Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3895
Peer-review started: August 1, 2019
First decision: August 9, 2019
Revised: September 23, 2019
Accepted: October 5, 2019
Article in press: October 5, 2019
Published online: November 26, 2019
Processing time: 117 Days and 0.2 Hours
Warthin’s tumor (WT) is composed of several cysts that are lined with tall, bilayered oncocytic columnar cells and lymphoid stroma. Within WT, the two components rarely transform into carcinoma or lymphoma, and when it does, carcinoma is the most common type. Approximately 28 cases of lymphoma with WT have been reported, most of which were non-Hodgkin lymphomas, and only a few cases were Hodgkin lymphomas. In the present report, we studied a case of diffuse large B cell lymphoma (DLBCL) arising from follicular lymphoma (FL) with WT in the parotid gland and its immunophenotypic and genetic features.
A 67-year-old man presented with a slowly enlarging right cheek mass for 12 years, and the mass began to change in size over a 2-mo time period. Over time, the patient felt mild local pain and right cheek discomfort. His medical history included a hepatitis B virus infection for 20 years and 30 years of smoking. Gross examination of the excised specimen showed a gray-red and gray-white appearance and a soft texture lobulated external surface neoplasm that measured 9 cm × 8 cm × 7 cm and was well circumscribed by relative normal parotid gland tissue. In cross section, the cut surfaces of the neoplasm were multicystic and had a homogeneous scaly appearance. A small fluid was discovered in the cyst. Bilateral oxyphilic, cuboidal or polygonal epithelium cells and lymphoid intraparenchymal components were observed. Many medium- to large-sized lymphoid cells were observed diffusely in part of the neoplasm, and a few secondary lymphoid follicles were observed at the center or edge of the neoplasm. Immunohistochemical staining showed that the columnar oncocytic cells were positive for AE1/AE3; neoplastic cells located in coarctate follicular were positive for CD20, Pax-5, bcl-2 and bcl-6; and the adjacent diffusely medium- to large-sized lymphoid cells were positive for Pax-5, bcl-6, CD20, MUM-1, bcl-2 and CD79a. The bcl-6 (3q27) break-apart rearrangement was observed, and an Epstein Barr virus test was negative in the tumor cells. The patient survived 6 months after being diagnosed without any treatment.
WT-associated lymphoma is a very rare neoplasm in the parotid gland. Most cases are B cell non-Hodgkin lymphomas and involve middle-age and older males. This case highlights the extremely rare association of DLBCL arising from FL with WT and the importance of deliberate evaluation of the WT intraparenchymal stroma. Molecular detection techniques have potential advantages in the diagnosis of lymphoma with WT.
Core tip: We present a case of diffuse large B cell lymphoma (DLBCL) that was transformed from follicular lymphoma in a Warthin’s tumor (WT) excision specimen. This is a very rare lesion with an extremely low incidence and unclear biological behavior. Therefore, we should carefully examine the tumor stroma during the pathological examination because the neoplastic cells can be confused with the normal reactive lymphoid component in WT. Requisite molecular detections are performed when the diagnosis of lymphoma with WT is dubious.
- Citation: Wang CS, Chu X, Yang D, Ren L, Meng NL, Lv XX, Yun T, Cao YS. Diffuse large B-cell lymphoma arising from follicular lymphoma with warthin’s tumor of the parotid gland - immunophenotypic and genetic features: A case report. World J Clin Cases 2019; 7(22): 3895-3903
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3895.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3895
Warthin’s tumor (WT) is associated with different lymphoproliferative disorders in the salivary glands. It is the second most common benign neoplasm of the salivary gland and occurs almost exclusively in the parotid gland. WT is composed of several cysts that are lined with tall, bilayered oncocytic columnar cells and lymphoid stroma. Within WT, the two composed parts rarely transform into carcinoma or lymphoma, and when it does, the carcinoma is the most common type. On occasion, some lymphomas contain the normal residual lymphoid stroma, and the majority of the stroma of WT is entirely replaced by neoplastic cells. However, the lymphoid stroma of WT comprises systemic lymphoid tissue based on the foundation that most lymphomas arising from WT are disseminated disorders. Approximately 28 cases of lymphomas with WT have been reported in the English literature, and most of them were non-Hodgkin lymphomas; only a few cases were Hodgkin lymphomas (Table 1). Follicular lymphoma (FL) is actually a prevalent subtype. The diffuse large B cell lymphoma (DLBCL) arising from FL also involves the parotid gland and is the first known report. In this paper, we studied a case of B cell non-Hodgkin lymphoma that arose from the stroma of WT and transformed to DLBCL. In addition, the molecular genetic characteristics and immunotypes of these genes were studied.
| Ref. | Sex/Age (yr) | Location of tumor | Subtype | Mass size (cm) | Stage at diagnosis | Follow-up |
| Reiner et al[18], 1979 | M/56 | Right Parotid | DLBCL | NA | NA | DOD, 2 mo |
| Miller et al[10], 1982 | M/49 | Right Mandible | FL | NA | IA | NHL in regional LNs 3 mo later |
| Hall et al[7], 1985 | M/64 | Right Parotid | FL | NA | IA | Generalize NHL 7 mo later |
| Banik et al[3], 1985 | M/75 | Left Parotid | FL | NA | NA | NA |
| M/76 | Right Parotid | FL | NA | III A | NHL, 12 mo | |
| Griesser et al[4], 1986 | F/64 | Palate | FL | NA | NA | NA |
| M/82 | Left submandibular | DLBCL | NA | IIA | NA | |
| Franco et al[24], 1986 | M/NA | Parotid | Lennert | NA | NA | NA |
| Melato et al[13], 1986 | M/69 | Right Parotid | HL | NA | NA | DOD, 13 mo |
| Bunker et al[21], 1989 | F/63 | Left Parotid | SLL/CLL | NA | NA | NA |
| Giardini et al[6], 1990 | M/57 | Left and Right Parotid | FL | NA | IIA | NED, 36 mo |
| Medeiros et al[2], 1990 | M/71 | Left Parotid | FL | NA | IIIA | NED, 48 mo |
| Shikhani et al[8], 1993 | M/56 | Right Parotid | FL | 7 × 6 × 3 | IA | DOD, 84 mo |
| Badve et al[14], 1993 | M/76 | Left Parotid | HL | 2.2 × 1.7 × 1.5 | NA | NED, 24 mo |
| Park et al[1], 2000 | F/68 | Right Parotid | FL | 1.7 × 1.5 × 1.5 | IA | NHL in LN 5 yr later |
| M/55 | Right Parotid | FL | 8.5 × 4 × 3.3 | IIA | Without treatment, 2 mo | |
| Marioni et al[23], 2004 | F/61 | Right Parotid | MALT | 6 × 4.7 × 2.5 | NA | NED, 11 mo |
| Pescarmona et al[25], 2005 | M/66 | upper right cervical lymph node | TCL, NOS | NA | IV | DOD, 5 mo |
| Saxena et al[20], 2005 | M/60 | Left Parotid | SLL/CLL | NA | IV A | CR |
| Gorai et al[15], 2007 | M/102 | Left Mandible | DLBCL | Diameter 2-3 | NA | DOD, 10 mo |
| Liu et al[26], 2008 | M/62 | Left Parotid | TCL, NOS | Diameter 3.5 | NA | NA |
| Giaslakiotis et al[27], 2009 | M/81 | Right Parotid | T-LBL | NA | IV B | DOD, 3mo |
| Liu et al[12], 2013 | M/78 | Left Parotid | LRCHL | 6 × 4 × 3 | IV B | DOD, 7 mo |
| Özkök et al[16], 2013 | M/60 | Left Parotid | DLBCL | 9 × 6 × 3.2 | IIIA | NED, 6 mo |
| Chu et al[17], 2015 | M/83 | Left Parotid | DLBCL(EBV+) | 2.7 × 2 × 2 | IIIA | DOD, 14 mo |
| Arcega et al[22], 2015 | M/70 | Left Parotid | MCL | 4.1 × 2.2 × 2.1 | IV A | NA |
| Napoli et al[11], 2015 | M/73 | Left Parotid | NLPHL | Diameter 4.5 | IIA | CR, 6 doses of rituximab and radiotherapy |
| Jawad et al[19], 2017 | M/80 | Right Parotid | SLL/CLL | 5.5 × 3.2 × 1.2 | NA | DOD, 22 mo |
| Present case | M/67 | Right Parotid | DLBCL | 9 × 8 × 7 | IIIA | NED, 6 mo |
A 67-year-old man was admitted to the hospital with a slowly enlarging right cheek mass for 12 years. At first, the mass was about the size of a peanut without any discomfort; the mass grew slowly and began to change in size over 2 mo before he was admitted to our hospital. Over time, the patient felt mild local pain and right cheek discomfort.
The patient had no history of the present illness.
His medical history included a hepatitis B virus infection for 20 years and 30 years of smoking.
His family histories and previous clinical and genetic disease histories were unremarkable.
A solitary, hard mass approximately 10.0 cm × 10.0 cm × 8.0 cm in size at the tail of the right parotid gland was examined, and there were no swollen lymph nodes in the adjacent region.
There were no special laboratory examination results.
Contrast-enhanced computed tomography (CT) scans of the neck and parotid gland demonstrated a heterogeneous echo-textured mass in the right parotid gland measuring 10 cm × 10 cm × 8 cm without pathologic cervical adenopathy. The facial nerve function was intact.
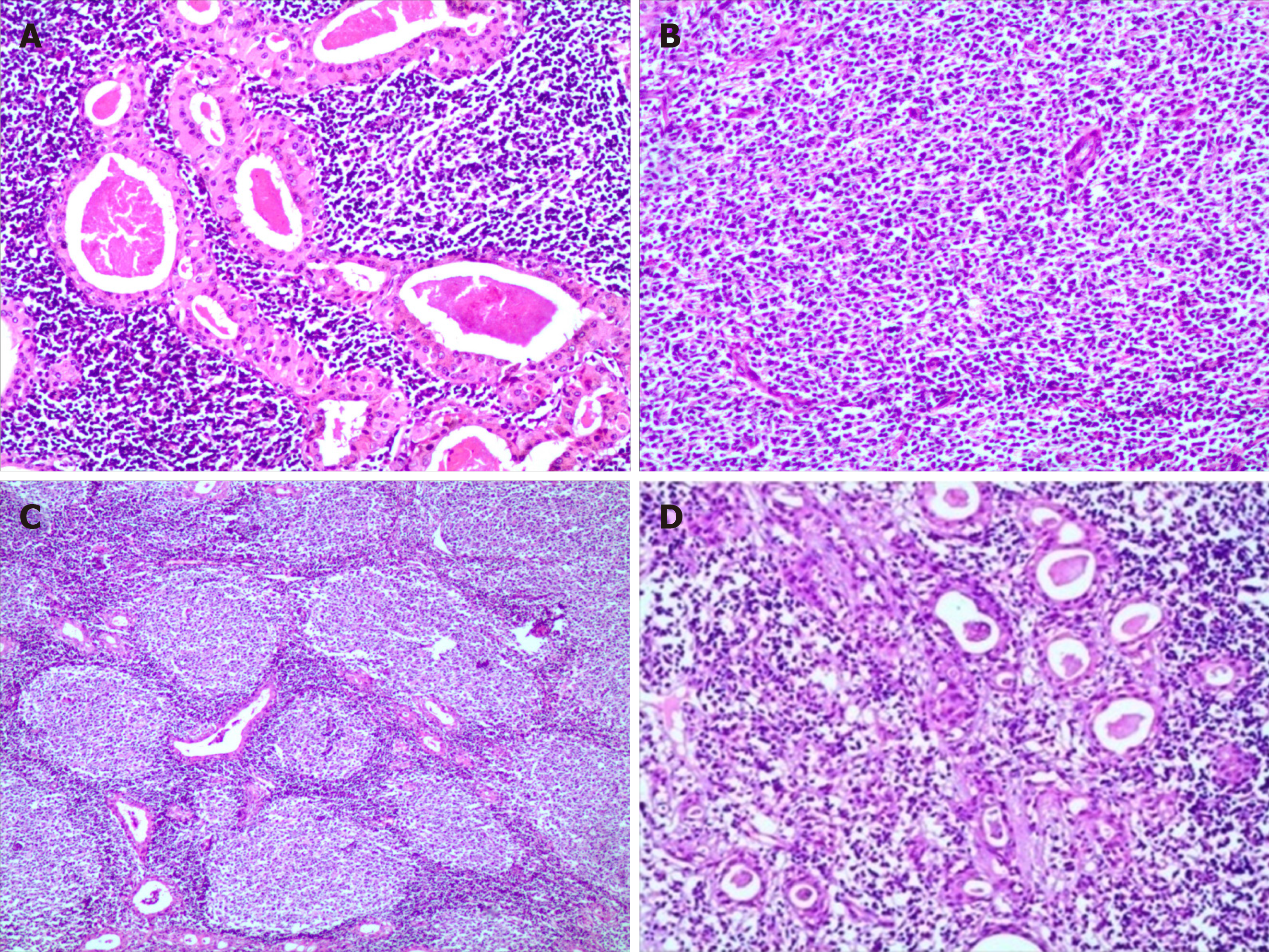
Gross examination of the excised specimen showed a gray-red and gray-white appearance, and a soft-textured, lobulated external surface neoplasm that measured 9 cm × 8 cm × 7 cm was surrounded by relative normal parotid gland tissue. In cross section, the cut surfaces of the neoplasm were multicystic and had a homogeneous scaly appearance, and a small amount of fluid was discovered in the cyst. Microscopically, bilayered oxyphilic, cuboidal or polygonal epithelium cells and lymphoid intraparenchymal components were observed, which are typical WT histological characteristics (Figure 1A). Simultaneously, many medium- to large-sized lymphoid cells were observed diffusely in other parts of the neoplasm, and a few secondary lymphoid follicles were observed at the center or edge of the neoplasm (Figure 1B and 1C). The lymphoid cells had irregular nuclear contours, indistinct nucleoli, and scant cytoplasm with atypia contours and nucleus. At the border of the epithelium, lymphoepithelial lesions were identified (Figure 1D). Necrosis was observed locally, and mitoses were approximately 3-4/10 high-power fields.
At the same time, immunohistochemical staining was performed according to the reported literature. Formalin-fixed paraffin-embedded sample blocks were cut into 3 μm-thick sections and used for immunohistochemical staining. High-pressure heating was performed for antigen retrieval in 10 mmol citrate buffer (pH 6.0). The primary antibodies were added and incubated overnight at 4 °C or 37 °C for 3 h according to the manufacturer’s instructions. The secondary antibodies were added and incubated on the next day and then visualized with DAB.
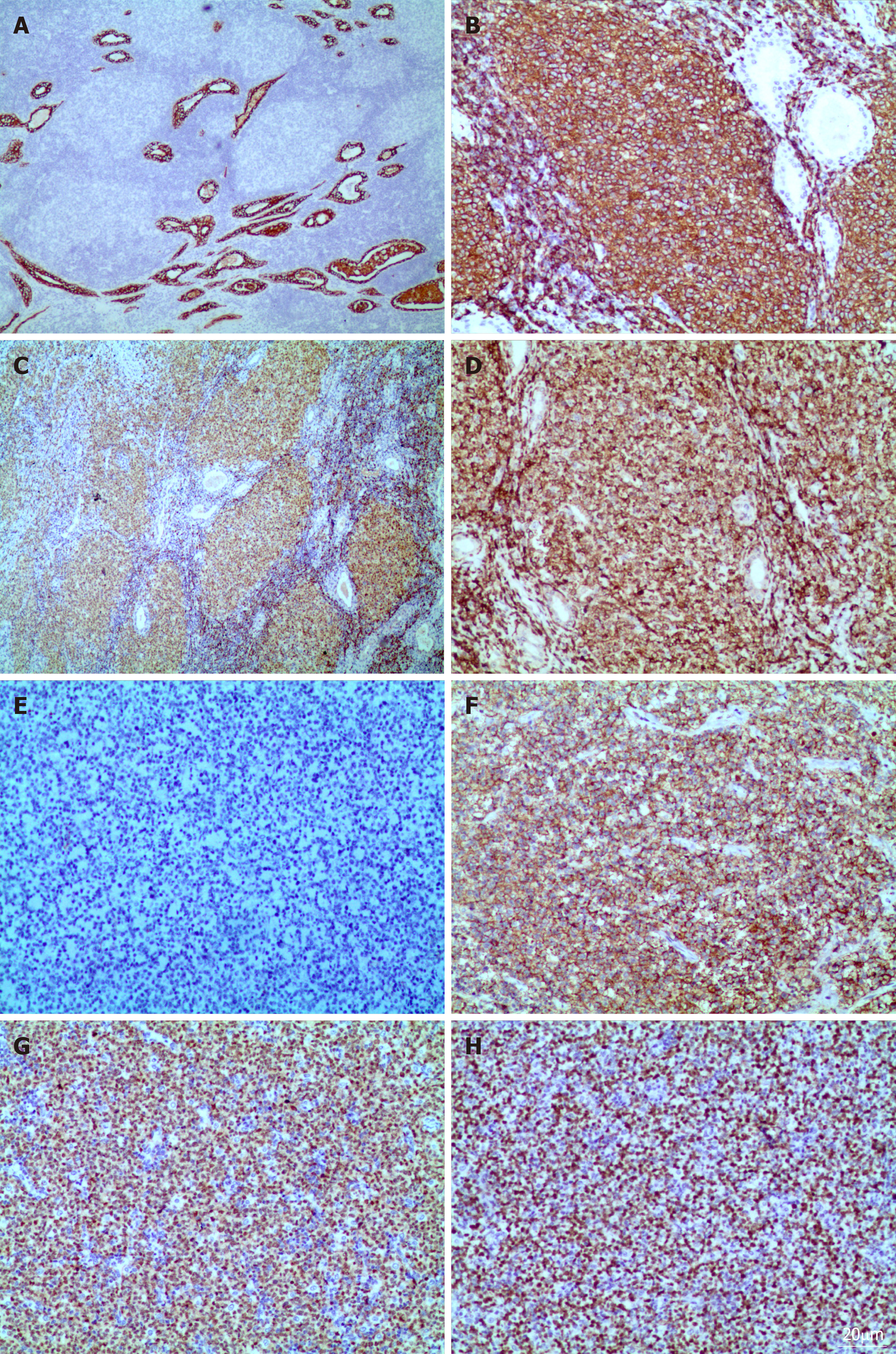
Immunohistochemical staining showed that the columnar oncocytic cells were positive for AE1/AE3 (Figure 2A) and CK18; neoplastic cells located in coarctate follicles were positive for CD20 (Figure 2B), Pax-5 (Figure 2C), bcl-2 (Figure 2D) and bcl-6, weakly positive for CD3, CD5 and CD45RO, and negative for CD5, CD21, CD10 (Figure 2E), CyclinD1, CD15, CD30, CD1a, ALK, CD4, CD123, CD56, S-100, and human melanoma black 45. The adjacent diffusely medium- to large-sized lymphoid cells were positive for Pax-5, bcl-6, CD20 (Figure 2F), MUM-1 (Figure 2G), bcl-2, Foxp1, andCD79a and negative for CD5, CD21, CD10, CyclinD1, CD3, and CD45RO. The Ki-67 proliferation index was estimated at approximately 80% (Figure 2H).
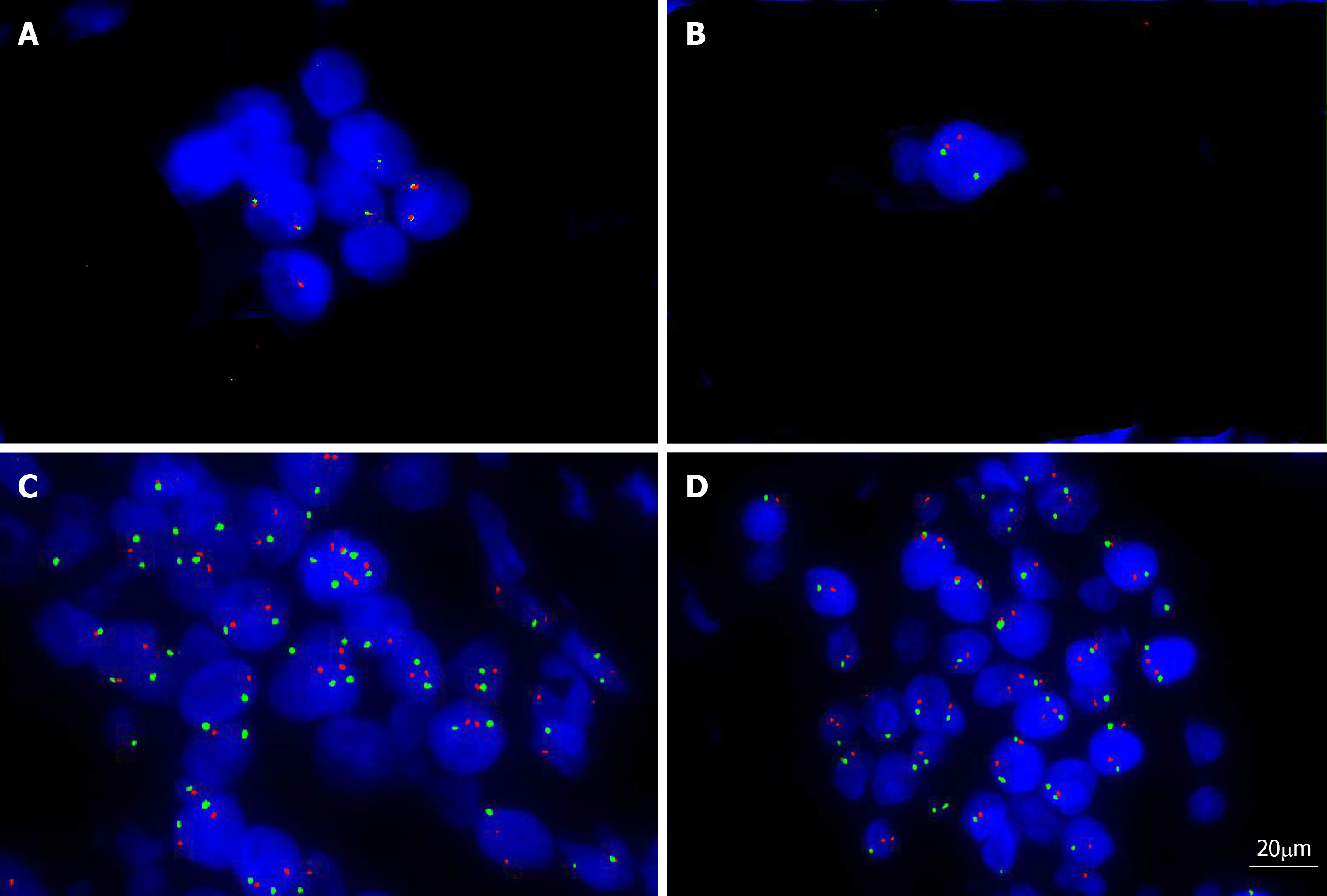
Fluorescent in situ hybridization (FISH) analysis with the dual-color dual fusion MYC (8q24), bcl-2 break-apart rearrangement (18q21), bcl2/IGH fusion translocation t(14; 18), bcl-6 break-apart rearrangement (3q27) and MYC/IGH fusion translocation t(14;18) probes were performed on the tissue mass. The results showed that there were no fusion genes or break-apart rearrangements, except for bcl-6 (3q27) (Figure 3A-D).
Epstein Barr virus (EBV) DNA was also detected using EBV-encoded RNA chromogenic in situ hybridization, and the result was negative in tumor cells. According to the histological features, immunophenotype and FISH results, the patient was diagnosed with “WT with diffuse large B-cell lymphoma (non-germinal center formation germinal center blastocyte type) that was transformed from FL in the parotid gland”.
The patient underwent a right superficial parotidectomy, and the tissue was sent for pathological examination. No swollen lymph nodes or neoplasms were found along the right carotid arteries and sternocleidomastoid muscle.
Soon after, full-body fluorine-18 fluorodeoxyglucose positron emission tomography/CT were performed to judge the clinical stage and extent of the lesion, and no potential lesions were detected. Bone marrow was not involved, which was detected by biopsy. The patient was classified as stage 3A. The patient did not receive any treatment because his health was too poor to tolerate any medicine. The patient survived 6 mo after diagnosis.
Malignant transformation in WT is rare and only occurs in approximately 1% of WT cases. It can affect either the epithelial or lymphoid component. In the majority of cases, this transformation occurs in the epithelium, and few cases involve the lymphoid stroma. Lymphoma originating from the stromal component of the parotid gland is extremely rare, especially in WT. According to the English and Chinese literature, 28 cases of WT with lymphoma have been reported to date. We concluded that the most common subtypes of lymphoma were FLs (10 cases)[1-10], followed by Hodgkin’s lymphoma (4 cases)[11-14] and diffuse large B cell lymphoma (6 cases)[4,15-18]. The others were small lymphocytic lymphoma/chronic lymphocytic leukemia (3 cases)[19-21], mantle cell lymphoma (1 case)[22], extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (1 case)[23], Lennert lymphoma (1 case)[24], peripheral T-cell lymphoma (2 cases)[25,26], and T lymphoblastic leukemia/lymphoma (1 case)[27]. In these patients, 4 cases of T-cell lymphoma and 25 cases of B-cell lymphoma were observed. The age of reported patients with lymphoma and WT ranged from 49 to 102 years, with an average age of 69 ± 11.3 years. There were 25 males (86.2%) and 4 females (13.8%), and the ratio was 6.3:1, indicating that this kind of disease is most likely involved in middle-age and older males. In these cases, only 1 case involved the bilateral parotid gland, and the others involved a single site. One case involved the upper right cervical lymph node. All of these cases were diagnosed accurately after WT excision. In our case, the patient was an older man and had a 12-year clinical history, which is consistent with the reported literature. He was promptly diagnosed after the excision of the parotid gland mass.
Due to the low incidence, the previously reported cases spanned a long time period, and the evidence of diagnosis was different. Molecular detection, FISH analysis, gene rearrangement and flow cytometry (FCM) analysis were not performed in most of these cases. Only a few cases were reported for FCM detection[19], immunoglobulin monoclonal gene rearrangement[1,21], PCR detection[23] or detection of bcl-2 mcr/JH fusion DNA sequences[1]. Our case was detected by the FISH method, and the result showed that only bcl-6 break-apart rearrangement (3q27) was observed in tumor cells of DLBCL. We reviewed the published paper and found that the CD10 was detected in 1 case and the result was negative[17], the others were not mentioned for detection. Bcl-2 rearranged were detected in 2 cases and the results revealed that the bcl-2 gene was rearranged, consistent with the presence of the t (14; 18) (q32; q21) translocation[1,2]. Our case showed that no bcl-2 break-apart rearrangement (18q21) and bcl2/IGH fusion translocation t(14; 18). CD10 staining positive and bcl2 gene rearrange were typically seen in FL which was developed in the lymph nodes. And CD10 expression and bcl-2 status were reported in few cases, especially in malignant lymphomas which were occurred in WT.
The mechanism of malignant transformation in WT is not fully understood. Smoking was presumed to be a first-hand factor with WT because smokers have an estimated risk 8 times that of nonsmokers. However, we reviewed the literature, and the results showed that only 5 patients had a history of smoking[12,16,17,19], and the smoking history of 2 patients exceeded 50 years[12,17]; the others did not mention smoking history. Therefore, smoking may not be the key cause of lymphoma with WT. Another etiological factor may be radiation exposure, as lymphomas arising within a WT occur after receiving previous radiation[28]. In our case, the patient had a 30-year history of smoking and no history of previous radiation exposure, so other unknown factors may cause the lymphoma involving WT.
The relationship between EBV infection and lymphoma with the parotid gland is unknown; based on a previous report, only 1 case of EBV-positive DLBCL of an elderly patient with WT was reported, and its clinical course was aggressive[17]; the others were negative for EBV infection, including our case. The mechanism of EBV infection in malignant lymphomas occurred in WT was unclear for the low incidence.
The prognosis of lymphomas with WT is discrepant because the incidence is too low. The reported literature showed that the valuable prognosis index had a high mitotic rate and a high Ki-67 index or a large number of positive cells. Although the Ki-67 index was up to 80% in our case, the patient remained alive 6 months after the operation.
As we have known that the lymphoid component of WT is part of the systemic lymphoid tissue, many lymphocytes will be found in the excised parotid gland, so lymphomas with WT arise from lymphoid cell transformation or chronic inflammation. The extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue may be the most common subtype neoplasm in WT; however, only 1 case of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue has been reported, and the majority of lymphomas with WT are FCL. Second, another query or debate is whether the lymphoma is coincidental or arises from the lymphoid stroma of WT; few patients with lymphoma in WT were reported to have systemic lymphoma by systemic fluorine-18 fluorodeoxyglucose positron emission tomography/CT span.
In conclusion, this case is the first reported case of DLBCL that was transformed from FL in a WT excision specimen. This is a very rare lesion with an extremely low incidence and unclear biological behavior. Therefore, we should carefully examine the tumor stroma during the pathological examination because the neoplastic cells can be confused with the normal reactive lymphoid component in WT. Requisite molecular detections are performed when the diagnosis of lymphoma with WT is dubious.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saito M, Yamashita K S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Park CK, Manning JT, Battifora H, Medeiros LJ. Follicle center lymphoma and Warthin tumor involving the same anatomic site. Report of two cases and review of the literature. Am J Clin Pathol. 2000;113:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Medeiros LJ, Rizzi R, Lardelli P, Jaffe ES. Malignant lymphoma involving a Warthin's tumor: a case with immunophenotypic and gene rearrangement analysis. Hum Pathol. 1990;21:974-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Banik S, Howell JS, Wright DH. Non-Hodgkin's lymphoma arising in adenolymphoma--a report of two cases. J Pathol. 1985;146:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Griesser GH, Hansmann ML, Bogman MJ, Pielsticker K, Lennert K. Germinal center derived malignant lymphoma in cystadenolymphoma. Virchows Arch A Pathol Anat Histopathol. 1986;408:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Colby TV, Dorfman RF. Malignant lymphomas involving the salivary glands. Pathol Annu. 1979;14 Pt 2:307-324. [PubMed] |
| 6. | Giardini R, Mastore M. Follicular non Hodgkin's lymphoma in adenolymphoma: report of a case. Tumori. 1990;76:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Hall G, Tesluk H, Baron S. Lymphoma arising in an adenolymphoma. Hum Pathol. 1985;16:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Shikhani AH, Shikhani LT, Kuhajda FP, Allam CK. Warthin's tumor-associated neoplasms: report of two cases and review of the literature. Ear Nose Throat J. 1993;72:264-269, 272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Romero M, González-Fontal GR, Duarte M, Saavedra C, Henao-Martínez AF. Small clonal B-cell population in the bone marrow as a possible tool in the diagnosis of occult primary parotid lymphoma. Colomb Med (Cali). 2016;47:59-62. [PubMed] |
| 10. | Miller R, Yanagihara ET, Dubrow AA, Lukes RJ. Malignant lymphoma in the Warthin's tumor. Report of a case. Cancer. 1982;50:2948-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Di Napoli A, Mallel G, Bartolazzi A, Cavalieri E, Becelli R, Cippitelli C, Ruco L. Nodular Lymphocyte-Predominant Hodgkin Lymphoma in a Warthin Tumor of the Parotid Gland: A Case Report and Literature Review. Int J Surg Pathol. 2015;23:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Liu YQ, Tang QL, Wang LL, Liu QY, Fan S, Li HG. Concomitant lymphocyte-rich classical Hodgkin's lymphoma and Warthin's tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e117-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Melato M, Falconieri G, Fanin R, Baccarani M. Hodgkin's disease occurring in a Warthin's tumor: first case report. Pathol Res Pract. 1986;181:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Badve S, Evans G, Mady S, Coppen M, Sloane J. A case of Warthin's tumour with coexistent Hodgkin's disease. Histopathology. 1993;22:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Gorai S, Numata T, Kawada S, Nakano M, Tamaru J, Kobayashi T. Malignant lymphoma arising from heterotopic Warthin's tumor in the neck: case report and review of the literature. Tohoku J Exp Med. 2007;212:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Ozkök G, Taşlı F, Ozsan N, Oztürk R, Postacı H. Diffuse Large B-Cell Lymphoma Arising in Warthin's Tumor: Case Study and Review of the Literature. Korean J Pathol. 2013;47:579-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Chu CY, Pan SC, Chang KC. EBV-positive diffuse large B-cell lymphoma of the elderly involving Warthin tumor. Pathol Int. 2015;65:677-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Reiner M, Goldhirsch A, Luscieti PR, Pedrinis E, Kaplan E, Cavalli F. Warthin's tumor with Sjögren's syndrome and non-Hodgkin's lymphoma. Ear Nose Throat J. 1979;58:345-350. [PubMed] |
| 19. | Jawad H, McCarthy P, O'Leary G, Heffron CC. Presentation of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma in a Warthin Tumor: Case Report and Literature Review. Int J Surg Pathol. 2018;26:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Saxena A, Memauri B, Hasegawa W. Initial diagnosis of small lymphocytic lymphoma in parotidectomy for Warthin tumour, a rare collision tumour. J Clin Pathol. 2005;58:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Bunker ML, Locker J. Warthin's tumor with malignant lymphoma. DNA analysis of paraffin-embedded tissue. Am J Clin Pathol. 1989;91:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Arcega RS, Feinstein AJ, Bhuta S, Blackwell KE, Rao NP, Pullarkat ST. An unusual initial presentation of mantle cell lymphoma arising from the lymphoid stroma of warthin tumor. Diagn Pathol. 2015;10:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Marioni G, Marchese-Ragona R, Marino F, Poletti A, Ottaviano G, de Filippis C, Staffieri A. MALT-type lymphoma and Warthin's tumour presenting in the same parotid gland. Acta Otolaryngol. 2004;124:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Franco V, Aragona F, Manzella G. [Lennert lymphoma arising in a cystadenolymphoma]. Pathologica. 1986;78:263-268. [PubMed] |
| 25. | Pescarmona E, Perez M, Faraggiana T, Granati L, Baroni CD. Nodal peripheral T-cell lymphoma associated with Warthin's tumour. Histopathology. 2005;47:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Liu HX, Sun YM. [Peripheral T-cell lymphoma, NOS arising in Warthin tumor: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2008;37:425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Giaslakiotis K, Androulaki A, Panagoulias G, Kyrtsonis MC, Lazaris AC, Kanakis DN, Patsouris ES. T cell lymphoblastic lymphoma in parotidectomy for Warthin's tumor: case report and review of the literature. Int J Hematol. 2009;89:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol. 1980;388:13-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 3.3] [Reference Citation Analysis (0)] |