Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3859
Peer-review started: April 23, 2019
First decision: September 9, 2019
Revised: October 9, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 219 Days and 19.2 Hours
Macrophage activation syndrome (MAS) is defined as a specific secondary hemophagocytic lymphohistiocytosis that refers particularly to those triggered by autoimmune diseases. MAS is a rare and highly lethal complication of systemic lupus erythematosus (SLE), which can be associated with, or mimic, disease flare. However, the data regarding the clinical course, management and outcome of SLE with MAS is limited, especially in adults. Lack of clinical recognition of the disease often leads to poor prognosis.
We report a 36-year-old Chinese woman without relevant past medical history who was admitted to hospital with a 6-d history of jaundice and a high fever of 39.4°C lasting one day. Abdominal magnetic resonance imaging excluded obstructive jaundice, no infection was identified and empiric superior antibiotic treatment (meropenem) showed no clinical improvement. However, newly emerged pancytopenia and respiratory failure endangered the patient’s life. Autoimmune work-up finally led to the diagnosis of SLE, which initially presented as MAS and manifested respiratory failure, although neither bone marrow biopsy nor lymph node biopsy showed hemophagocytosis. To our knowledge, such a scenario has never been reported in detail before. The patient had a favorable reaction to combination treatment with corticosteroid and cyclosporine A and has been in clinical remission during the 1-year follow up period.
Respiratory failure and MAS can be an onset of SLE. Early diagnosis and appropriate treatment are extremely important for a better prognosis.
Core tip: We report a 36-year-old Chinese woman diagnosed with systemic lupus erythematosus who initially presented with macrophage activation syndrome (MAS) and manifested respiratory failure. The administration of corticosteroid and cyclosporine A improved her respiratory depression along with all other symptoms. The clinical characteristics of MAS show great heterogeneity, and sufficient knowledge of these syndromes and early diagnosis are essential to improve prognosis. Corticosteroids are the mainstay of initial treatment for MAS.
- Citation: Sun J, Wang JW, Wang R, Zhang H, Sun J. Respiratory failure and macrophage activation syndrome as an onset of systemic lupus erythematosus: A case report. World J Clin Cases 2019; 7(22): 3859-3865
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3859.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3859
Macrophage activation syndrome (MAS) is defined as a specific secondary hemophagocytic lymphohistiocytosis (HLH) that refers particularly to those triggered by autoimmune diseases, most commonly, systemic juvenile idiopathic arthritis (s-JIA)[1]. MAS has also been reported at the onset of adult-onset Still’s disease, systemic lupus erythematosus (SLE), rheumatoid arthritis, systemic vasculitides, and Sjogren’s syndrome[2]. The reported prevalence of MAS associated with SLE ranges from 0.9% to 4.6%[3], and the mortality rate of MAS complicating adult SLE has been reported to range from 4.5%-9.8%[2,4,5]. The typical signs and symptoms of patients with MAS are fever, lymphadenopathy, hepatosplenomegaly and hemorrhagic manifestations. Abnormal laboratory tests include cytopenia, coagulopathy, hypertriglyceridemia and hyperferritinemia[4]. The incidence of MAS in rheumatic disorders is approximately 4.2% and the mortality rate is 40%[6]. Here, we report a 36-year-old Chinese woman who was diagnosed with SLE and MAS concurrently with the main manifestation of respiratory failure. To our knowledge, such a scenario has never been reported in detail before.
A 6-d history of jaundice and a high fever of 39.4°C lasting one day.
The patient observed mild jaundice in her eyes and skin 6 d before admission, and an emerging high fever of 39.4°C lasting 1 d brought her to our hospital.
No previous illnesses.
Unremarkable.
Physical examination revealed moderate skin and sclera jaundice, as well as enlarged bilateral cervical lymph nodes, which were freely movable and non-tender. Splenomegaly exceeding 3.5 cm below the costal margin was noted.
On admission, the patient showed marked liver damage with alanine transaminase 224 U/L (normal 9-50 U/L), aspartate aminotransferase 409 U/L (15-40 U/L), direct bilirubin 129.4 μmol/L (0-6.8 μmol/L), and total bilirubin 160.8 μmol/L (3.42-20.5 μmol/L).
Abdominal magnetic resonance imaging (MRI) showed multiple enlarged lymph nodes in the hilar area.
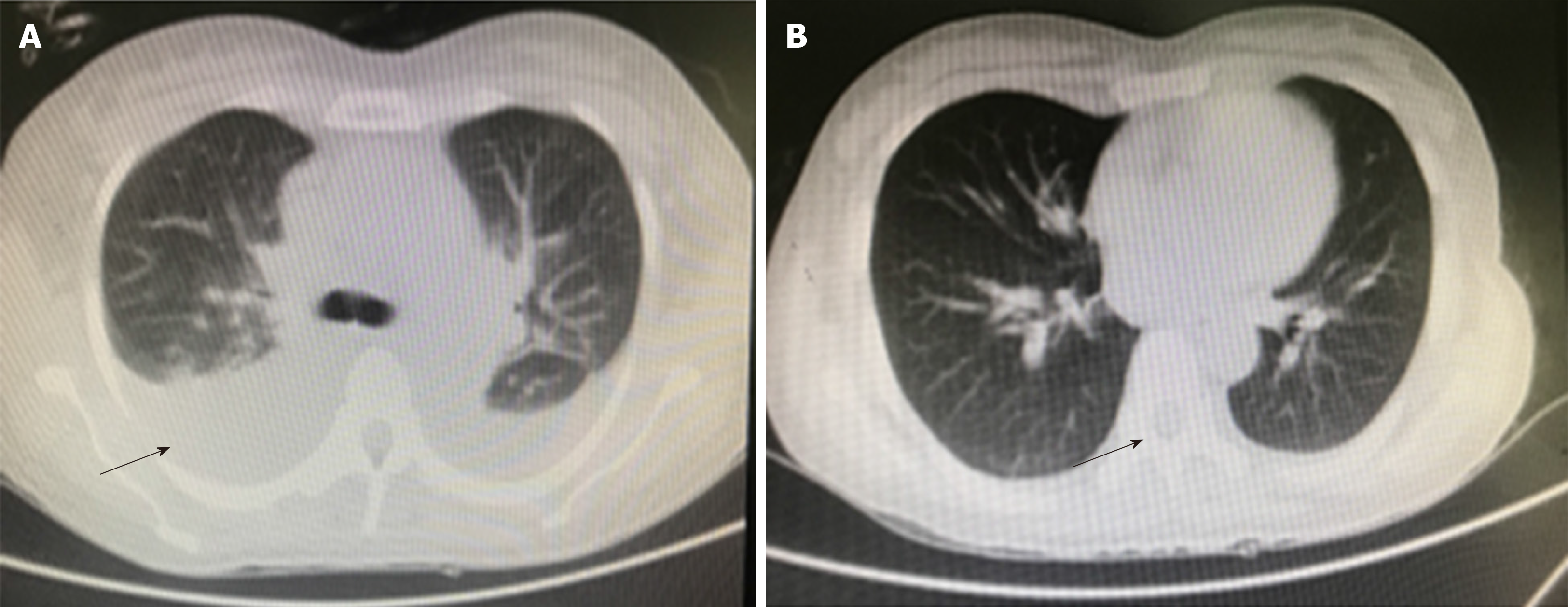
Obstructive jaundice was initially suspected. However, abdominal MRI disproved this possibility. Severe infection was then suspected due to decreased white blood cells of 3.15 × 109/L (3.5-9.5 × 109/L) and continuing high grade fever. However, erythrocyte sedimentation rate and C-reactive protein were within the normal range, but an elevated ferritin level of 10620 μg/L (20-110 μg/L) was observed. Laboratory tests for wide range infection screening did not identify any infections (Table 1). Even after liver protection treatment and empiric superior antibiotic therapy (meropenem), the patient had continued high fever and exacerbated liver function damage, which were complicated by newly emerged pancytopenia (Table 1) and middle level seroperitoneum, as well as type I respiratory failure supported by arterial blood gas analysis of PO2: 55 mmHg (80-100 mmHg), PCO2: 32 mmHg (35-45 mmHg). Computed tomography suggested bilateral pleural effusion (Figure 1); therefore, the patient was offered assisted breathing and closed chest drainage. Considering the elevated serum ferritin level and early hematological involvement, the possibility of MAS was suspected and more laboratory tests for MAS and autoimmune diseases were conducted (Table 2).
| Items | Result | Reference values |
| Sedimentation (mm/h) | 12 | 0-34 |
| C-reactive protein (mg/L) | 5.6 | 0-10 |
| WBC (× 109/L) | 2.41 | 3.5-9.5 |
| Neutrophils | 1.82 | 1.8-6.3 |
| PLT (× 109/L) | 9 | 125-350 |
| Hb (g/L) | 66 | 115-150 |
| Triglyceride (mmol/L) | 4.29 | < 1.7 |
| Fibrinogen (mg/dL) | 61 | 200-400 |
| D-dimer (mg/L) | 2.43 | < 0.55 |
| Total bilirubin (μmol/L) | 201.9 | 3.42-20.5 |
| Direct bilirubin (μmol/L) | 155.2 | 0-6.8 |
| Hepatitis virus DNA | Not detected | |
| HIV, EB-virus and cytomegalovirus | Not detected | |
| IgG and IgM of M. pneumoniae | Negative | Negative |
| Blood cultures for pathogens | Negative | Negative |
| T-SPOT and acid-resistant staining | Negative | Negative |
| Full-set tumor markers | Negative | Negative |
| Items | Result | Reference values |
| ANA antibody | 1:320(+) | Negative |
| Anti-Ro-52 | ++ | Negative |
| Anti-SS-A | +++ | Negative |
| Anticardiolipin IgM | + | Negative |
| Anti-dsDNA | Negative | Negative |
| Anti-SM | Negative | Negative |
| Complement C3 (g/L) | 0.439 | 0.79-1.52 |
| Complement C4 (g/L) | 0.18 | 0.16-0.38 |
| ANCA | Negative | Negative |
| MPO+PR3 | Negative | Negative |
| IgG4 (g/L) | 0.164 | 0.03-2.01 |
| Soluble CD25 (pg/mL) | 8516 | < 6400 |
| Crushed red blood cells and Coombs test | Negative | Negative |
| Natural killer cell function | Not able to complete | |
| Lymph node biopsy | Kikuchi lymphadenitis, CD68/CD163 positive by immunohistochemistry analysis | Negative |
Respiratory failure and MAS as an onset of SLE.
Intravenous methylprednisolone therapy of 1.5 mg/kg/d was initiated on the 7th d, and 150 mg/d of cyclosporine A was added on the 10th d. On the 14th d, methylprednisolone was reduced to 1.0 mg/kg/d and 80 g intravenous immunoglobulin was initiated on the 10th d. To our delight, the patient had a prompt favorable reaction to this treatment. Her fever subsided on the 8th d, all disease indicators improved, the SPO2 increased to 96% and plural effusion improved (Figure 1). Oral corticosteroid and cyclosporine A were maintained to achieve long-term remission.
Following hospital discharge, the patient was in clinical remission during the 1-year follow-up period.
MAS is defined as a specific secondary HLH that refers particularly to those triggered by autoimmune diseases. A defect in perforin-mediated cytotoxicity is the underlying mechanism[7]. Perforin mediates not only the killing of target cells, but also apoptosis of autologous cells. The decreased killing efficiency of target cells due to the gene defect leads to reactive proliferation of natural killer (NK) cells and T cells, and stimulates the excess release of pro-inflammatory factors by macrophages[8]. In addition, the apoptotic pathway is blocked, resulting in further accumulation and uninterrupted hyper-stimulation of immune cells and then a waterfall release of inflammatory factors and eventually the so-called “inflammatory storms”[8].
Pulmonary involvement in MAS has been described previously[4,7], and symptoms include cough, dyspnea and respiratory failure, especially in cases triggered by respiratory viruses[9]. However, clinical data are obscure and may make limited sense for clinical work. The specific pathological process of hydrothorax and respiratory failure in SLE-MAS has not yet been elucidated. A possible mechanism may be that inflammatory factors act on the lung capillaries, causing inflammatory exudation and deterioration of gas exchange in the lungs, leading to pleural effusion and respiratory failure. Pulmonary infection may be an additional etiology at the early stage of the episode or may even be the trigger, and it may also merge during the episode and jointly aggravate the pulmonary condition. It has been reported that Mycoplasma pneumoniae infection has been linked to several extra-respiratory systems[10,11]; thus, it is important for clinicians to exclude the possibility of infection when MAS is suspected, especially in the presence of respiratory failure.
Our patient was diagnosed with MAS and underlying SLE concurrently. For early recognition of MAS, it should be emphasized that a high ferritin level and/or a rapid ferritin increase seem to indicate a diagnosis of MAS rather than active rheumatic disease alone[12,13]. Studies have shown that hyperferritinemia has the best sensitivity and specificity for indicating MAS and the relative reduction in platelet count appears to be the best early marker for identifying underlying SLE activity and MAS onset, following exclusion of thrombocytopenia caused by SLE disease activity itself[14]. Our case showed no macrophage hemophagocytosis in two bone marrow biopsies. There is consensus that pathologic proof of hemophagocytosis is not vital for the diagnosis of MAS/HLH and the absence of hemophagocytosis should not delay treatment of MAS/HLH[1,4,15,16]. The recovery of our patient supports this. Even histiocytic hemophagocytosis itself is not necessarily abnormal, as histiocytes or macrophages can phagocytose aged or dying hematopoietic cells to maintain tissue homeostasis. Thus, it is important to define distinctive histiocytes in bone marrow to diagnose MAS[2].
Our patient fulfilled all the diagnostic criteria for HLH-2004, with the exception of hemophagocytosis and NK cell function. Despite studies showing discrepancies with respect to MAS characteristics, laboratory tests and therapeutic response between children and adults[2], many clinical guidelines and treatment trials have focused on pediatric patients due to lower morbidity in adults. Even the HLH-2004 criteria were originally created for children[17], but are now widely used as diagnostic criteria for adults. New diagnostic guidelines such as the 2005 s-JIA-MAS guidelines by Ravelli et al[18], the 2009 childhood-onset-SLE-MAS criteria by Parodi et al[14], and the 2016 EULAR/ACR/PRINTO-MAS criteria for s-JIA-MAS[19], are all focused on pediatrics. A scoring system known as the HScore was designed to help clinicians diagnose hemophagocytic syndrome[20], yet its robustness and efficiency in adults remain to be tested. The absence of standardized diagnostic criteria for adults may result in frequent missed or incorrect diagnoses, and consequently poor prognosis[7]. Furthermore, the pathogenic and pathogenesis of each MAS episode may vary due to different triggers[2,21], and some researchers have found it important to formulate a robust set of specific diagnostic criteria and therapeutic strategies aimed at different etiologies[14,21]. Large samples and high-quality analysis are required for this purpose.
Some experts have proposed the following triple simultaneous approach for the treatment of HLH: Support measures; The elimination of triggers (mainly infection); Suppression of the inflammatory response and cell proliferation (neoplasia)[7]. With regard to the treatment of SLE-MAS, there are currently no unified guidelines. Corticosteroids are thought to be the mainstay of initial treatment irrespective of the etiology, and can be administered alone or in combination with adjuvant drugs including methotrexate, cyclophosphamide, cyclosporine, tacrolimus, intravenous immunoglobulin and etoposide[2,4]. Drug combinations should be given according to the etiology and characteristics of the episode. Physicians may also administer biological treatments such as rituximab, infliximab, etanercept, anti-interleukin 1r (anakinra) and interleukin-6 (tocilizumab), when patients show no response to first-line treatments[1,4,21].
MAS should be considered when continued high fever complicated by multi-system damage occurs. International and multidisciplinary efforts for a robust set of specific diagnostic criteria and therapeutic strategies for SLE-MAS in adults are urgently needed, as early diagnosis and treatment are extremely critical for optimal prognosis[5].
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gheita TA, Poddighe D S-Editor: Dou Y L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Cron RQ, Davi S, Minoia F, Ravelli A. Clinical features and correct diagnosis of macrophage activation syndrome. Expert Rev Clin Immunol. 2015;11:1043-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol. 2014;66:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Vilaiyuk S, Sirachainan N, Wanitkun S, Pirojsakul K, Vaewpanich J. Recurrent macrophage activation syndrome as the primary manifestation in systemic lupus erythematosus and the benefit of serial ferritin measurements: a case-based review. Clin Rheumatol. 2013;32:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Gavand PE, Serio I, Arnaud L, Costedoat-Chalumeau N, Carvelli J, Dossier A, Hinschberger O, Mouthon L, Le Guern V, Korganow AS, Poindron V, Gourguechon C, Lavigne C, Maurier F, Labro G, Heymonet M, Artifoni M, Viau AB, Deligny C, Sene T, Terriou L, Sibilia J, Mathian A, Bloch-Queyrat C, Larroche C, Amoura Z, Martin T. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: A study of 103 episodes in 89 adult patients. Autoimmun Rev. 2017;16:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Kim JM, Kwok SK, Ju JH, Kim HY, Park SH. Reactive hemophagocytic syndrome in adult Korean patients with systemic lupus erythematosus: a case-control study and literature review. J Rheumatol. 2012;39:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Moradinejad MH, Ziaee V. The incidence of macrophage activation syndrome in children with rheumatic disorders. Minerva Pediatr. 2011;63:459-466. [PubMed] |
| 7. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 960] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 8. | Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Karras A, Thervet E, Legendre C; Groupe Coopératif de transplantation d'Ile de France. Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation. 2004;77:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Matthys I, Borsboom D, Steyaert S, Vervloet D, Cornelis K, Vanderstraeten E, Kindt S, Dewint P, Lambrecht V, Sinnaeve P, Van Steenkiste C. A plethora of manifestations following a Mycoplasma pneumoniae infection: a case report. Acta Clin Belg. 2019;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. 2018;30:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Poddighe D, Cavagna L, Brazzelli V, Bruni P, Marseglia GL. A hyper-ferritinemia syndrome evolving in recurrent macrophage activation syndrome, as an onset of amyopathic juvenile dermatomyositis: a challenging clinical case in light of the current diagnostic criteria. Autoimmun Rev. 2014;13:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Gilboa M, Bornstein G, Ben-Zvi I, Grossman C. Macrophage activation syndrome complicating rheumatic diseases in adults: case-based review. Rheumatol Int. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Parodi A, Davì S, Pringe AB, Pistorio A, Ruperto N, Magni-Manzoni S, Miettunen P, Bader-Meunier B, Espada G, Sterba G, Ozen S, Wright D, Magalhães CS, Khubchandani R, Michels H, Woo P, Iglesias A, Guseinova D, Bracaglia C, Hayward K, Wouters C, Grom A, Vivarelli M, Fischer A, Breda L, Martini A, Ravelli A; Lupus Working Group of the Paediatric Rheumatology European Society. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. 2009;60:3388-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Ahn SS, Yoo BW, Jung SM, Lee SW, Park YB, Song JJ. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Semin Arthritis Rheum. 2017;47:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Liu AC, Yang Y, Li MT, Jia Y, Chen S, Ye S, Zeng XZ, Wang Z, Zhao JX, Liu XY, Zhu J, Zhao Y, Zeng XF, Li ZG. Macrophage activation syndrome in systemic lupus erythematosus: a multicenter, case-control study in China. Clin Rheumatol. 2018;37:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3599] [Article Influence: 199.9] [Reference Citation Analysis (1)] |
| 18. | Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, Viola S, Martini A. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, Aricò M, Avcin T, Behrens EM, De Benedetti F, Filipovic L, Grom AA, Henter JI, Ilowite NT, Jordan MB, Khubchandani R, Kitoh T, Lehmberg K, Lovell DJ, Miettunen P, Nichols KE, Ozen S, Pachlopnik Schmid J, Ramanan AV, Russo R, Schneider R, Sterba G, Uziel Y, Wallace C, Wouters C, Wulffraat N, Demirkaya E, Brunner HI, Martini A, Ruperto N, Cron RQ; Paediatric Rheumatology International Trials Organisation; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology Collaborative Study Group; Histiocyte Society. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 20. | Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 925] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 21. | Lerkvaleekul B, Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key. Open Access Rheumatol. 2018;10:117-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |