Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3866
Peer-review started: August 2, 2019
First decision: September 23, 2019
Revised: October 12, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 116 Days and 1.6 Hours
Gastric duplication cysts (GDCs) are extremely uncommon lesions and the definitive diagnosis of GDCs is challenging for gastrointestinal specialists. It is important that a differential diagnosis is performed to rule out the possibility of other diseases, mainly malignancies with a cystic component. Despite the use of multiple diagnostic modalities including endoscopy, the preoperative diagnosis of GDCs is challenging.
A 53-year-old female patient with a GDC was confirmed by positron emission tomography/computed tomography (PET/CT) instead of more conventional procedures such as endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA). We propose that 18F-FDG-PET/CT has higher accuracy than EUS-FNA and may be an effective technique for the characterization of duplication cysts.
Preoperative diagnosis of GDCs in adults is difficult largely due to their rarity and the absence of characteristic findings. In addition, few endoscopists include GDCs in the differential diagnosis when they encounter a lesion with cystic characteristics. 18F-FDG-PET/CT with additional imaging data, may complement EUS-FNA in the diagnosis of GDCs.
Core tip: Gastric duplication cysts (GDCs) are rare congenital gastrointestinal abnormalities. This is the first case report in which both endoscopic ultrasonography-guided fine needle aspiration and positron emission tomography/computed tomography approaches were used in the diagnosis and intervention of GDCs. Even though a panel of imaging modalities is available to consolidate the diagnosis, it is still recommended that a different operative approach to resect the cystic lesion is chosen, as most cases with symptomatic manifestations and/or complications have the possibility of malignant transformation at the time of presentation.
- Citation: Hu YB, Gui HW. Diagnosis of gastric duplication cyst by positron emission tomography/computed tomography: A case report. World J Clin Cases 2019; 7(22): 3866-3871
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3866
Gastric duplication cysts (GDCs) are extremely uncommon lesions and the definitive diagnosis of GDCs is difficult. It is important that a differential diagnosis is performed to rule out the possibility of other diseases, mainly malignancies with a cystic component. Despite the use of multiple diagnostic modalities including endoscopy, the preoperative diagnosis of GDCs is challenging. Some reports have proposed endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) as the most accurate technique for the identification of cystic lesions[1]. However, it is challenging to retrieve high quality cytological and histological samples of the lesion. In addition to the risk of infection and bleeding, EUS-FNA is also associated with a potential risk of disseminating the malignant cells along the needle tract and into the peritoneum. Here we report a unique case in which both EUS-FNA and positron emission tomography/computed tomography (PET/CT) approaches were used in the diagnosis and intervention of GDCs. The rarity of this disease and the misdiagnosis by EUS-FNA prompted us to report this case.
A 53-year-old woman presented to our gastroenterology department with mild epigastralgia a few hours after consuming a considerable amount of meat.
The patient’s symptoms started with mild epigastralgia, which had worsened in the last 2 h.
The patient had no previous medical history.
The patient’s temperature was 36.8 °C, heart rate was 75 bpm, respiratory rate was 15 breaths per minute, blood pressure was 130/70 mmHg and oxygen saturation in room air was 99%. A physical examination revealed mild tenderness without any peritoneal signs.
All hematological parameters were within normal ranges, including serum levels of amylase, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9.
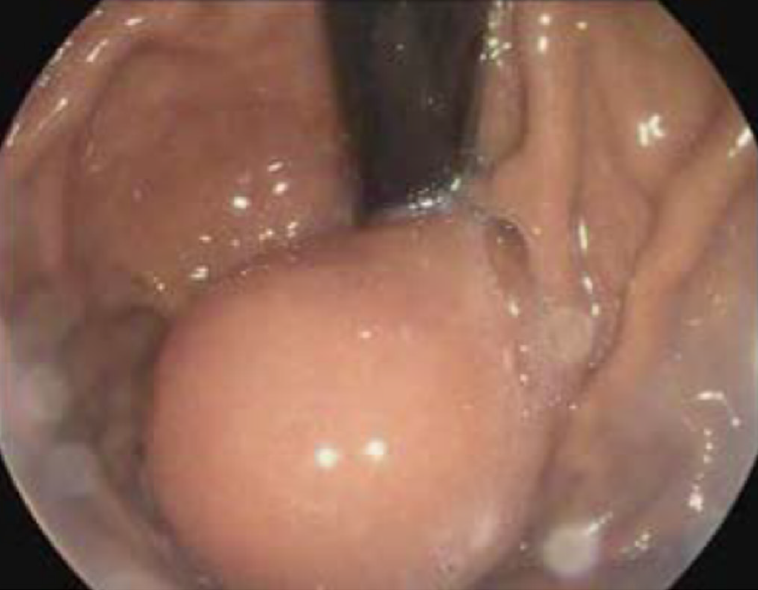
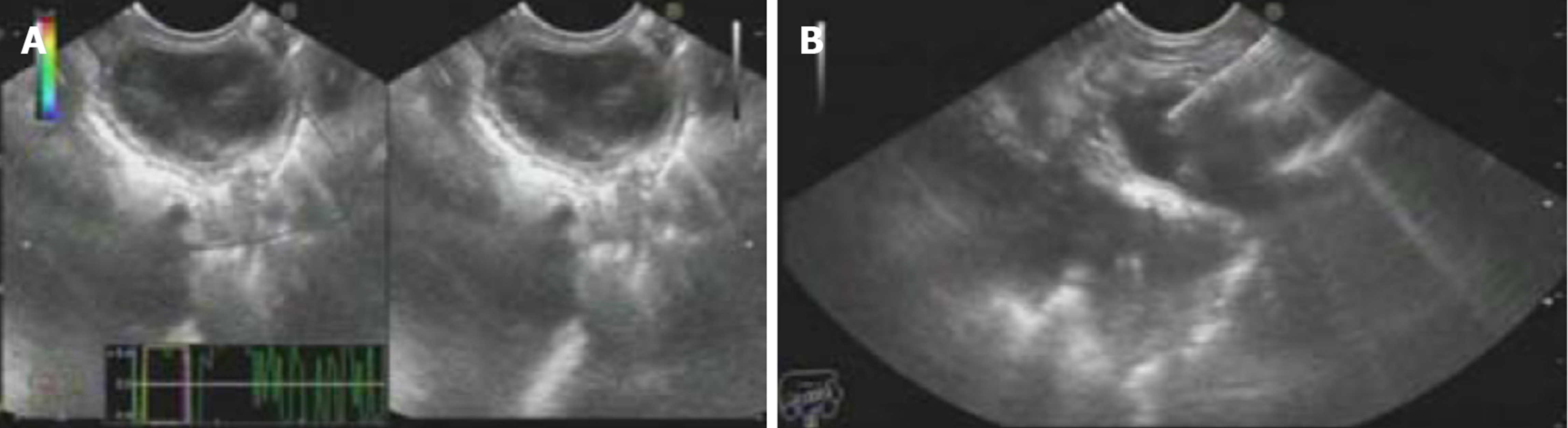
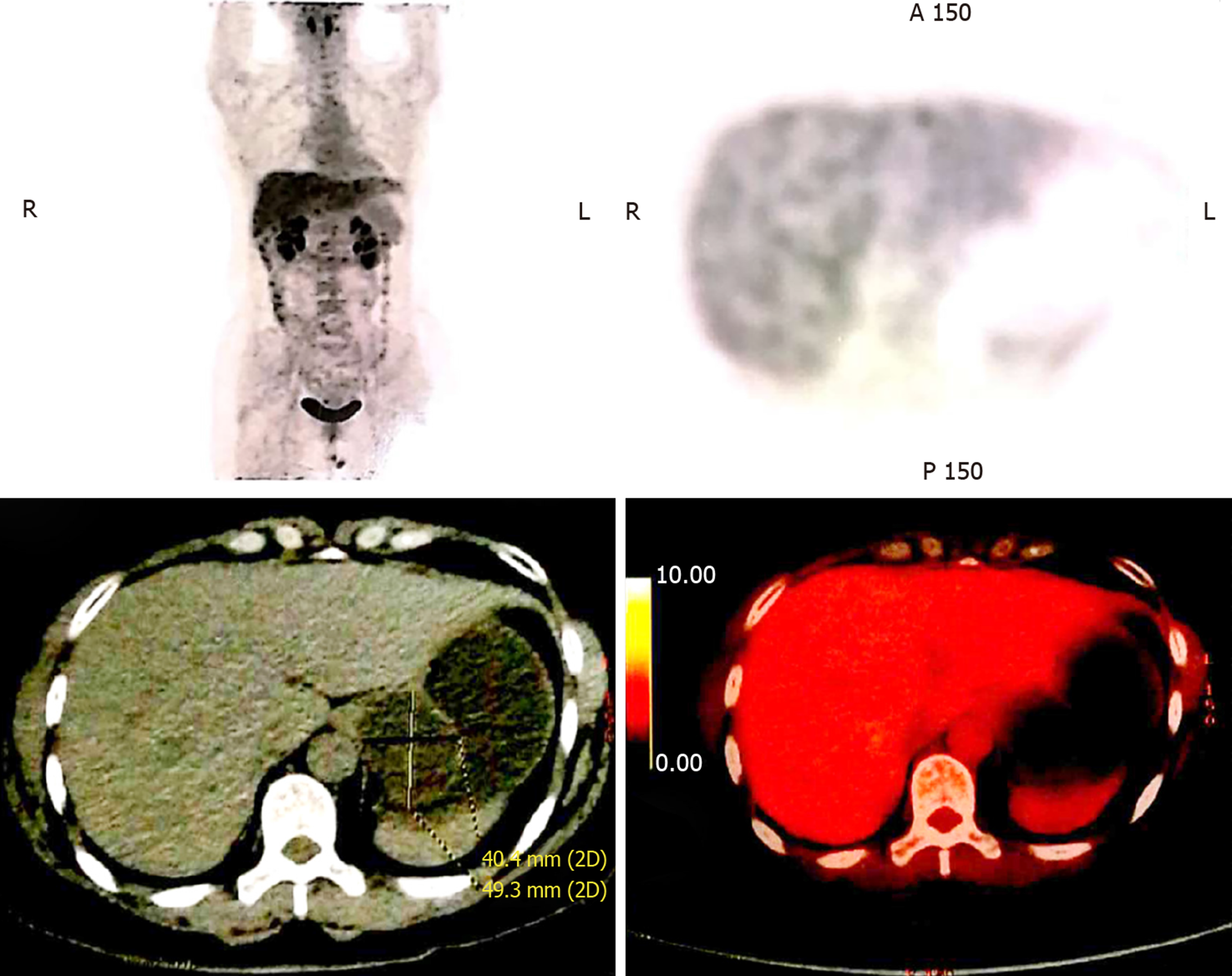
Computed tomography (CT) revealed a cystic lesion apparently originating from the submucosa of the stomach, which was well-defined and had a homogeneous density. The lesion showed no intensity on contrast enhanced CT. Upper endoscopy revealed a subepithelial mass with a smooth surface at the greater curvature of the gastric cardia and fundus (Figure 1). EUS showed a 3.0 cm × 3.0 cm hypoechoic cystic lesion, apparently located outside the serosa of the stomach (Figure 2). Neither abdominal nor mediastinal enlarged lymph nodes were identified. Some mucoid fluid and small tissue fragments of the cystic wall were retrieved by EUS-FNA. The cytological smear showed foamy histiocytes, and histopathologic examination showed mucinous materials. However, the cyst fluid from FNA showed CEA levels over 1500 ng/mL and CA-199 levels over 1200 U/mL. This suggested the possible diagnosis of a mucinous cystic neoplasm. Before surgery, the patient was referred for PET/CT. A 4.9 cm × 4.0 cm cystic mass showed no significant 18F-fluorodeoxyglucose (18F-FDG) uptake (Figure 3), which suggested that the gastric cystic lesion could be a GDC.
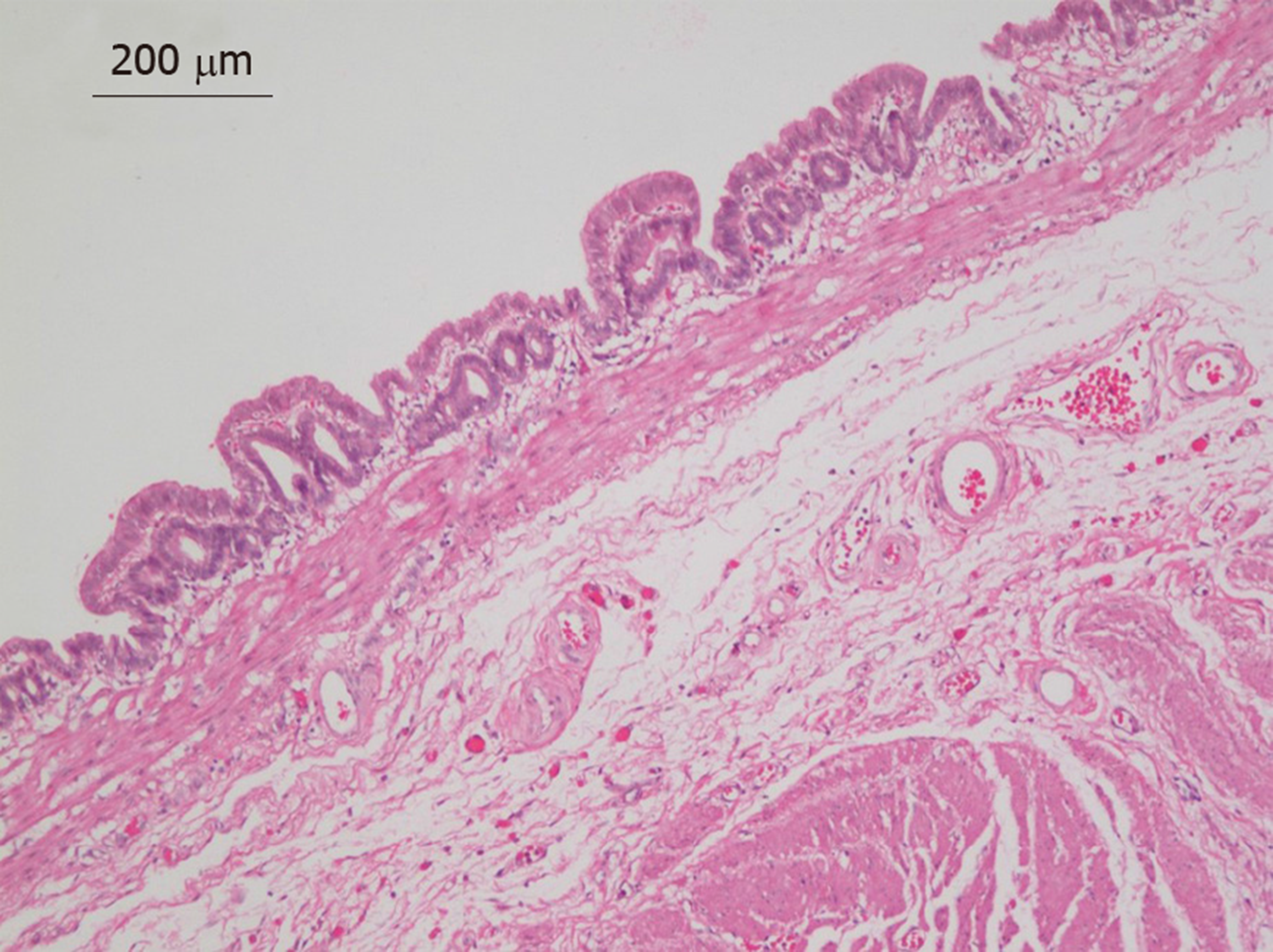
Pathologic examination of the surgical specimen revealed macroscopically a cystic lesion 3.5 cm × 3.5 cm × 3 cm in size with a mucoid content. Under the microscope, the inner side of the cystic lesion comprised a single layer of columnar epithelium with gastric glandular formation (Figure 4). The immunohistochemistry results showed CK7(+), Villin(+), CDX-2(+), CK20(-), ER(-), PR(-), PAX-8(-), and CD10(-). These features were consistent with a diagnosis of GDC.
Considering the malignant potential of the cystic lesion based on its increasing size, a proximal gastrectomy was planned. Laparoscopic resection revealed the cystic mass contiguous to the greater curvature of the stomach, indicating the gastric origin of the cystic lesion, rather than pancreatic or left adrenal gland origin. The cyst was completely resected with partial wedge resection of the stomach.
The patient had an uneventful postoperative recovery and was discharged on the ninth postoperative day. The patient has been well since hospital discharge, and there is no evidence of recurrence during the 1-year follow-up period.
GDCs are rare congenital gastrointestinal abnormalities[2]. These cysts are usually diagnosed during early childhood, as duplication cysts tend to be symptomatic at this age. Conversely, these cysts are usually asymptomatic during adulthood, and their diagnosis is challenging. Malignant transformation of these lesions has been reported[3-5]; thus, accurate diagnosis is required. A recent report suggested that EUS-FNA may play a major role in the diagnosis of GDCs as it has a higher accuracy rate compared to traditional imaging techniques[6]. However, the cystic lesion in our case was thought to share the layer of the submucosa of the proper stomach wall. This feature led to EUS misdiagnosis where the cystic lesion was concluded to exist outside the proper gastric wall. In fact, it was in the three-layered hyperechoic submucosa, which was mistaken to be the five-layered hyperechoic serosa. As observed in our case, the cystic lesion was found inside the gastric wall by surgery, rather than outside the gastric wall as shown by EUS. Although a cytological examination using EUS-FNA was performed in this case, the cellular and tissue samples showed nonspecific mucinous materials, which led to the misinterpretation of a mucinous cystic neoplasm without a final diagnosis. Of note, new technologies such as EUS-guided fine needle biopsy (EUS-FNB) appear to have comparable pathologic diagnostic yield to EUS-FNA[7]. Further investigation is required to confirm whether EUS-FNB can extract more pathological tissue than EUS-FNA, and cause less complications in patients with cystic lesions[8].
PET/CT using FDG, a glucose analogue, may have the ability to detect hypermetabolic neoplastic cells and help resolve the discrepancies obtained using different methods[9-11]. In this case, the PET/CT scan showed normal FDG uptake in the cystic lesion, suggesting that this lesion was more likely a benign lesion. Moreover, PET/CT can suggest the possibility of GDC at preoperative diagnosis and with higher accuracy, compared to EUS-FNA. This may be partially due to the fact that duplication cysts are lined by columnar epithelium with gastric glandular formation similar to the adjacent proper gastric wall. Therefore, duplication cysts and the proper gastric wall showed the same 18F-FDG uptake rate in the PET/CT scan. Imaging techniques such as CT and enhanced CT are unable to provide an accurate diagnosis of these lesions, and perform rather poorly in terms of lesion characterization. Hence, 18F-FDG-PET/CT with higher accuracy has been considered for the diagnosis of GDC.
The histiocytes in GDCs observed on EUS-FNA are easily mistaken for mucinous epithelial cells of cystic neoplasms[12]. Mucin-producing well-differentiated adenocarcinoma of the pancreas or stomach may also mimic mucinous material similar to GDCs[13]. Ancillary examinations, such as the level of CEA, CA19-9 and amylase, etc., also lends insufficient support to the differential diagnoses regarding the malignancy of cystic lesions[14]. Definitive diagnosis is often achieved during pathologic and/or microscopic examination post-excision. If GDCs are diagnosed as benignant lesions, endoscopic submucosal dissection, which is less invasive, may be recommended. Otherwise, laparoscopic resection or surgical resection may be the preferred treatment[15].
In summary, preoperative diagnosis of GDCs in adults remains difficult as these lesions are rare with no clear characteristic findings. In medical practice, very few endoscopists would consider GDCs in the differential diagnosis when they encounter a lesion with cystic characteristics. Technically, 18F-FDG-PET/CT with additional imaging data, may complement EUS-FNA in the diagnosis of GDCs[16,17]. Even though there is currently a panel of imaging modalities to assist the diagnosis, it is still recommended that a different operative approach is chosen for cystic lesions, as most cases with symptomatic manifestations and/or complications have the possibility of malignant transformation at the time of presentation.
We thank Prof. Dong Kuang, Department of Pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Caboclo JF, Skok P, Vorobjova T S-Editor: Dou Y L-Editor: Webster JR E-Editor: Liu JH
| 1. | Napolitano V, Pezzullo AM, Zeppa P, Schettino P, D'Armiento M, Palazzo A, Della Pietra C, Napolitano S, Conzo G. Foregut duplication of the stomach diagnosed by endoscopic ultrasound guided fine-needle aspiration cytology: case report and literature review. World J Surg Oncol. 2013;11:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Abdalkader M, Al Hassan S, Taha A, Nica I. Complicated Gastric Duplication Cyst in an Adult Patient: Uncommon presentation of an uncommon disease. J Radiol Case Rep. 2017;11:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review. J Clin Pathol. 2004;57:428-431. [PubMed] |
| 4. | Geng YH, Wang CX, Li JT, Chen QY, Li XZ, Pan H. Gastric foregut cystic developmental malformation: case series and literature review. World J Gastroenterol. 2015;21:432-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Yamasaki A, Onishi H, Yamamoto H, Ienaga J, Nakafusa Y, Terasaka R, Nakamura M. Asymptomatic adenocarcinoma arising from a gastric duplication cyst: A case report. Int J Surg Case Rep. 2016;25:16-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Seijo Ríos S, Lariño Noia J, Abdulkader Nallib I, Lozano León A, Vieites Pérez-Quintela B, Iglesias García J, Domínguez Muñoz JE. [Adult gastric duplication cyst: diagnosis by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA)]. Rev Esp Enferm Dig. 2008;100:586-590. [PubMed] |
| 7. | Crinò SF, Larghi A, Bernardoni L, Parisi A, Frulloni L, Gabbrielli A, Parcesepe P, Scarpa A, Manfrin E. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology. 2019;30:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Kandel P, Wallace MB. Recent advancement in EUS-guided fine needle sampling. J Gastroenterol. 2019;54:377-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Alongi P, Laudicella R, Gentile R, Scalisi S, Stefano A, Russo G, Grassedonio E, Albano D, Pompei G, Rossi F, Raimondo D, Ganduscio G, Midiri M, Sinagra E. Potential clinical value of quantitative fluorine-18-fluorodeoxyglucose-PET/computed tomography using a graph-based method analysis in evaluation of incidental lesions of gastrointestinal tract: correlation with endoscopic and histopathological findings. Nucl Med Commun. 2019;40:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Kwon Y, Park E, Pahk K, Kim S, Kim MJ, Graf D, Park S. Preoperative assessment of malignant potential of gastrointestinal stromal tumor by dual-time-point 18F-fluorodeoxyglucose positron emission tomography imaging: Usefulness of standardized uptake value and retention index. J Cancer Res Ther. 2019;15:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Iliaz R, Cavus B, Yegen G, Alcin G, Gulluoglu M, Karaca C, Demir K, Besısık F, Kaymakoglu S, Turkmen C, Akyuz F. Should we worry about incidental gastrointestinal tract involvement in positron emission tomography/computed tomography as gastroenterologist? Acta Gastroenterol Belg. 2018;81:471-475. [PubMed] |
| 12. | Namdaroglu OB, Argon A, Aydogan S, Ozturk AM, Yakan S, Yildirim M, Erkan N. Gastric duplication cyst in adult: Challenge for surgeons. J Minim Access Surg. 2017;13:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yoda T, Furihata M, Nagao S, Wada T. An Adult Gastric Duplication Cyst Mimicking a Gastrointestinal Stromal Tumor. Intern Med. 2016;55:2401-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sultan M, Karanovic D, Chalhoub W, Ajmera A, Maufa F, Zeck JC, Shafa S, Johnson L, Haddad N. Gastric Duplication Cyst With Elevated Amylase: An Unusual Presentation Mimicking Pancreatic Cystic Neoplasm. ACG Case Rep J. 2015;2:86-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Izumi H, Yoshii H, Abe R, Mukai M, Nomura E, Ito H, Sugiyama T, Tajiri T, Makuuchi H. Successful laparoscopic resection for gastric duplication cyst: a case report. J Med Case Rep. 2019;13:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Tind S, Vestergaard S, Farahani ZA, Hess S. Positron emission tomography/computer tomography in gastrointestinal malignancies: current potential and challenges. Minerva Chir. 2017;72:397-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Bargiggia S, Dainese E, Parente F. Primary jejunal malignant melanoma detected by (18)F-FDG PET/CT and enteroscopy. Dig Liver Dis. 2017;49:1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |