with Scr. Among all clinical indicators, the difference in UAER is the most obvious, and it is also the most commonly used indicator for diagnosing DKD[5]. The studies of Duvnjak et al[21] and Keyzer et al[22] revealed that after the occurrence of DKD, renal blood flow self-regulation function decreased. Glomerular function was in a high perfusion and high filtration state. The dysfunction of endothelial cells leads to the damage of glomerular filtration barrier and eventually leads to the continued formation of microalbuminuria.
Unlike the laboratory indicators, ultrasound can more intuitively indicate the changes in kidney morphology, blood flow, and elasticity caused by DKD. This study found that with the progress of DKD, the renal parenchymal thickness increased first and then decreased. This is because in the early stage of DKD, the glomerular basement membrane is thickened and the mesangial matrix is increased, resulting in an increase in the thickness of the renal parenchyma. With the further development of DKD, the glomerular structure is gradually destroyed, and the occluded glomerulus is increased, which leads to the reduction of the kidney and the decrease of the thickness of the renal parenchyma[17,23]. The changes of renal artery RI are significantly associated with the changes in renal hemodynamics[24,25]. With the progress of DKD, the glomerular hyperperfusion state gradually deteriorates, the basement membrane thickens, and the glomerulus hardens. Ultrasound showed a decrease in diastolic blood flow velocity and an increase in renal artery RI. Similarly, as the glomerular sclerosis changes gradually, the renal cortical elasticity and the renocortical YM gradually increases[26,27].
By comparing data between different groups, it was found that the diabetes duration, UAER, Scr, BUN, Ccr, CysC, renocortical YM, renal artery RI, and renal parenchymal thickness were different between the early DKD group and medium DKD group. However, further logistic regression showed that only UAER, renocortical YM, and renal parenchymal thickness were the independent factors in the development from early DKD to medium DKD. This is mainly because Scr, BUN, Ccr, and other indicators are susceptible to interference from diet, exercise, and other factors. Only when the glomerular filtration rate is reduced to a certain extent will they change significantly[28].
UAER is the most commonly used DKD diagnosis and staging indicator in clinical practice. The results of logistic regression confirm the value of UAER. The result that renocortical YM and renal parenchymal thickness became the independent influencing factors suggested that ultrasound assessment of renal elasticity and morphological also had the potential to identify early and medium DKD. This study performed a further ROC analysis to explore the accuracy of UAER, renocortical YM, and renal parenchymal thickness in identifying early and medium DKD. The results showed that the accuracy of UAER was the highest. It is indeed an excellent indicator for identifying early and medium DKD. It can be used as a criterion for identifying early and medium DKD in clinic. The accuracies of renocortical YM and renal parenchymal thickness were similar to that of UAER (both AUC > 0.9), and the differences were not statistically significant. It statistically demonstrated that the assessment of renal elasticity and morphological changes by ultrasound can be used to identify early and medium DKD as well as UAER.
The early diagnosis of DKD is very important. If early DKD can be detected and early intervention started, patients will get a good prognosis. However, it is difficult to diagnose early DKD in clinic, because the strong compensatory capacity of the kidney makes the pathological changes of kidney and UAER changes not obvious. By comparing the data from the simple diabetes group and early DKD group, the differences of clinical data, laboratory indicators, and ultrasound indicators were similar. There were only differences in the diabetes duration, UAER, and renocortical YM. Further logistic regression analysis found that the diabetes duration, UAER, and renocortical YM were the independent factors. It suggests that the three indicators have certain value for the identification of early DKD. The commonly used renal function and ultrasound indicators are not independent factors, and it is difficult to be used for the diagnosis of early DKD. That also indicated the current clinical difficulty in the diagnosis of early DKD. We performed ROC analysis to further explore the ability of these three indicators in diagnosing early DKD. The results showed that UAER (AUC = 0.744), diabetes duration (AUC = 0.757), and renocortical YM (AUC = 0.782) were not highly accurate for the identification of early DKD and simple diabetes. However, the combination of UAER, diabetes duration, and renocortical YM based on logistic regression model found that the combination significantly improved the accuracy (AUC > 0.9). The accuracy of the combination was significantly higher than that of the three indicators independently. It suggested that it was hopeful to diagnose early DKD by combining UAER, diabetes duration, and renocortical YM based on logistic regression model. It may help to prompt early clinical intervention and improve patient prognosis.
This study proposes the diagnosis of early DKD through a combined model of diabetes duration, UAER, and renocortical YM. However, its clinical application value is still unclear. The next step of the study is to conduct a prospective study to verify the accuracy and reliability of the combined model and to make reasonable adjustments to the protocol based on the obtained results, in order to obtain a clinically applicable accurate diagnosis method of early DKD.
In conclusion, the evaluation of renal elasticity by ElastPQ can make an accurate differential diagnosis of early and medium DKD, and the accuracy of differential diagnosis of early DKD and simple diabetes is significantly improved after combined with diabetes duration and UAER. Therefore, ElastPQ is of great value for improving the accuracy of early DKD diagnosis. It is expected to help clinical diagnosis in the early DKD and help timely intervention.
Diabetic kidney disease (DKD) is one of the common causes of end-stage renal disease. Accurate and timely diagnosis of early DKD are significant for improving prognosis and reducing mortality. Kidney biopsy is the golden standard in DKD diagnosis, but it is an invasive examination method that cannot be used as a screening tool. To date, DKD is often diagnosed by biochemical indicators such as urinary albumin excretion rate (UAER), serum creatinine, and blood urea nitrogen, but these fail to make accurate diagnosis of early DKD because of the strong compensatory capacity of kidney.
Elastography point quantification (ElastPQ) is a new shear wave elastography technology that displays the elasticity of renal tissue and quantifies tissue elasticity through Young's Modulus (YM). Recently, researchers have found that with the progression of DKD, the elasticity of the renal tissue gradually decreases. However, studies about the renal tissue elasticity of early DKD are still lacking, and it is uncertain whether ElastPQ can improve the accuracy of early DKD diagnosis.
In this study, the ElastPQ technique was used to measure the changes of renal elasticity in patients with different stages of DKD. Our study aims to explore the value of ElastPQ in improving the accuracy of early DKD diagnosis.
A total of 69 patients with type 2 diabetes who underwent renal biopsy were recruited and divided into early DKD group and medium DKD group. At the same time, 40 patients with simple diabetes were enrolled as the diabetes group. The basic data, clinical indicators, and ultrasound indicators of each patient were recorded and compared among the three groups. Multivariate logistic regression was used to analyze the influencing factors of the development of simple diabetes into early DKD, and early DKD into medium DKD. Receiver operating characteristic curves were used to test the accuracy of potential indicators in identifying early DKD and medium DKD, as well as early DKD and simple diabetes.
Our study demonstrated that the consistency of YM in renal cortex, medulla, and renal sinus was excellent. Multivariate logistic regression showed that UAER, renal cortical YM, and renal parenchymal thickness were the independent influencing factors of the development of early DKD into medium DKD. Receiver operating characteristic analysis showed that they were accurate in identifying early DKD and medium DKD. As for the development from simple diabetes into early DKD, diabetes duration, UAER, and renal cortical YM were independent factors. However, the accuracy of UAER, diabetes duration, and renocortical YM for the identification of early DKD and simple diabetes were limited. Logistic regression model was used to combine UAER, diabetes course, and renal cortical YM, and it found that the accuracy of the combined indicators was significantly higher than any of the three indicators individually in identifying early DKD and simple diabetes.
ElastPQ is of great value to improve the accuracy of early DKD diagnosis. After combined with diabetes duration and UAER, it is expected to predict accurately early DKD, which is helpful for early clinical diagnosis of DKD.
In order to explore further the clinical application value of the combined model of diabetes duration, UAER, and renocortical YM in early DKD diagnosis, this study plans to verify the accuracy and reliability of the combined model through prospective studies. Based on the results, the protocol should be adjusted reasonably in order to obtain a clinically applicable accurate diagnosis method of early DKD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arville B, Kantsevoy S S-Editor: Wang JL L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Tong L, Adler SG. Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2018;13:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Lin CH, Chang YC, Chuang LM. Early detection of diabetic kidney disease: Present limitations and future perspectives. World J Diabetes. 2016;7:290-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 3. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1792] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 4. | Tain YL, Hsu CN. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int J Mol Sci. 2017;18:E381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Chen C, Wang C, Hu C, Han Y, Zhao L, Zhu X, Xiao L, Sun L. Normoalbuminuric diabetic kidney disease. Front Med. 2017;11:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Futrakul N, Futrakul P. Biomarker for early renal microvascular and diabetic kidney diseases. Ren Fail. 2017;39:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32 Suppl 2:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 557] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Grenier N, Gennisson JL, Cornelis F, Le Bras Y, Couzi L. Renal ultrasound elastography. Diagn Interv Imaging. 2013;94:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Lin HY, Lee YL, Lin KD, Chiu YW, Shin SJ, Hwang SJ, Chen HC, Hung CC. Association of Renal Elasticity and Renal Function Progression in Patients with Chronic Kidney Disease Evaluated by Real-Time Ultrasound Elastography. Sci Rep. 2017;7:43303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Goya C, Kilinc F, Hamidi C, Yavuz A, Yildirim Y, Cetincakmak MG, Hattapoglu S. Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am J Roentgenol. 2015;204:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Samir AE, Allegretti AS, Zhu Q, Dhyani M, Anvari A, Sullivan DA, Trottier CA, Dougherty S, Williams WW, Babitt JL, Wenger J, Thadhani RI, Lin HY. Shear wave elastography in chronic kidney disease: a pilot experience in native kidneys. BMC Nephrol. 2015;16:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Ling W, Lu Q, Lu C, Quan J, Ma L, Li J, He D, Liu J, Yang J, Wen T, Wu H, Zhu H, Luo Y. Effects of vascularity and differentiation of hepatocellular carcinoma on tumor and liver stiffness: in vivo and in vitro studies. Ultrasound Med Biol. 2014;40:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2371] [Cited by in RCA: 2483] [Article Influence: 206.9] [Reference Citation Analysis (0)] |
| 14. | Doshi SM, Friedman AN. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2017;12:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 15. | Peride I, Rădulescu D, Niculae A, Ene V, Bratu OG, Checheriță IA. Value of ultrasound elastography in the diagnosis of native kidney fibrosis. Med Ultrason. 2016;18:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1094] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 17. | Hassan K, Loberant N, Abbas N, Fadi H, Shadia H, Khazim K. Shear wave elastography imaging for assessing the chronic pathologic changes in advanced diabetic kidney disease. Ther Clin Risk Manag. 2016;12:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Bob F, Grosu I, Sporea I, Bota S, Popescu A, Sima A, Şirli R, Petrica L, Timar R, Schiller A. Ultrasound-Based Shear Wave Elastography in the Assessment of Patients with Diabetic Kidney Disease. Ultrasound Med Biol. 2017;43:2159-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Yassine HN, Trenchevska O, Dong Z, Bashawri Y, Koska J, Reaven PD, Nelson RW, Nedelkov D. The association of plasma cystatin C proteoforms with diabetic chronic kidney disease. Proteome Sci. 2016;14:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Ji F, Zhang S, Jiang X, Xu Y, Chen Z, Fan Y, Wang W. Diagnostic and prognostic value of galectin-3, serum creatinine, and cystatin C in chronic kidney diseases. J Clin Lab Anal. 2017;31:e22074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Duvnjak L, Perković MN, Blaslov K. Dipeptidyl peptidase-4 activity is associated with urine albumin excretion in type 1 diabetes. J Diabetes Complications. 2017;31:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Keyzer CA, de Jong MA, Fenna van Breda G, Vervloet MG, Laverman GD, Hemmelder M, Janssen WM, Lambers Heerspink HJ, Navis G, de Borst MH; Holland Nephrology Study (HONEST) Network. Vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease (ViRTUE study): rationale and study protocol. Nephrol Dial Transplant. 2016;31:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Takata T, Koda M, Sugihara T, Sugihara S, Okamoto T, Miyoshi K, Hodotsuka M, Fujise Y, Matono T, Okano J, Hosho K, Iyama T, Fukui T, Fukuda S, Munemura C, Isomoto H. Left Renal Cortical Thickness Measured by Ultrasound Can Predict Early Progression of Chronic Kidney Disease. Nephron. 2016;132:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Grupp C, Koziolek MJ, Wallbach M, Hoxhold K, Müller GA, Bramlage C. Difference between renal and splenic resistive index as a novel criterion in Doppler evaluation of renal artery stenosis. J Clin Hypertens (Greenwich). 2018;20:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Liu J, Zhou F, Wang W, Chen N. PGC-1α ameliorates kidney fibrosis in mice with diabetic kidney disease through an antioxidative mechanism. Mol Med Rep. 2018;17:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Bilgici MC, Bekci T, Genc G, Tekcan D, Tomak L. Acoustic Radiation Force Impulse Quantification in the Evaluation of Renal Parenchyma Elasticity in Pediatric Patients With Chronic Kidney Disease: Preliminary Results. J Ultrasound Med. 2017;36:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Yan P, Zhang Z, Xu Y, Wan Q, Ma H, He J. [Relationship between serum cystatin-C levels and vibrating perception threshold in patients with Type 2 diabetes mellitus]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |