Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3887
Peer-review started: April 30, 2019
First decision: August 1, 2019
Revised: October 17, 2019
Accepted: October 30, 2019
Article in press: October 30, 2019
Published online: November 26, 2019
Processing time: 211 Days and 6.7 Hours
Thyroxine-binding globulin (TBG; the gene product of SERPINA7) is the main transporter of thyroid hormones in humans. Mutations in the TBG gene may lead to inherited TBG deficiency. There have been 28 reported mutations that associate with complete TBG deficiency (TBG-CD). Here we identified a novel frameshift mutation causing early termination of the TBG protein and TBG-CD in a Chinese family.
A 46-year-old Chinese man was referred to our hospital with normal free thyroxine, free triiodothyronine, thyrotropin, but lower total thyroxine and total triiodothyronine, and undetectable serum TBG, indicative of TBG-CD. Blood samples were obtained from the patient’s family members and thyroid function and serum TBG were evaluated. Genomic DNA from peripheral blood was sequenced to detect possible TBG mutation(s). Quantitative PCR high-resolution melting curve analysis was used to screen TBG-Poly (L283F) among 117 Chinese men. A novel mutation of TBG (p.Phe135Alafs*21), a 19-nucleotide insertion in exon 1, was identified, which resulted in a truncated TBG protein product and caused TBG-CD. The other mutation, identified in the proband’s father, is a known polymorphism, TBG-Poly (L283F). The frequency of the TBG-Poly allele among 117 unrelated Han Chinese men from northeast China was 21.37%.
A novel mutation in the TBG gene associated with the TBG-CD phenotype was identified in a Chinese family. Additionally, it was found that 21.37% of Chinese males had TBG-Poly (L283F).
Core tip: We present herein a novel thyroxine-binding globulin (TBG) mutation in exon 1, c.381_382 ins TTGCAGATAGGAAATGCCC (p.Phe135Alafs*21), which was associated with complete TGB deficiency in a Chinese family. This 19-nucleotide insertion in exon 1 resulted in a frameshift and a premature stop codon at position 155 of the protein coding sequence. TBG deficiency is often misdiagnosed as hypothyroidism. Clinical awareness is needed to correctly diagnose affected individuals and avoid unnecessary treatment. Genomic testing is a method to identify the mutation carriers and provide appropriate genetic counseling for affected individuals.
- Citation: Dang PP, Xiao WW, Shan ZY, Xi Y, Wang RR, Yu XH, Teng WP, Teng XC. Novel frameshift mutation causes early termination of the thyroxine-binding globulin protein and complete thyroxine-binding globulin deficiency in a Chinese family: A case report. World J Clin Cases 2019; 7(22): 3887-3894
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3887.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3887
Thyroxine-binding globulin (TBG) is the main thyroid hormone transport protein in humans, carrying approximately 75% of the total thyroxine (TT4) and 70% of the total triiodothyronine (TT3) present in serum[1-3]. The human TBG gene belongs to the serpin family of genes and is located on the long arm of the X-chromosome (Xq22.2)[4,5]. Abnormalities in TBG are caused by mutations in the TBG gene, and demonstrate an X-linked pattern of inheritance[6-8]. Based on the serum levels of TBG in hemizygotes expressing only the mutant allele, TBG defects are classified as complete TBG deficiency (TBG-CD), partial TBG deficiency (TBG-PD), and TBG excess (TBG-E)[1].
To date, 28 TBG mutations that cause TBG-CD have been identified: 7 intron region mutations, 20 exon mutations, and 1 mutation involving both an intron and an exon. These 28 mutations include 14 single nucleotide substitutions, 12 nucleotide deletions, 1 deletion-insertion, and 1 single nucleotide insertion. Additionally, 19 TBG gene mutations result in TBG-PD; all of them are single nucleotide substitutions, with 17 in exon regions, 1 in an intron, and 1 in the downstream enhancer region of the TBG gene. Furthermore, 3 single nucleotide substitutions in TBG have been identified as gene polymorphisms that do not cause changes in TBG levels[9-14]. As yet, no large insertional mutations that associate with TBG-CD have been reported in TBG.
Here, we report a novel TBG mutation in exon1, c.381_382 ins TTGCAGATAGGAAATGCCC (p.Phe135Alafs*21), which was associated with TBG-CD in a Chinese family. This 19-nucleotide insertion in exon 1 resulted in a frameshift and a premature stop codon at position 155 of the protein coding sequence; the mutation is termed TBG-CDC. The proband and his brother are hemizygous for the mutation, and manifested the TBG-CD phenotype. The proband’s mother is heterozygous for this mutation, but displayed the same TBG-CD phenotype as her affected sons. The proband’s father has a single nucleotide substitution in exon 3, c.909G>T (p.Leu303Phe), which is known as TBG-Poly (L283F). The proband’s father had low TT4 and TT3, but a normal amount of thyrotropin (TSH), and his serum TBG level was between normal and affected hemizygous, which is inconsistent with previous reports that this polymorphism causes no changes in TBG levels.
A 46-year-old Chinese man had a health examination at a local hospital. He had normal free thyroxine (FT4), free triiodothyronine (FT3), and TSH, but low TT4 and TT3. The patient was then referred to our hospital for additional diagnosis.
The patient underwent physical examination a month ago and abnormal thyroid function was found.
The patient had a free previous medical history.
The patient’s thyroid gland was normal, without nodules, as measured by thyroid gland ultrasound. The patient denied symptoms of weakness, drowsiness, and intolerance to cold.
Upon re-evaluation of the patient’s thyroid function, we detected low serum TT4 and TT3 levels, normal FT4, FT3, and TSH levels, and undetectable serum TBG (the lower limit of detection of the assay was 3.5 μg/mL; normal range: 14–31 μg/mL).
To find out if other people in the proband's family have similar performance, blood samples were obtained from the patient’s family members, including his paternal grandfather, parents, younger brother, and nephew. To identify the prevalence of the TBG-Poly (L283F) variant in Chinese men, serum and whole blood samples were obtained from 117 unrelated Han Chinese men from northeastern China.
Serum TT4, TT3, FT3, FT4, TSH, thyroglobulin antibodies (TgAb), and thyroperoxidase antibodies (TPOAb) were measured using electro-chemiluminescence immunoassays performed on a Cobas Elesys 601 instrument. TT4, TT3, FT3, FT4, TSH, TgAb, TPOAb were analyzed using the competition principle, and TSH using the sandwich principle (Catalog number: 26047103 for TSH, 23816601 for TT3, 25785701 for FT3, 24807703 for TT4, 24682503 for FT4, 22084502 for TPOAb, and 23015003 for TgAb, Roche Diagnostics GmbH, Mannheim, Germany). Serum TBG and thyroglobulin (Tg) were measured using a solid–phase, competitive chemiluminescent enzyme immunoassay on an Immulite 2000 Xpi instrument (Catalog number: L2KTB2 for TBG and L2KTY2 for Tg, Siemens Healthcare Diagnostics Products limited, United Kingdom). Genomic DNA was isolated from peripheral blood using a DNA Kit (TIANGEN, Beijing, China), and targeted sequences were amplified by PCR. Gene regions including exons 1–4 of the TBG gene and intron-exon boundaries were sequenced (3730XL; Applied Biosystems, Carlsbad, California). Quantitative PCR high-resolution melting curve analysis was used to screen the TBG-Poly (L283F) variant among 117 Chinese men; the results were verified by direct DNA sequencing. The primers used in PCR amplification and sequencing are shown in Table 1.
| Primer ID | Primer sequence |
| Sequencing | |
| Exon 1 | |
| SERPINA7-F | AGAGAAACCCCTGCTCAG |
| SERPINA7-R | TTTCCTGGACTCATTCACAG |
| Exon 2 | |
| SERPINA7-F | GGTACCTAACTCTGTGGTGA |
| SERPINA7-R | CATAGCTGTTGGGTAGTTCA |
| Exon 3 | |
| SERPINA7-F | TGGTTATCAATACTCAGGGAAG |
| SERPINA7-R | TCTAGCTTAGGAGGAGTCAC |
| Exon 4 | |
| SERPINA7-F | ACTACATTTAGCAGAGGAAAC |
| SERPINA7-R | CAAAGTTCAGCCAGGGTT |
| qPCR-HRM | |
| TBG-Poly of exon 3 | |
| Forward primer | AAAGTGTGGCTCCAAGGTCA |
| Reverse primer | GGTGATTGCCATGTGTTCCC |
| Sanger sequencing | |
| TBG-Poly of exon 3 | |
| Forward primer | AGAGAGAAGGAGAGAATCATAAGC |
| Reverse primer | TGGAAAGTTTCAGACCATTGTC |
| TBG-Xq22G>A | |
| Forward primer | GTTGGGAAACTGGAAGGAGA |
| Reverse primer | AGAGGTGGAAAGGGGAAGAG |
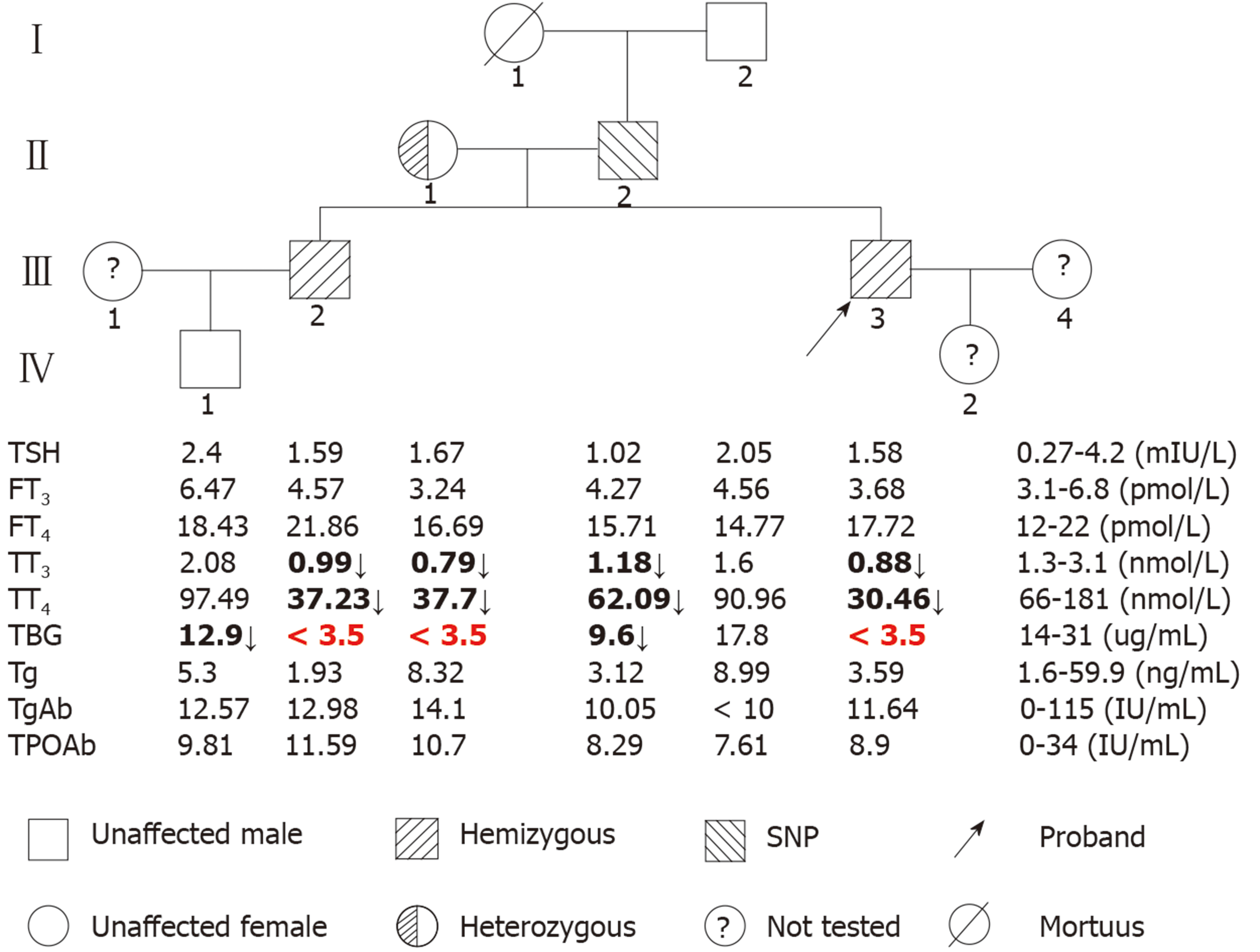
The pedigrees and results of the thyroid function tests (TFTs) of the family members are shown in Figure 1. The proband (III-3), his brother (III-2), and his mother (II-1) had low serum TT4 and TT3 levels but normal TSH concentrations, and serum TBG was undetectable, which is characteristic of TBG-CD. The proband’s father (II-2) had low TT4 and TT3, but normal TSH; his serum TBG level was between normal and affected hemizygous (Figure 1), which indicated TBG-PD. The proband’s grandfather (I-2) and nephew (IV-1) had normal TFTs. All family members had normal thyroglobulin (Tg), TgAb, and TPOAb levels.
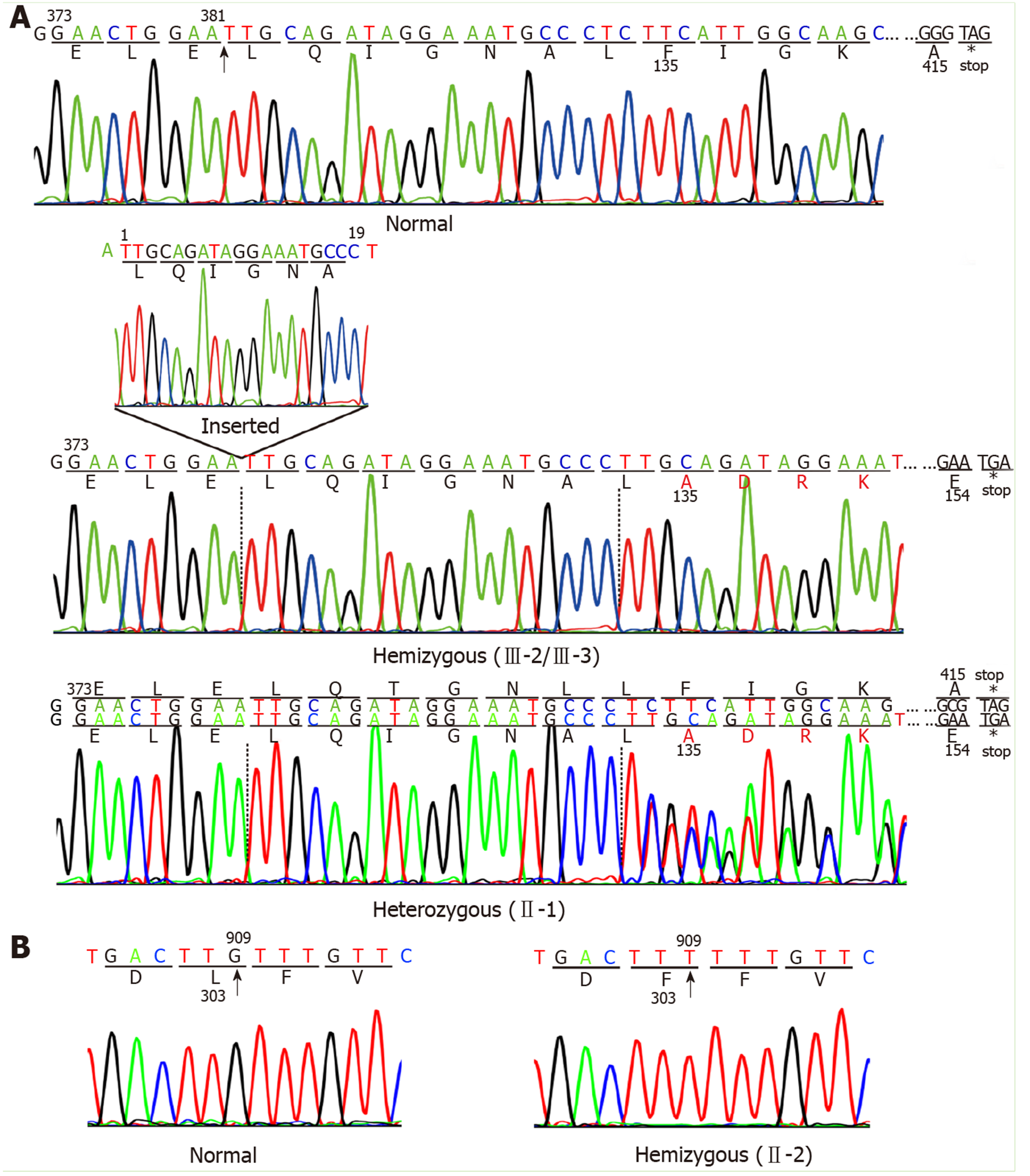
Two mutations in the TBG gene were identified in this Chinese family. One, a novel mutation, was identified in the index III-3, III-2, and II-1. This mutation is a 19-nucleotide insertion, occurring between cDNA positions 381 and 382 (c.381_382insTTGCAGATAGGAAATGCCC) in exon 1. This mutation changes the phenylalanine at codon 135 to alanine, following which there are 19 amino acids and then an early termination codon at position 155, leading to premature termination of TBG (Figure 2A). This mutation results in a truncated protein containing only the first 134 amino acids; in comparison, the wild-type TBG protein (TBG-C) is 395 amino acids long, excluding the 20 amino acid signal peptide. As expected, the III-3 and III-2 are hemizygous for the TBG mutation, while II-1 is heterozygous, demonstrating that the mutation follows a pattern of X-linked inheritance.
The other TBG mutation is a single nucleotide substitution (TTG→TTT) in codon 303 in exon 3 (p.Leu303Phe), which was identified in the II-2 (Figure 2B). This variant was previously known as TBG polymorphism, TBG-Poly (L283F). The II-2 carried the TBG-Poly (L283F) polymorphism and presented with TBG-PD. He had low TT4 and TT3 levels, with his serum TBG levels falling between normal and affected hemizygous levels (Figure 1).
The frequency of the TBG-Poly allele among the 117 unrelated Han Chinese men was found to be 21.37%.
In the present study, we identify a novel mutation, named TBG-CDC, in TBG (p.Phe135Alafs*21) from a Chinese family. This mutation is a 19-nucleotide insertion, located between cDNA positions 381 and 382 in exon 1, and is the first reported large nucleotide fragment insertional mutation of the TBG gene that results in TBG-CD.
This mutation resulted in a truncated protein that contained only the first 134 of the 395 amino acids of the mature TBG-C, and lacked 66% of the carboxyl terminal amino acids. The carboxyl terminus of TBG is important for intracellular transport and protein synthesis[15]. We used Mutation Taster software to predict the disease-causing potential of this mutation[16]. The prediction suggested that the truncated TBG protein lost two glycosylation sites (at positions 165 and 253), and two thyroxine binding sites (at positions 293 and 398). Glycosylation plays an important role in the processing, folding, and secretion of TBG, and the thyroxine binding sites of TBG are associated with the transport of thyroid hormone in blood[17-19]; we therefore speculate that this truncation of TBG may lead to TBG-CD, and may result in a reduction in TT3 and TT4 levels. However, the mechanism for the failure to detect immunoreactive TBG in the serum of these patients harboring this mutation remains unknown. We speculate that three reasons may be concerned. The first one may be the impaired synthesis of the truncated TBG proteins. The 19-nucleotide insertion in exon 1 is supposed to affect the transcription or the translation of TBG, leading to the failure of synthesis of mutant TBG. The second one may be the impaired secretion of the truncated TBG proteins. Most of the truncated TBG molecules previously reported were not secreted[20-22]. They remained in the rough endoplasmic reticulum and were rapidly degraded within the cells, or had impaired intracellular transport in the blood. And the last reason may be associated with the TBG antibodies used in the present study, which can only bind to the truncated region, but not to the non-truncated region, and hence results in no detection of serum TBG.
Since inherited TBG defects follow an X-linked pattern, TBG-CD is fully manifested in hemizygous males but only partially in heterozygous females[1]. However, the TBG-CD phenotype has been reported in two heterozygous females with selective inactivation of the X-chromosome carrying the normal TBG alleles[23], and in two females with XO Turner’s syndrome[24,25].
In the family we studied, the II-1 also manifested TBG-CD, but the molecular basis for her TBG-CD remains unclear. The proband’s mother has normal stature and fertility, and therefore XO Turner’s syndrome is unlikely, although the condition cannot be ruled out without additional investigation. We speculate that selective inactivation of the X-chromosome containing the TBG-C allele may be associated with her phenotype presentation.
The TBG-Poly (L283F) variation is a common polymorphism, with high prevalence in different races and regions, including 16% of French Canadian males[26], 50% of Australian Aboriginal males[27], 20% of the Japanese population[28], 31% of the Han Chinese population in Taiwan[29], and 21.37% of Chinese men in northeastern China. Previous studies have reported that TBG-Poly (L283F) causes no changes in biological properties[27]. It is worth noting that the majority of complete TBG defects are associated with nonsense mutations that produce truncated protein, or with missense mutations associated with the presence of the L283F polymorphism (TBG-Poly)[26,29]. In the present study, we report that the II-2 presented with the TBG–PD phenotype. Whether the TBG-Poly (L283F) is responsible for lower TBG levels as well as lower levels of TT3 and TT4 remains unclear. Ferrara et al[12] previously reported a mutation in the TBG gene enhancer region, the Xq22G>A mutation, which is located 20 kb downstream of the TBG gene; this mutation has been associated with the TBG-PD phenotype. However, the Xq22G>A mutation was not detected in the II-2. Since only TBG coding regions and adjacent intron regions were sequenced in the present study, abnormalities in other transcriptional regulatory elements associated with this gene might be involved in the variable phenotypes seen in the Chinese male with TBG-Poly (L283F); these possibilities merit further investigation.
TBG deficiency may produce alterations in total thyroid hormone concentration in serum, whereas free THs remain unchanged. So TBG deficiency is often misdiagnosed as hypothyroidism. Clinical awareness is needed to correctly diagnose affected individuals and avoid inappropriate treatment. Genomic testing is a method to identify the mutation carriers and provide appropriate genetic counseling for affected individual[29,30].
In conclusion, a novel TBG mutation, p.Phe135Alafs*21, was identified in a Chinese family. This mutation is a 19-nucleotide insertion in exon 1, produces truncated TBG protein, and is termed “TBG-CDC”. The fact that the proband’s father had the TBG-Poly (L283F) variant and presented as TBG-PD merits further investigation. The allelic frequency of TBG-Poly (L283F) was found to be 21.37% in 117 unrelated Chinese males in northeastern China.
We gratefully acknowledge all of the subjects who participated in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fatima SS, Kumar B S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Refetoff S. Inherited thyroxine-binding globulin abnormalities in man. Endocr Rev. 1989;10:275-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Refetoff S, Feingold KR, Anawalt B, Boyce A. Thyroid Hormone Serum Transport Proteins. Feingold KR, Anawalt B, Boyce A. Endotext. South Dartmouth: MDText.com, Inc 2000; . |
| 3. | Robbins J, Rall JE. Zone electrophoresis in filter paper of serum I 131 after radioiodide administration. Proc Soc Exp Biol Med. 1952;81:530-536. [PubMed] |
| 4. | Trent JM, Flink IL, Morkin E, van Tuinen P, Ledbetter DH. Localization of the human thyroxine-binding globulin gene to the long arm of the X chromosome (Xq21-22). Am J Hum Genet. 1987;41:428-435. [PubMed] |
| 5. | Mori Y, Miura Y, Oiso Y, Hisao S, Takazumi K. Precise localization of the human thyroxine-binding globulin gene to chromosome Xq22.2 by fluorescence in situ hybridization. Hum Genet. 1995;96:481-482. [PubMed] |
| 6. | Nicoloff JT, Dowling JT, Patton DD. Inheritance of Decreased Thyroxine-Binding By the thyroxine-binding globulin. J Clin Endocrinol Metab. 1964;24:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Marshall JS, Levy RP, Steinberg AG. Human thyroxine-binding globulin deficiency. A genetic study. N Engl J Med. 1966;274:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Nikolai TF, Seal US. X-chromosome linked inheritance of thyroxine-binding globulin deficiency. J Clin Endocrinol Metab. 1967;27:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Pappa T, Ferrara AM, Refetoff S. Inherited defects of thyroxine-binding proteins. Best Pract Res Clin Endocrinol Metab. 2015;29:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Fang YL, Wang CL, Liang L. [Partial thyroxine binding globulin deficiency in test tube infants: report of cases and literature review]. Zhonghua Er Ke Za Zhi. 2016;54:428-432. [PubMed] |
| 11. | Berger HR, Creech MK, Hannoush Z, Watanabe Y, Kargi A, Weiss RE. A Novel Mutation Causing Complete Thyroid Binding Globulin Deficiency (Tbg-Cd Mia) in a Male with Coexisting Graves Disease. AACE Clin Case Rep. 2017;3:e134-e139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ferrara AM, Pappa T, Fu J, Brown CD, Peterson A, Moeller LC, Wyne K, White KP, Pluzhnikov A, Trubetskoy V, Nobrega M, Weiss RE, Dumitrescu AM, Refetoff S. A novel mechanism of inherited TBG deficiency: mutation in a liver-specific enhancer. J Clin Endocrinol Metab. 2015;100:E173-E181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Moeller LC, Appiagyei-Dankah Y, Köhler B, Biebermann H, Janssen OE, Führer D. Two Novel Mutations in the Serpina7 Gene Are Associated with Complete Deficiency of Thyroxine-Binding Globulin. Eur Thyroid J. 2015;4:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Soheilipour F, Fazilaty H, Jesmi F, Gahl WA, Behnam B. First report of inherited thyroxine-binding globulin deficiency in Iran caused by a known de novo mutation in SERPINA7. Mol Genet Metab Rep. 2016;8:13-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345-2355. [PubMed] |
| 16. | Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2146] [Cited by in RCA: 2363] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 17. | Murata Y, Magner JA, Refetoff S. The role of glycosylation in the molecular conformation and secretion of thyroxine-binding globulin. Endocrinology. 1986;118:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kambe F, Seo H, Mori Y, Murata Y, Janssen OE, Refetoff S, Matsui N. An additional carbohydrate chain in the variant thyroxine-binding globulin-Gary (TBGAsn-96) impairs its secretion. Mol Endocrinol. 1992;6:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proc Natl Acad Sci USA. 2006;103:13321-13326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Li P, Janssen OE, Takeda K, Bertenshaw RH, Refetoff S. Complete thyroxine-binding globulin (TBG) deficiency caused by a single nucleotide deletion in the TBG gene. Metabolism. 1991;40:1231-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Janssen OE, Refetoff S. In vitro expression of thyroxine-binding globulin (TBG) variants. Impaired secretion of TBGPRO-227 but not TBGPRO-113. J Biol Chem. 1992;267:13998-14004. [PubMed] |
| 22. | Miura Y, Kambe F, Yamamori I, Mori Y, Tani Y, Murata Y, Oiso Y, Seo H. A truncated thyroxine-binding globulin due to a frameshift mutation is retained within the rough endoplasmic reticulum: a possible mechanism of complete thyroxine-binding globulin deficiency in Japanese. J Clin Endocrinol Metab. 1994;78:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Okamoto H, Mori Y, Tani Y, Nakagomi Y, Sano T, Ohyama K, Saito H, Oiso Y. Molecular analysis of females manifesting thyroxine-binding globulin (TBG) deficiency: selective X-chromosome inactivation responsible for the difference between phenotype and genotype in TBG-deficient females. J Clin Endocrinol Metab. 1996;81:2204-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Refetoff S, Selenkow HA. Familial thyroxine-binding globulin deficiency in a patient with Turner's syndrome (XO). Genetic study of a kindred. N Engl J Med. 1968;278:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Reutrakul S, Janssen OE, Refetoff S. Three novel mutations causing complete T(4)-binding globulin deficiency. J Clin Endocrinol Metab. 2001;86:5039-5044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Mori Y, Takeda K, Charbonneau M, Refetoff S. Replacement of Leu227 by Pro in thyroxine-binding globulin (TBG) is associated with complete TBG deficiency in three of eight families with this inherited defect. J Clin Endocrinol Metab. 1990;70:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Takeda K, Mori Y, Sobieszczyk S, Seo H, Dick M, Watson F, Flink IL, Seino S, Bell GI, Refetoff S. Sequence of the variant thyroxine-binding globulin of Australian aborigines. Only one of two amino acid replacements is responsible for its altered properties. J Clin Invest. 1989;83:1344-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Takeda K, Iyota K, Mori Y, Tamura Y, Suehiro T, Kubo Y, Refetoff S, Hashimoto K. Gene screening in Japanese families with complete deficiency of thyroxine-binding globulin demonstrates that a nucleotide deletion at codon 352 may be a race specific mutation. Clin Endocrinol (Oxf). 1994;40:221-226. [PubMed] |
| 29. | Su CC, Wu YC, Chiu CY, Won JG, Jap TS. Two novel mutations in the gene encoding thyroxine-binding globulin (TBG) as a cause of complete TBG deficiency in Taiwan. Clin Endocrinol (Oxf). 2003;58:409-414. [PubMed] |
| 30. | Pappa T, Moeller LC, Edidin DV, Pannain S, Refetoff S. A Novel Mutation in the TBG Gene Producing Partial Thyroxine-Binding Globulin Deficiency (Glencoe) Identified in 2 Families. Eur Thyroid J. 2017;6:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |