Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3934
Peer-review started: September 6, 2019
First decision: October 24, 2019
Revised: October 30, 2019
Accepted: November 20, 2019
Article in press: November 20, 2019
Published online: December 6, 2019
Processing time: 91 Days and 11.4 Hours
The incidence of acute kidney injury (AKI) in patients with sepsis is high, and the prognosis of patients with septic AKI is poor. The early diagnosis and treatment of septic AKI is of great significance in improving the prognosis of patients with sepsis.
To explore the value of contrast-enhanced ultrasound (CEUS), serum creatinine (Scr), and other indicators in the early diagnosis of septic AKI.
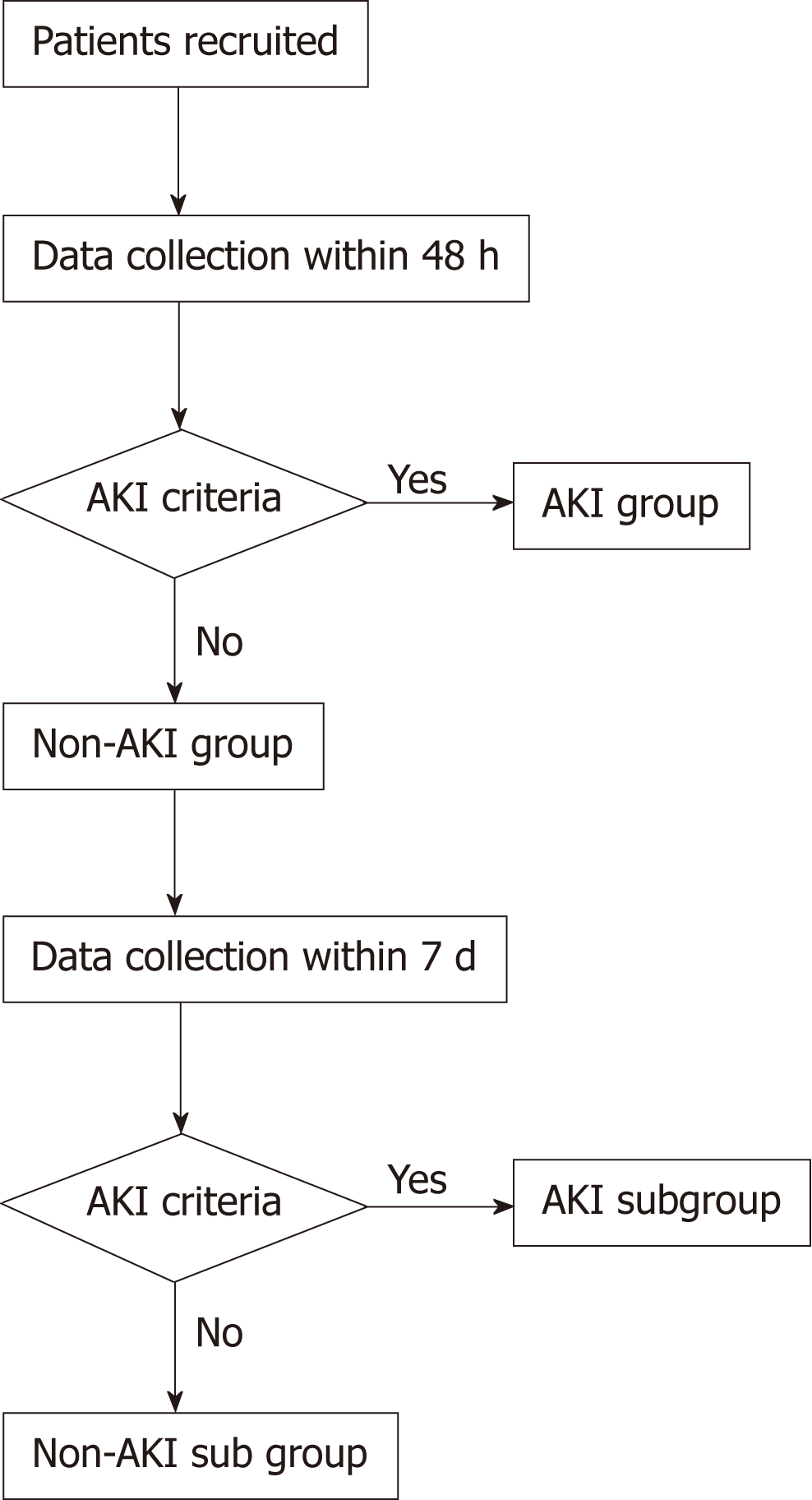
Ninety patients with sepsis during hospitalization at Tongji Hospital of Tongji University were recruited as subjects. Each patient was recorded with relevant basic data, clinical indicators, and CEUS results. The patients were divided into AKI group and non-AKI group according to the results of renal function diagnosis after 48 h. On the 7th day, the renal function of the non-AKI group was re-evaluated and the patients were further divided into AKI subgroup and non-AKI subgroup. The differences of the indicators in different groups were compared, and the diagnostic value of each indicator and their combination for septic AKI was analyzed.
Systemic inflammatory response score (2.58 ± 0.75), blood lactic acid (3.01 ± 1.33 mmol/L), Scr (141.82 ± 27.19 μmol/L), blood urea nitrogen (4.41 ± 0.81mmol/L), and rise time (10.23 ± 2.63 s) in the AKI group were higher than those in the non-AKI group. Peak intensity (PI) (10.78 ± 3.98 dB) and wash in slope (WIS) (1.07 ± 0.53 dB/s) were lower than those in the non-AKI group. The differences were statistically significant (P < 0.05). The PI (12.83 ± 3.77 dB) and WIS (1.22 ± 0.68 dB/s) in the AKI subgroup were lower than those in the non-AKI subgroup, and the differences were statistically significant (P < 0.05). The area under curve (AUC) of Scr for the diagnosis of septic AKI was 0.825 with a sensitivity of 56.76% and a specificity of 100%. The AUCs of WIS and PI (0.928 and 0.912) were higher than those of Scr. Their sensitivities were 100%, but the specificities were 71.70% and 75.47%. The AUC of the combination of three indicators for the diagnosis of septic AKI was 0.943, which was significantly higher than the AUC diagnosed by each single indicator. The sensitivity was 94.59%, and the specificity was 81.13%.
The combination of Scr, PI, and WIS can improve the diagnostic accuracy of septic AKI. PI and WIS are expected to predict the occurrence of early septic AKI.
Core tip: Early detection of risk factors for septic acute kidney injury (AKI) and early intervention are important for the treatment of patients with sepsis. Currently, serum creatinine (SCr) is used as the criterion for AKI diagnosis. But SCr has obvious hysteresis and poor sensitivity, and it is difficult to predict the occurrence of early septic AKI. In this study, contrast-enhanced ultrasound techniques were utilized to explore the value of early diagnosis of septic AKI, and it revealed that the combination of SCr, peak intensity, and wash in slope (contrast-enhanced ultrasound indicators) can improve the early diagnosis of septic AKI.
- Citation: Wang XY, Pang YP, Jiang T, Wang S, Li JT, Shi BM, Yu C. Value of early diagnosis of sepsis complicated with acute kidney injury by renal contrast-enhanced ultrasound. World J Clin Cases 2019; 7(23): 3934-3944
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/3934.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.3934
Sepsis is prone to cause multiple organ dysfunction[1,2]. Studies have reported that the incidence of acute kidney injury (AKI) is higher in patients with sepsis, while the prognosis of septic AKI is poor[3,4]. Early detection of risk factors for septic AKI and early intervention are of great importance. At present, AKI related clinical guidelines use urine volume and serum creatinine (Scr) as the diagnosis criteria. Urine volume is easily affected by some factors such as diuretic and rehydration status, so the increase of Scr level becomes the main criterion[5,6]. However, Scr is less sensitive to the diagnosis of AKI, and the glomerular filtration rate usually has increased more than 50% when Scr begins to rise. It has obvious hysteresis and is difficult to reveal kidney injury in time[7]. Most patients have suffered severe AKI when they are diagnosed, which seriously delays treatment onset[8]. Therefore, exploring a more sensitive detection indicator for AKI is the focus of current research. Studies have reported that blood lactic acid (Lac), C-reactive protein (CRP), and other biochemical indicators may be related to the occurrence of AKI, but its ability to predict AKI is controversial[9,10].
Contrast-enhanced ultrasound (CEUS) can display tissue microcirculation and has the characteristics of convenient operation and no nephrotoxicity. It has been widely used in the monitoring of renal microcirculation under various situations such as kidney transplantation, hypertensive nephropathy, and extracorporeal circulation[11]. Animal experiments have revealed that CEUS can detect changes in renal cortical microcirculation in early ischemia/reperfusion injury, suggesting the possibility of AKI[12], but its role in AKI assessment and diagnosis has not been clinically confirmed.
In this study, patients with sepsis were defined as subjects, and statistical analysis of general data, biochemical indicators, and CEUS indicators were conducted to investigate the diagnostic value for septic AKI. The aim of this study is to realize early intervention in the development of septic AKI, thereby improving patient prognosis.
Patients who developed sepsis during hospitalization in Tongji Hospital of Tongji University from September 2016 to January 2019 were recruited as subjects. Inclusion criteria were as follows: (1) Meet the diagnostic criteria for sepsis[13]; and (2) Age ≥ 18-years-old. Exclusion criteria were as follows: (1) Kidney transplantation, renal benign/malignant tumor, and renal vascular disease before admission; (2) Severe heart failure within 72 h; (3) Patient gave up treatment halfway; (4) Pregnant women, lactating women, patients with mental disabilities; and (5) Poor results of CEUS. The study was approved by the clinical trial ethics committee of Tongji Hospital of Tongji University. All subjects were informed and signed informed consent.
After diagnosis of sepsis, the patient's hourly urine volume, mean arterial pressure (MAP), Lac, central venous oxygen saturation (ScvO2), Acute physiological chronic health status scoring system II, and systemic inflammatory response score (SIRS) were recorded. Renal function indicators [Scr, blood urea nitrogen (BUN)] were measured using Modular-P800 automatic biochemical analyzer (Roche, Switzerland) and supporting reagents. CRP was detected by IAMMGE analyzer (Beckman, Brea, CA, United States) and supporting reagents. Serum amyloid A (SAA) was detected by BNII analyzer (Siemens, Munich, Germany) and supporting reagents.
A MyLab Twice Color Doppler Ultrasound system (Esaote, Italy) with a convex array probe (frequency 4 MHz) was used in this study. CEUS was performed using Sonovue ultrasound microbubble contrast agent. The patient was placed in the lateral position, and after the image was clear, the probe was fixed to the largest renal long axis section and kept. The CEUS mode was activated after the contrast agent was injected. The sonographer continuously observed the contrast process of the renal parenchyma in real time until the enhancement intensity was weakened to near the pre-test level. Then the entire process of CEUS was saved. Dynamic analysis of the region of interest was performed using offline software. The region of interest was placed perpendicular to the renal cortex of the sound beam, avoiding the thicker blood vessels. The software automatically formed a time-intensity curve (TIC) and CEUS quantitative data, including peak intensity (PI), rise time (RT), wash in slope (WIS), time from peak to one half, and area under the curve (AUC).
Patients completed a 6 h bundle treatment firstly, and they were divided into AKI group and non-AKI group according to the renal function and hourly urine volume within 48 h. Renal function and hourly urine volume of the patients in non-AKI group were recorded again on day 7 and further classified into AKI subgroup and non-AKI subgroup according to AKI diagnostic criteria[14] (Figure 1).
The statistical analysis was performed using SPSS (Armonk, NY, IL, United States) software (version 19.0) and Medcalc software (version 19.0.2, Ostend, Belgium). The numerical data were expressed as mean ± SD, and the comparison between the two groups was compared using the t test. The categorical variables were expressed as number and percentage, and the comparison between the two groups was performed by the χ2 test. Receiver operating characteristic (ROC) curve was used to analyze the diagnostic efficacy of septic AKI. The combination of potential indicators was established based on logistic regression model to explore the diagnostic value of septic AKI. Statistical significance was defined as 2-tailed P < 0.05 for all tests.
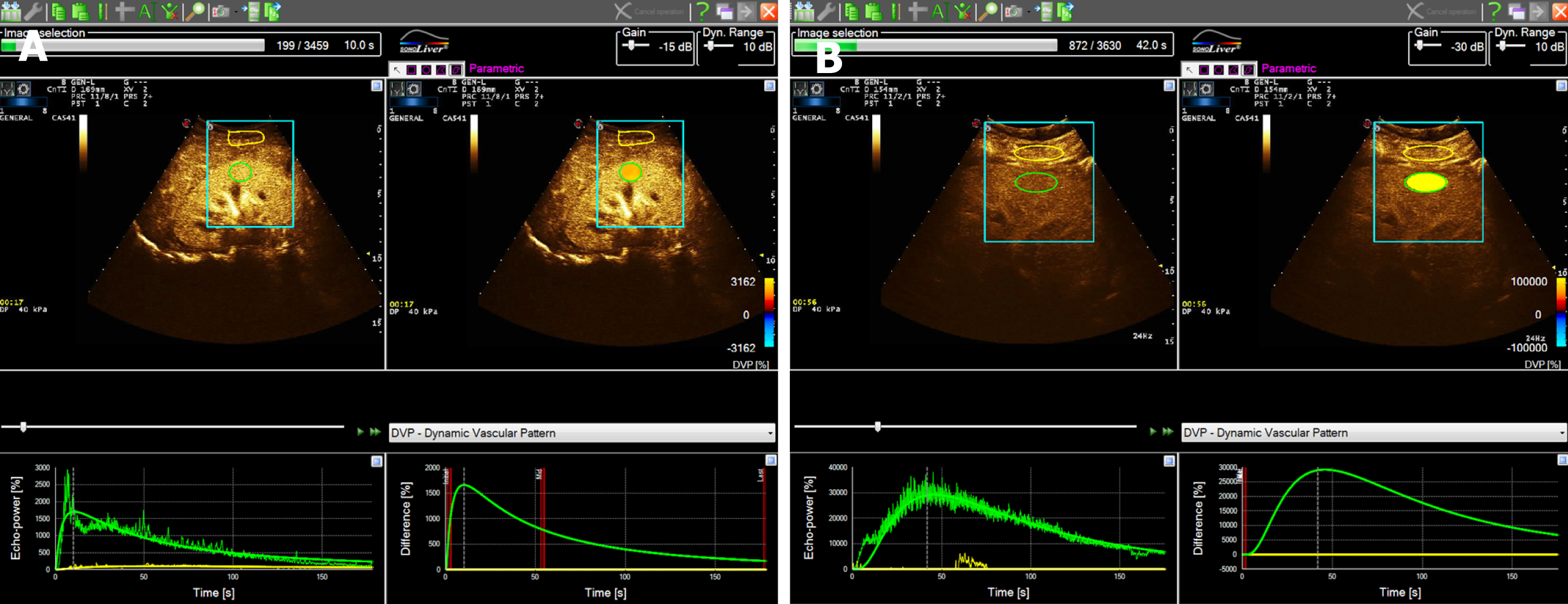
A total of 90 patients with sepsis were included in the study and were divided into the AKI group (n = 24) and the non-AKI group (n = 66) according to the renal function and hourly urine volume within 48 h. The renal function and hourly urine volume of patients in the non-AKI group were recorded again on day 7, and they were further divided into the AKI subgroup (n = 13) and non-AKI subgroup (n = 53) according to the AKI diagnostic criteria. The difference in CEUS between septic AKI and non-AKI sepsis was shown in Figure 2. The TIC of patients with non-AKI sepsis showed a rapid rise to a peak and a slow decline. The TIC in patients with septic AKI showed a slow rise to a peak and a slower decline. Compared with patients with non-AKI sepsis, patients with septic AKI showed a decreased PI, prolonged RT, and decreased WIS
The clinical characteristics between the non-AKI group and AKI group were compared in Table 1. The age, gender, body mass index, Acute physiological chronic health status scoring system II, MAP, ScvO2, CRP, SAA, and 24 h urine volume were similar between the two groups, and the differences were not statistically significant (P > 0.05). The SIRS, Lac, Scr, and BUN in the AKI group were significantly higher than those in the non-AKI group, and the differences were statistically significant (P < 0.05). In the CEUS indicators, the RT in the AKI group was higher than that in the non-AKI group, and the PI and WIS were lower than those in the non-AKI group. The differences were statistically significant (P < 0.05). The time from peak to one half and AUC were similar between the two groups, which were not statistically significant (P > 0.05).
| Non-AKI group, n = 66 | AKI group, n = 24 | t/χ2 value | P value | |
| Age in yr | 65.38 ± 12.93 | 66.92 ± 10.24 | 0.526 | 0.600 |
| Gender, male/female | 36/30 | 14/10 | 0.102 | 0.749 |
| BMI in kg/m2 | 22.72 ± 3.82 | 23.01 ± 2.98 | 0.336 | 0.738 |
| APACHE II | 20.91 ± 3.52 | 21.27 ± 3.38 | 0.433 | 0.666 |
| MAP in mmHg | 78.73 ± 8.39 | 77.38 ± 9.21 | 0.658 | 0.512 |
| SIRS | 2.25 ± 0.53 | 2.58 ± 0.75 | 2.325 | 0.022 |
| Lac in mmol/L | 2.43 ± 1.02 | 3.01 ± 1.33 | 2.193 | 0.031 |
| ScvO2 | 0.71 ± 0.05 | 0.72 ± 0.04 | 0.882 | 0.380 |
| CRP in mg/L | 158.34 ± 63.82 | 167.28 ± 48.92 | 0.622 | 0.535 |
| SAA in mg/L | 358.83 ± 89.93 | 325.18 ± 72.02 | 1.649 | 0.103 |
| Scr in μmol/L | 91.43 ± 23.92 | 141.82 ± 27.19 | 8.519 | 0.000 |
| BUN in mmol/L | 3.22 ± 1.03 | 4.41 ± 0.81 | 5.108 | 0.000 |
| 24 h urine volume in mL | 2062.63 ± 227.83 | 2002.52 ± 201.63 | 1.140 | 0.258 |
| PI in dB | 20.72 ± 8.27 | 10.78 ± 3.98 | 5.640 | 0.000 |
| RT in s | 8.37 ± 3.82 | 10.23 ± 2.63 | 2.199 | 0.030 |
| WIS in dB/s | 1.73 ± 0.82 | 1.07 ± 0.53 | 3.667 | 0.000 |
| TPH | 61.82 ± 21.82 | 58.32 ± 18.89 | 0.696 | 0.488 |
| AUC | 1083.28 ± 482.72 | 937.92 ± 392.73 | 1.323 | 0.189 |
Renal function was re-evaluated on the 7th day for non-AKI group patients. The incidence of AKI was 19.70% (13/66). Patients were divided into the AKI subgroup (n = 13) and non-AKI subgroup (n = 66). The comparison of clinical data on admission between AKI subgroup and non-AKI subgroup is shown in Table 2. The PI and WIS in the AKI subgroup were lower than those in the non-AKI subgroup, and the differences were statistically significant (P < 0.05). The SIRS, Lac, Scr, BUN, and RT were similar between the two groups, and the differences were not statistically significant (all P > 0.05).
| Non-AKI subgroup, n = 53 | AKI subgroup, n = 13 | t/χ2 value | P value | |
| SIRS | 2.18 ± 0.44 | 2.31 ± 0.38 | 1.283 | 0.203 |
| Lac in mmol/L | 2.29 ± 0.88 | 2.61 ± 0.92 | 1.507 | 0.135 |
| Scr in μmol/L | 89.82 ± 12.81 | 92.71 ± 11.06 | 0.980 | 0.330 |
| BUN in mmol/L | 3.02± 0.91 | 3.38 ± 0.96 | 1.636 | 0.105 |
| PI in dB | 15.82 ± 3.92 | 12.83 ± 3.77 | 3.232 | 0.002 |
| RT in s | 7.92 ± 3.31 | 8.79 ± 3.27 | 1.106 | 0.272 |
| WIS, dB/s | 1.92 ± 0.81 | 1.22 ± 0.68 | 3.774 | 0.000 |
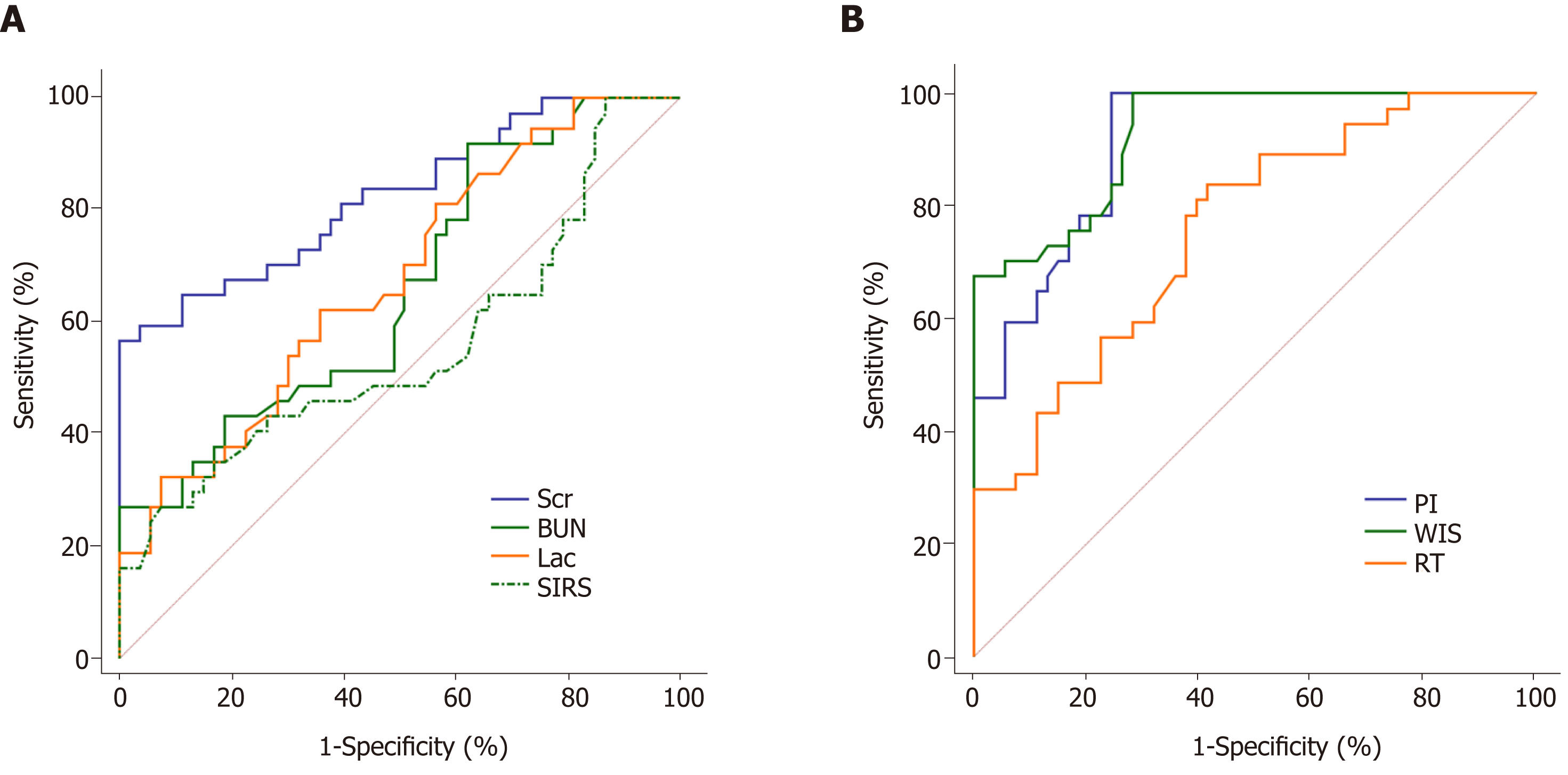
The results of ROC analysis for the diagnosis of septic AKI by SIRS, Lac, Scr, BUN, PI, RT, and WIS are shown in Table 3 and Figure 3. It revealed that SIRS, Lac, BUN, and RT had lower accuracy in the diagnosis of septic AKI (all AUC < 0.8). The AUC of Scr was 0.825, which was higher than other potential indicators. Furthermore, its diagnostic specificity was 100%. However, the sensitivity was low (only 56.76%). In the CEUS indicators, both WIS and PI had high diagnostic value. The AUC of WIS and PI were 0.928 and 0.912, respectively. In contrast to Scr, the sensitivities of PI and WIS were both 100%, but their specificities were poor (PI: 75.47%, WIS: 71.70%).
| AUC | 95%CI | Cut-off point | Sensitivity | Specificity | |
| SIRS | 0.555a | 0.446 - 0.660 | 2.69 | 27.03% | 92.45% |
| Lac | 0.674a | 0.567 - 0.769 | 2.67 mmol/L | 62.16% | 64.15% |
| Scr | 0.825a | 0.730 - 0.897 | 129.98 μmol/L | 56.76% | 100.00% |
| BUN | 0.658a | 0.551 - 0.755 | 2.84 mmol/L | 37.74% | 91.89% |
| PI | 0.912 | 0.833 - 0.961 | 15.93 dB | 100.00% | 75.47% |
| RT | 0.758a | 0.656 - 0.842 | 8.1 s | 83.78% | 58.49% |
| WIS | 0.928 | 0.853 - 0.972 | 1.48 dB/s | 100.00% | 71.70% |
| Combination | 0.943 | 0.873 - 0.981 | 0.48 | 94.59% | 81.13% |
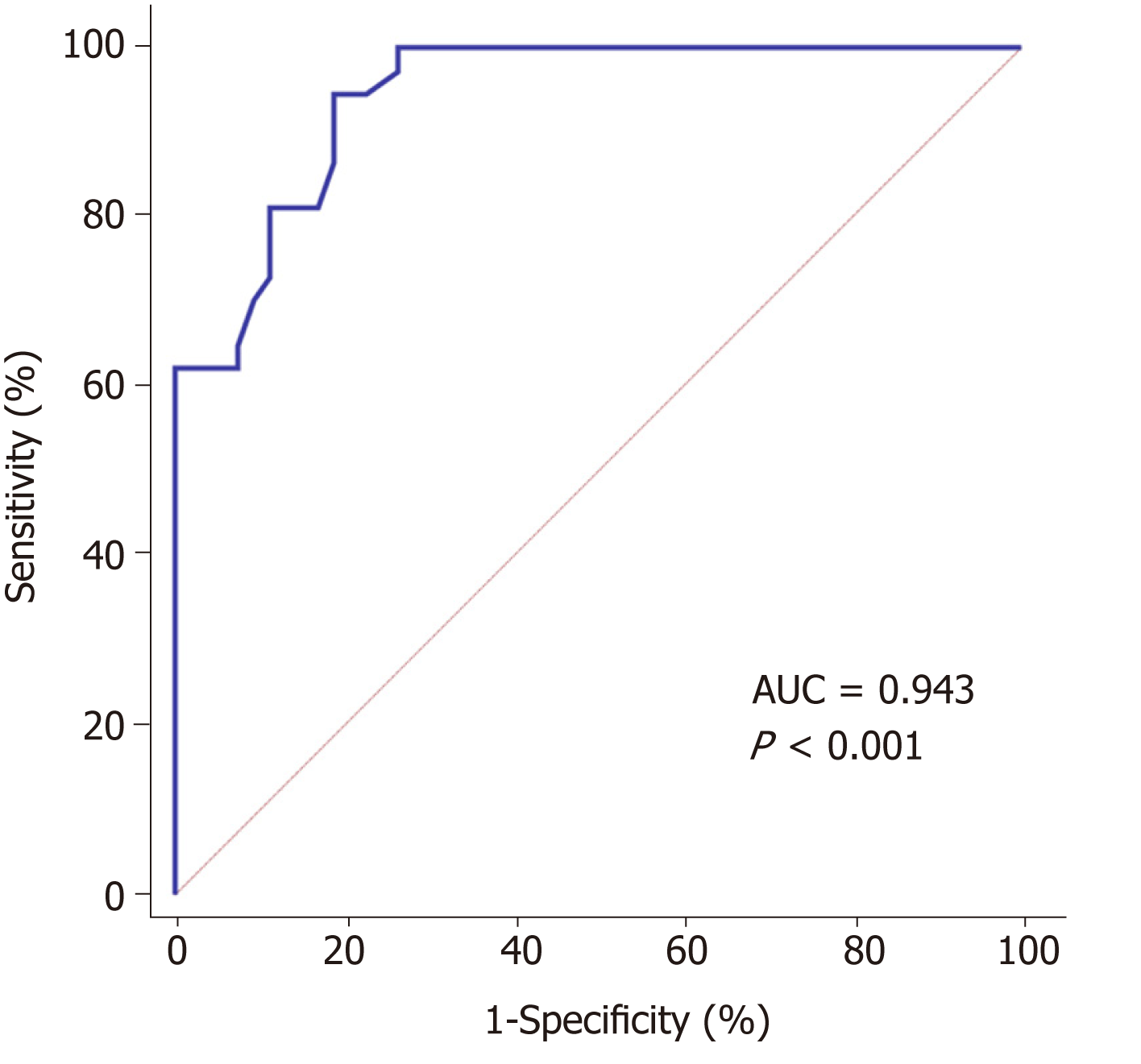
Since the specificity of Scr for the diagnosis of septic AKI was 100%, the sensitivities of WIS and PI were 100%. This study performed a combined diagnosis of Scr, PI, and WIS based on logistic regression model. It revealed that the accuracy of the combination for the diagnosis of septic AKI was highest (AUC = 0.943, 95%CI: 0.873-0.981), which was significantly greater than the AUC of Scr (z = 2.398, P = 0.017). In addition, it was greater than the AUCs of WIS and PI, but the differences were not statistically significant (WIS: z = 0.483, P = 0.629; PI: z = 0.910, P = 0.363). The best diagnostic point was 0.48, and the sensitivity was 94.59%, specificity was 81.13% (Figure 4).
Sepsis often leads to multiple organ dysfunction, and the kidney is one of the most susceptible organs[15]. The study of Mehta et al[16] revealed that 44% of patients suffered septic AKI, and their mortality rate was 74.5%, which was significantly higher than sepsis patients who did not develop AKI. Early diagnosis or prediction of the occurrence of septic AKI and early intervention is critical to improve the prognosis of patients with sepsis. Scr and urine volume are commonly used diagnostic indicators of AKI at the current stage[17], but Scr and urine volume are systemic indicators, which are unable to reveal the status of single kidney and have obvious hysteresis[18]. In addition, some factors in the treatment process will affect the patient's urine volume, including the patient's own condition and tolerance and the patient's medication and rehydration, which make it difficult to guarantee the diagnostic value of AKI[19]. Parikh et al[20] reported that the rise of Scr significantly lags behind the decrease of renal blood volume, and the sensitivity of AKI diagnosis is insufficient. Petrovic et al[21] reported that when Scr changes significantly, the kidney has already undergone large-scale irreversible damage. At that time, intervention has missed the best treatment window, which may be one of the causes of poor prognosis in patients with septic AKI. In recent years, studies have examined potential indicators for the early diagnosis of septic AKI and have found some that can reveal the extent of tissue perfusion and inflammatory response related to the occurrence of AKI[22]. However, there are controversies about the clinical accuracy and clinical applicability[23].
CEUS is a commonly used tool for clinical detection of tissue perfusion. Stenberg et al[24] found that CEUS can be sensitive to changes in renal cortical blood vessels, which may become a potential method for early warning of septic AKI, but its diagnostic value has not been confirmed. Therefore, in this study, we analyzed the patient's clinical indicators and CEUS results, explored the method of early diagnosis of septic AKI, and analyzed the application value of multi-index combined diagnosis in order to increase the possibility of early diagnosis of septic AKI.
The development of AKI is complicated, and some patients have a significant increase of Scr levels in a short time. According to the international guidelines for the management of sepsis and septic shock, AKI can be diagnosed by Scr growth of ≥ 26.5 μmol/L within 48 h. Accordingly, patients in this study were divided into the AKI group and non-AKI group. Comparative analysis of the clinical indicators between the two groups found that the SIRS in the AKI group was significantly higher than that in the non-AKI group. Since the SIRS can reveal the severity of the inflammatory response in the body, its increase may be related to the occurrence of AKI. This conclusion is similar to the study by Messmer et al[25]. The core reason may be that SIRS describes the pathophysiological changes of sepsis caused by infection. The release of inflammatory factors may cause damage to renal tubular epithelial cells and further trigger septic AKI. Excessive immune and inflammatory mechanisms may accelerate this process[26,27]. However, there are no significant differences in CRP, SAA, and other inflammation-related indicators between the two groups. We believed that SIRS is more comprehensive in assessing the severity of the inflammatory response in the body. It is more suitable to reveal the degree of kidney damage than other indicators.
Previous studies have shown that changes in indicators such as Lac, Scr, and BUN are associated with changes in renal function, and renal dysfunction in patients with sepsis may be associated with the occurrence of AKI[28]. In this study, Lac, Scr, and BUN in the AKI group were significantly higher than in the non-AKI group. An increase in the level of Lac, Scr, or BUN at admission indicated that the patient had a severe renal injury and was more likely to develop septic AKI. Patients with severe sepsis often have hypotension, which will affect blood perfusion in tissues and organs[29]. The combination of renal hemodynamic instability and hypoperfusion is one of the mechanisms of high incidence of septic AKI[30,31]. MAP can be used to reveal the perfusion of the whole body. The study by Bagshaw et al[32] believes that MAP is important for the occurrence and prognosis of AKI. However, in the study by Deruddre et al[33], there was no significant difference in MAP between patients with septic AKI and simple sepsis. Similarly, the relationship between ScvO2 and AKI is also highly controversial[34].
CEUS is sensitive to changes in renal blood perfusion. An animal study by Stock et al[35] has confirmed that detection of cortical RT is better than Scr in AKI prediction. This study compared the CEUS indicators between the two groups, and found that the RT of the AKI group was significantly greater than that of the non-AKI group and that the PI and WIS were significantly lower than those of the non-AKI group. Compared with the CEUS characteristics of the non-AKI group, the PI of the AKI group decreased, and the TIC had the characteristics of slow rise and slow decline. The reason may be that CEUS can display local tissue perfusion, and renal perfusion was insufficient in patients with septic AKI. Compared with sepsis patients without AKI, the filling and discharge rate of contrast agent in the kidney were decreased. However, there were no significant differences in MAP and ScvO2 between the two groups, which are similar to the results of Deruddre et al[33]. The possible reason is that MAP and ScvO2 levels mainly reveal systemic blood perfusion and oxygen supply, while CEUS can reveal renal blood flow in real time and indicate renal microcirculation blood perfusion. It suffers less interference and can be more sensitive to indicate changes in renal blood perfusion, and even the occurrence of AKI.
Scr is the main reference index for clinical diagnosis of AKI at this stage, but it has a certain hysteresis. In patients who have not been diagnosed with septic AKI within 48 h, some in the early stages of AKI will not be identified by Scr. Therefore, the growth of Scr within 7 d is often supplemented by the 48 h Scr currently. That is, if patients with an increase in Scr < 26.5 µmol/L within 48 h, it can be diagnosed as AKI as well if the increase of Scr was 1.5 times higher than the baseline within 7 d. According to this feature, the sepsis patients without AKI within 48 h were re-diagnosed according to the Scr on day 7 and were divided into AKI subgroup and non-AKI subgroup. By comparing the indicators on admission, the SIRS, Lac, Scr, and BUN were similar between the two subgroups. It is suggested that SIRS, Lac, Scr, and BUN cannot indicate the subclinical changes in early septic AKI. ROC analysis also suggested that SIRS, Lac, Scr, and BUN were less accurate as indicators for the diagnosis of septic AKI. When the kidneys are less damaged, inflammation is lighter and renal dysfunction is not obvious, and it is difficult detect warning signs in the early stage of septic AKI. Among the CEUS indicators, the PI and WIS of the AKI subgroup were higher than those of the non-AKI subgroup, but the RT was similar in the two groups. These findings suggest that increased PI and WIS may be used to indicate the development of early septic AKI.
This study demonstrated that changes in CEUS results, SIRS, Lac, Scr, and BUN in patients with sepsis may suggest the occurrence of septic AKI. In order to clarify further the diagnostic ability of each indicator for septic AKI, this study conducted ROC analyses on the above indicators. The results showed that the diagnostic values of WIS and PI were higher (both AUC > 0.9), which were higher than the accuracy of Scr (AUC = 0.825). In addition, the specificity of Scr for the diagnosis of septic AKI was 100%, but the sensitivity was insufficient. It indicated that when Scr was used as a diagnostic criterion, although the occurrence of misdiagnosis could be effectively avoided, the rate of missed diagnosis was high. Besides, when WIS and PI were used as the diagnosis criteria, both of them have the characteristics of high sensitivity but insufficient specificity. It may be because CEUS is sensitive to changes in renal blood perfusion. A slight change in renal blood flow can cause changes in filling intensity and time. CEUS can sensitively detect blood flow changes in mild kidney injury. Therefore, its sensitivity to diagnose AKI is high, but it also means that it is prone to misdiagnosis.
According to the results of ROC analyses, it was found that Scr had an ideal specificity but insufficient sensitivity for the diagnosis of septic AKI, and its AUC was difficult to meet the diagnostic needs. Although WIS and PI had high diagnostic values (Both AUC > 0.9), their specificities were not enough. In order to explore a method that can accurately diagnose the occurrence of septic AKI, this study combined the indicators with high independent diagnostic accuracy and found that the combination of Scr, WIS, and PI for the diagnose of septic AKI had the highest accuracy (AUC = 0.943), which was significantly higher than the AUC of each indicator in diagnosing septic AKI individually. Its sensitivity and specificity was 94.59% and 81.13%, respectively. It suggested that the combination of Scr, WIS, and PI for the diagnosis of septic AKI can achieve higher accuracy. The combination of Scr, WIS, and PI may detect early septic AKI and allow clinicians to intervene as early as possible, thereby reducing the extent of kidney damage and improving prognosis.
This study only analyzed the ability of each indicator for diagnosing septic AKI based on the recruited patients. The actual value of combined diagnosis has not been verified. Therefore, this study further plans to use the combined diagnosis data to identify septic AKI patients and adjust the combined diagnosis according to the results in order to find a truly accurate diagnostic method that is truly suitable for clinical use.
In conclusion, this study found that SIRS, Lac, BUN, Scr, PI, WIS, and RT are valuable in diagnosing septic AKI. Among them, the diagnostic accuracy of Scr, PI, and WIS was higher, and the combination of the three indicators can improve the accuracy in diagnosing septic AKI, which may help the early diagnosis of septic AKI.
Patients with sepsis are more likely to develop acute kidney injury (AKI), which can lead to worse prognosis. Early diagnosis or prediction of AKI of patients with sepsis can greatly improve the prognosis. Currently, serum creatinine (Scr) and urine volume are commonly used diagnostic indicators of AKI, but they are insufficient, making it difficult to detect the occurrence of AKI in time and accurately. For that reason, finding a new way to detect the risk factors and intervene in an early time is the focus of the diagnosis and treatment of septic AKI.
Contrast-enhanced ultrasound (CEUS) can monitor microcirculatory blood perfusion. It has been widely used in many kidney-related diseases because of its simple operation and low nephrotoxicity. In animal experiences, CEUS has been proven to detect changes in renal cortical microcirculation in early ischemia/reperfusion injury, suggesting the possibility of applying it in AKI diagnosis. However, its role in AKI assessment and diagnosis has not been clinically confirmed.
In this study, we analyzed the diagnostic value of general data, biochemical indicators, and CEUS indicators in septic AKI. The aim of this study is to realize early diagnosis and intervention in the development of septic AKI, thereby improving patient prognosis.
Ninety patients who developed sepsis during hospitalization were recruited as subjects. The relevant basic data, clinical indicators, and CEUS results of each patient were measured and recorded. The patients were divided into the AKI group and non-AKI group according to the results of renal function diagnosis after 48 h. The renal function of non-AKI group was reassessed on the 7th day, and the patients in this group were then further divided into AKI subgroup and non-AKI subgroup. The differences of the indicators in different groups were compared, and the diagnostic value of each indicator and their combination for septic AKI were analyzed.
The systemic inflammatory response score (SIRS) , blood lactic acid (Lac) , Scr, blood urea nitrogen (BUN), and rise time (RT) in the AKI group were higher than those in the non-AKI group. Peak intensity (PI) and wash in slope (WIS) were lower than those in the non-AKI group. The differences were statistically significant (P < 0.05). PI and WIS in the AKI subgroup were lower than those in the non-AKI subgroup, and the differences were statistically significant (P < 0.05). The area under curve (AUC) of Scr for the diagnosis of septic AKI was 0.825 with a sensitivity of 56.76% and a specificity of 100%. The AUCs of WIS and PI (0.928 and 0.912) were higher than those of Scr. Their sensitivities were 100%, but the specificities were 71.70% and 75.47%, respectively. The AUC of the combination of three indicators for the diagnosis of septic AKI was 0.943, which was significantly higher than the AUC diagnosed by each single indicator. The sensitivity was 94.59%, and the specificity was 81.13%.
SIRS, Lac, BUN, Scr, PI, WIS, and RT are valuable in diagnosing septic AKI. The diagnostic accuracy of Scr, PI, and WIS was higher than the others, and the combination of the three indicators can improve the accuracy in diagnosing septic AKI.
In order to avoid the interference caused by the differences of individual and environmental factors in different patients, the further study plans to use the combined diagnosis data in more clinical septic AKI patients and adjust the combined diagnosis according to the results in order to find a truly accurate diagnostic method that is truly suitable for clinical use.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arisawa T, Kim ES S-Editor: Wang JL L-Editor: Filipodia E-Editor: Xing YX
| 1. | Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 2. | Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken). 2008;291:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and Multi-Organ Failure in Sepsis. Int J Mol Sci. 2017;18:E2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Carcillo JA, Halstead ES, Hall MW, Nguyen TC, Reeder R, Aneja R, Shakoory B, Simon D; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators. Three Hypothetical Inflammation Pathobiology Phenotypes and Pediatric Sepsis-Induced Multiple Organ Failure Outcome. Pediatr Crit Care Med. 2017;18:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Karsten J, Heinze H. [Ventilation as a trigger for organ dysfunction and sepsis]. Med Klin Intensivmed Notfmed. 2016;111:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Zhao N, Tian HH, Li Z, Wang T, Hao D, Qi ZJ, Lv CJ, Wang XZ. [Risk factors and early diagnosis of acute kidney injury in patients with sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 333] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 9. | Cheng X, Wu B, Liu Y, Mao H, Xing C. Incidence and diagnosis of Acute kidney injury in hospitalized adult patients: a retrospective observational study in a tertiary teaching Hospital in Southeast China. BMC Nephrol. 2017;18:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6993] [Cited by in RCA: 6391] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 11. | ARISE Investigators, ANZICS Clinical Trials Group; Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1316] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 12. | Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, Drudi FM, Malpassini F, Isidori A, Meloni FM, Calliada F, D'Ambrosio F. Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1988] [Article Influence: 248.5] [Reference Citation Analysis (1)] |
| 14. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3344] [Article Influence: 257.2] [Reference Citation Analysis (0)] |
| 15. | Chung KK. Sepsis and Multi-Organ Failure. J Burn Care Res. 2017;38:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Hatakeyama Y, Horino T, Nagata K, Kataoka H, Matsumoto T, Terada Y, Okuhara Y. Evaluation of the accuracy of estimated baseline serum creatinine for acute kidney injury diagnosis. Clin Exp Nephrol. 2018;22:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Hodgson LE, Sarnowski A, Roderick PJ, Dimitrov BD, Venn RM, Forni LG. Systematic review of prognostic prediction models for acute kidney injury (AKI) in general hospital populations. BMJ Open. 2017;7:e016591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55:1074-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 20. | Parikh A, Rizzo JA, Canetta P, Forster C, Sise M, Maarouf O, Singer E, Elger A, Elitok S, Schmidt-Ott K, Barasch J, Nickolas TL. Correction: Does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) and serum creatinine (sCr) for acute kidney injury (AKI) diagnosis. PLoS One. 2017;12:e0185772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Petrovic S, Bogavac-Stanojevic N, Lakic D, Peco-Antic A, Vulicevic I, Ivanisevic I, Kotur-Stevuljevic J, Jelic-Ivanovic Z. Cost-effectiveness analysis of acute kidney injury biomarkers in pediatric cardiac surgery. Biochem Med (Zagreb). 2015;25:262-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Prowle JR. Sepsis-Associated AKI. Clin J Am Soc Nephrol. 2018;13:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Xu C, Wang Z, Lu K, Jin H. Hotspot Analysis of Sepsis Literature. Med Sci Monit. 2018;24:5427-5436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Stenberg B, Wilkinson M, Elliott S, Caplan N. The prevalence and significance of renal perfusion defects in early kidney transplants quantified using 3D contrast enhanced ultrasound (CEUS). Eur Radiol. 2017;27:4525-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Messmer UK, Briner VA, Pfeilschifter J. Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells. Kidney Int. 1999;55:2322-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Xu C, Chang A, Hack BK, Eadon MT, Alper SL, Cunningham PN. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 2014;85:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 27. | Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817-5823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Pereira BJ, Barreto S, Gentil T, Assis LS, Soeiro EM, Castro I, Laranja SM. Risk factors for the progression of chronic kidney disease after acute kidney injury. J Bras Nefrol. 2017;39:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 30. | Mårtensson J, Bellomo R. Sepsis-Induced Acute Kidney Injury. Crit Care Clin. 2015;31:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Keir I, Kellum JA. Acute kidney injury in severe sepsis: pathophysiology, diagnosis, and treatment recommendations. J Vet Emerg Crit Care (San Antonio). 2015;25:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med. 2015;41:1862-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Stock E, Paepe D, Daminet S, Vandermeulen E, Duchateau L, Saunders JH, Vanderperren K. Contrast-Enhanced Ultrasound Examination for the Assessment of Renal Perfusion in Cats with Chronic Kidney Disease. J Vet Intern Med. 2018;32:260-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |