Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3851
Peer-review started: June 21, 2019
First decision: September 9, 2019
Revised: September 24, 2019
Accepted: October 5, 2019
Article in press: October 5, 2019
Published online: November 26, 2019
Processing time: 132 Days and 14.1 Hours
Pulmonary alveolar microlithiasis (PAM) is a rare idiopathic lung disease characterized by the accumulation of innumerable microliths. Currently, effective therapeutics for PAM are not available, and the only treatment for end-stage lung disease is lung transplantation (LuTx). Further, there are few reports that focus on LuTx for the treatment of PAM, and the follow-up reports of postoperative imaging are even rarer.
A 52-year-old man presented to Shanghai Pulmonary Hospital in 2017 after experiencing shortness of breath and exacerbation. The patient was diagnosed with PAM and referred for single-LuTx (SLuTx) on March 14, 2018. Preoperative imaging results from a chest X-ray demonstrated bilateral, diffuse, symmetrical, sandstorm-like radiopaque micronodules, and pneumothorax and a computed tomography scan revealed minute, calcified military nodules in both lungs. We performed a left SLuTx, and intraoperative pathology was consistent with PAM. One week after surgery, a chest X-ray revealed slight exudation of the left lung, and one month later, the left transplanted lung exhibited good dilation, mild pulmonary perfusion injury with local infection, and left pleural effusion. Fiberoptic bronchoscopy revealed left hyperplastic granulation at the left bronchial anastomosis. Multiple sputum cultures suggested the presence of Klebsiella pneumoniae and Acinetobacter baumannii. The last follow-up was conducted in April 2019; the patient recovered well.
This case presents the imaging findings of a patient with PAM before and after LuTx and confirms the effectiveness of LuTx for the treatment of this disease.
Core tip: Pulmonary alveolar microlithiasis (PAM) is a rare idiopathic lung disease characterized by the accumulation of innumerable microliths. Currently, there are no effective therapeutic drugs for PAM, and the only treatment for end-stage lung disease is lung transplantation (LuTx). Here we present a rare case of alveolar microlithiasis transplantation. This case highlights the importance of imaging findings of PAM before and after LuTx and confirms the effectiveness of LuTx for the treatment of PAM.
- Citation: Ren XY, Fang XM, Chen JY, Ding H, Wang Y, Lu Q, Ming JL, Zhou LJ, Chen HW. Single-lung transplantation for pulmonary alveolar microlithiasis: A case report. World J Clin Cases 2019; 7(22): 3851-3858
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3851.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3851
Pulmonary alveolar microlithiasis (PAM) is a rare idiopathic lung disease characterized by the accumulation of innumerable microliths that consist primarily of calcium and phosphorus in pulmonary alveoli[1]. PAM was first described by an Italian scientist in 1868, and more than 1000 cases have been reported worldwide[2]. Currently, there is no medical therapy available to alter the progression of PAM definitively, and lung transplantation (LuTx) is usually performed when patients are diagnosed with end-stage lung disease[3]. Here, we report a successful case of left single-LuTx (SLuTx) in a patient with PAM for the first time in China.
A 52-year-old man (weight, 55 kg; height, 172 cm) was referred for a LuTx after experiencing shortness of breath with chest tightness for 4 years and exacerbation for 10 d.
The patient coughed and expectorated since childhood and experienced chest tightness and shortness of breath that gradually increased after exercise since 2014. He was diagnosed with PAM by using fiberoptic bronchoscopy at Shanghai Pulmonary Hospital in 2017. The patient had three instances of pneumothorax in the left lung over a period of 6 months and received poor treatment. On March 14, 2018, the patient was evaluated for LuTx at Wuxi People’s Hospital.
The patient’s past medical history was unremarkable.
The patient’s brother experienced similar symptoms, although he had not been diagnosed at a hospital.
Both lungs had low respiratory sounds and slightly moist rales.
The results of liver and heart function tests were as follows: Albumin, 34.5 g/L (normal range: 35.0-53.0 g/L); lactic dehydrogenase, 246.0 U/L (normal range: 109.0-245.0 U/L); and globulin, 35.8 g/L (normal range: 17.0-33.5 g/L). Immunological parameters were as follows: Immunoglobulin A, 5.7 g/L (normal range: 0.7-5.0 g/L); immunoglobulin M, 3.03 g/L (normal range: 0.4-2.8 g/L); and complement C3, 786.0 mg/L (normal range: 790.0-1520.0). Tumor indices were as follows: CA125: 249.5 U/mL (normal range: < 35.0 U/mL) and C-reactive protein, 17.3 mg/L (normal range: 0-8.0 mg/L).
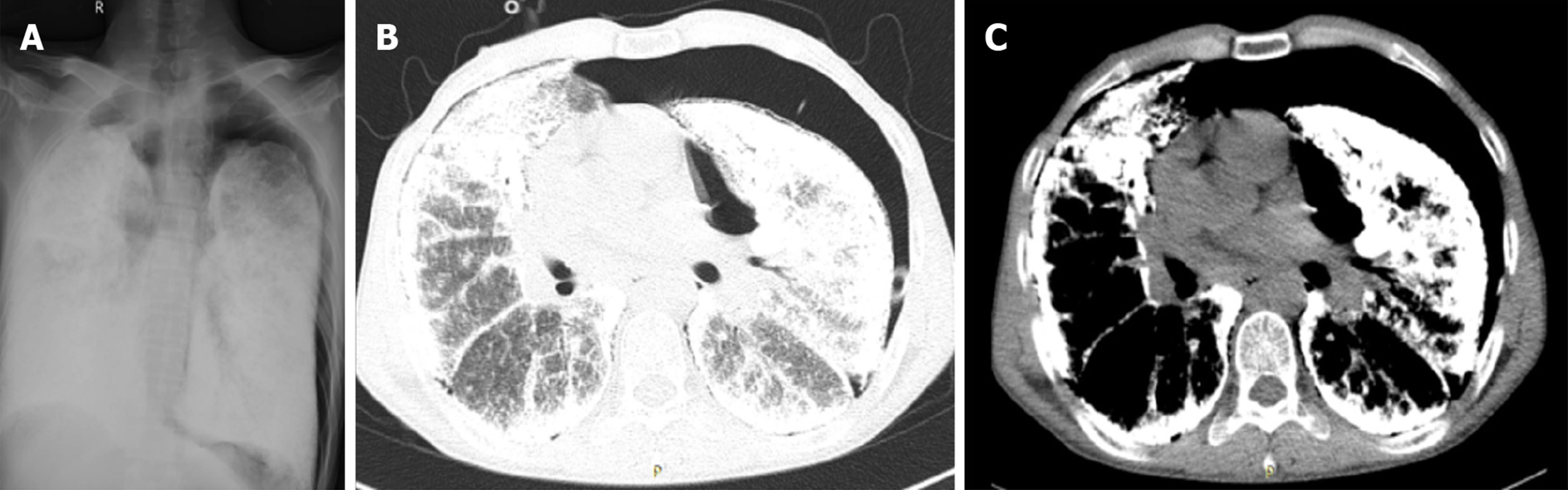
A preoperative chest X-ray demonstrated bilateral, diffuse, symmetrical, sandstorm-like radiopaque micronodules and pneumothorax, and a chest computed tomography (CT) scan revealed decreased diffuse transmittance and calcified minute miliary nodules in both lungs (Figure 1A-C). The clinical symptoms and imaging results were consistent PAM. After discussion and approval by the hospital ethics committee, the patient was placed on the waiting list for LuTx.
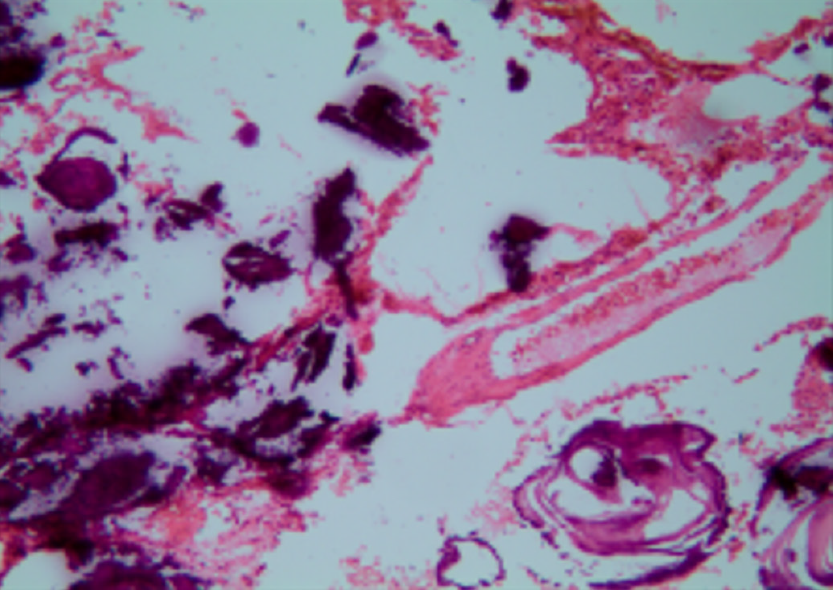
Intraoperative pathology revealed the accumulation of calcium salts in the alveoli (Figure 2). A final diagnosis of PAM was established.
On April 9, 2018, the lung from a 28-year-old donor (weight, 70 kg; height, 175 cm) became available for our patient. The donor was in good health and pronounced brain dead following intracerebral hemorrhage. The donor’s close relatives agreed to donate his organs. The patient’s and donor’s ABO and Rh blood groups were the same, their body type and chest circumference matched, and panel reactive antibody and human leukocyte antigen were negative. The donor’s lung was cut off according to the standard protocol, preserved with raffinose-low potassium dextran solution, and transported.
We performed a left SLuTx with extracorporeal membrane oxygenation (ECMO). During this procedure, few adhesions were noted in the left side of the chest. We observed diffuse consolidation of the left lung, and it had a firm, sandy texture. Intraoperative blood loss was 1600 mL, and transfusion of 1600 mL of blood was performed. The cooling time for the supply lung was 7.5 h.
After the operation, the patient was transferred to the intensive care unit (ICU). Postoperative intubation time was 3 d, and ECMO was removed 2 d later due to hypoxia. After 5 d, he was transferred from the ICU to the general ward for further treatment.
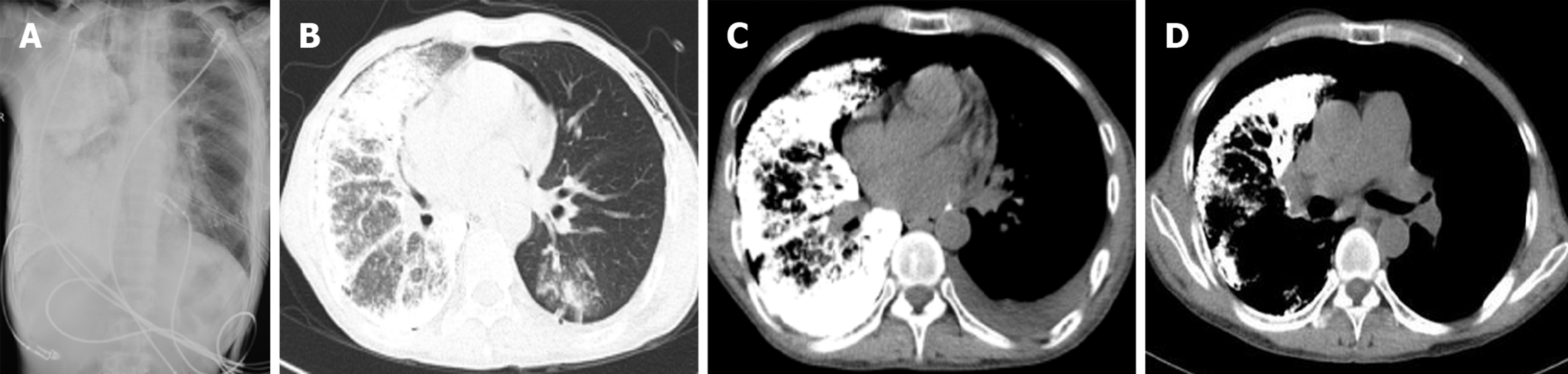
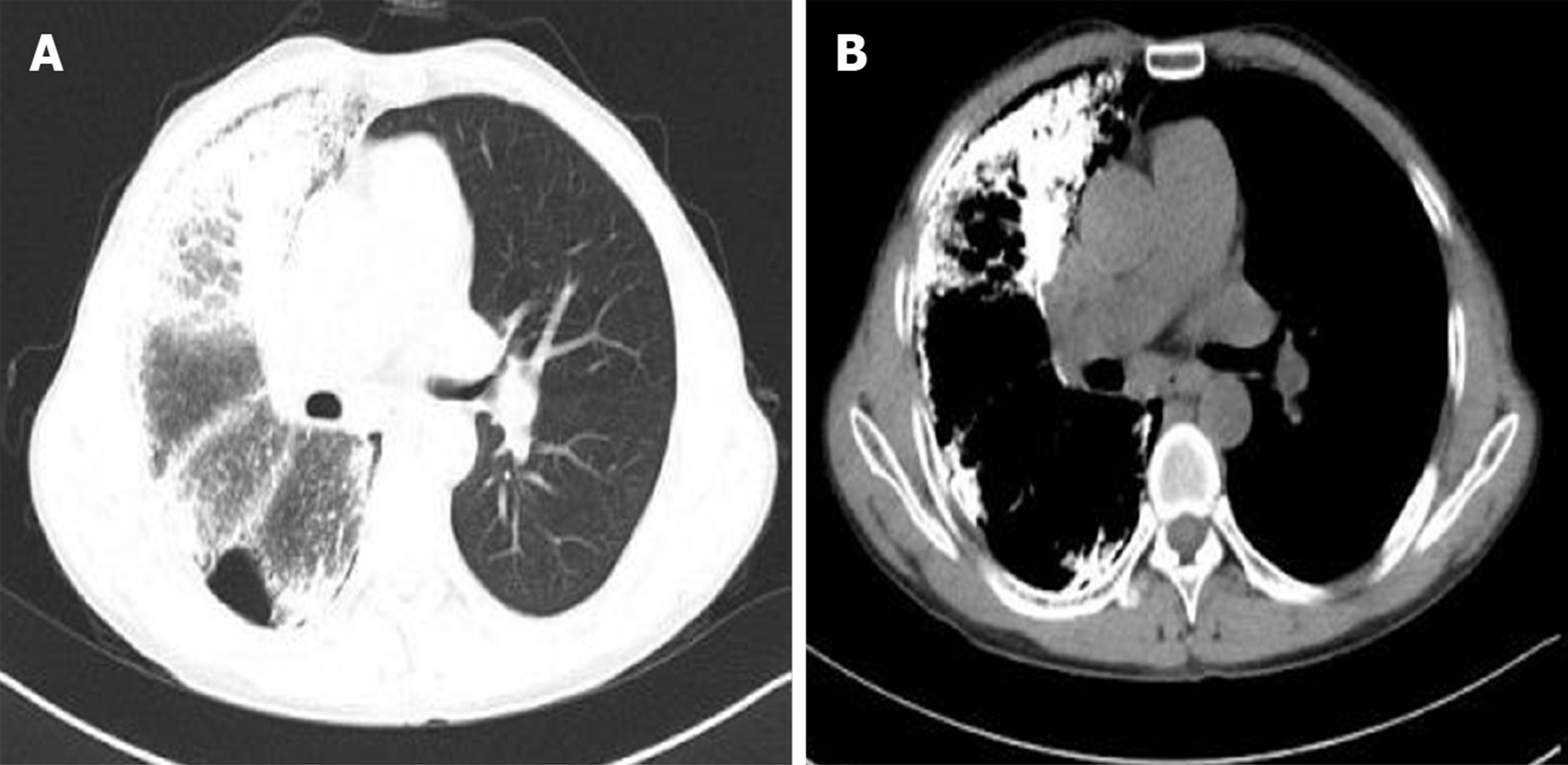
One week after surgery, a chest X-ray showed slight exudation of the left lung (Figure 3A), and one month later, the left transplanted lung showed good dilation, mild pulmonary perfusion injury with local infection (Figure 3B), and left pleural effusion (Figure 3C). Fiberoptic bronchoscopy revealed obstruction of the left bronchial anastomosis caused by hyperplastic granulation and the accumulation of yellow and white sticky moss. Multiple sputum cultures suggested the presence of Klebsiella pneumoniae and Acinetobacter baumanii. The patient was discharged from the hospital in a stable condition after treatment. In September, anastomotic stenosis was improved after bronchoscopic balloon dilatations were performed three times (Figure 3D). The last follow-up was conducted in April 2019, and the patient recovered well (Figure 4).
PAM is an autosomal recessive disease caused by mutations in the SLC34A2 gene, which lead to defects in the sodium phosphate-IIb cotransporter protein. These defects prevent the clearance of phosphate and calcium phosphate deposits from the extracellular fluid by alveolar type II epithelial cells[4]. PAM has a familial genetic tendency, and familial cases account for about 30%-50% of all cases. Additionally, there is a slight predominance among males[5]. Previous studies (Table 1) reported that five patients mentioned their family history in the literature, one of whom had a family history, and our patient stated that his brother had similar symptoms but had not been diagnosed in hospital.
| Ref. | Yr | Age | Family history | Double/Single LuTx | Complications | Outcome |
| Bonnette et al[14] | 1992 | 46 | NR | Double | No | Alive, NR |
| Stamatis et al[11] | 1993 | 32 | YES | Double | Major bleeding | Alive, 18 m |
| Raffa et al[15] | 1996 | 48 | NO | Single | Acute rejection, anastomotic stenosis | Alive, 12 m |
| Edelman et al[16] | 1997 | 56 | NO | Double | Major bleeding | Dead, POD 5 |
| 35 | NR | No | Alive, 32 m | |||
| Jackson et al[12] | 2001 | 53 | NR | Single | No | Alive, 90 m |
| Coulibaly et al[17] | 2009 | 43 | NR | Double | Infection | Dead, 3 m |
| Shadmehr et al[18] | 2009 | 32 | NR | Single | Hemodynamically instable, reperfusion edema | Dead, NR |
| Shigemura et al[19] | 2010 | 63 | NR | Double | No | Alive, 16 m |
| Samano et al[20] | 2010 | 47 | NO | Double | Reperfusion syndrome, shock | Alive, 12 m |
| Borrelli et al[21] | 2014 | 64 | NR | Single | NR | Alive, 60 m |
| Güçyetmez et al[22] | 2014 | 52 | NR | Double | NR | Alive, 12 m |
| Klikovits et al[9] | 2016 | 32 | NR | Double | PGD, Sepsis | Dead, 11 d |
| 52 | Reperfusion-edema | Alive, 74 d | ||||
| 34 | No | Alive, 67 d | ||||
| 52 | No | Alive, 35 d | ||||
| 52 | Atrial fibrillation | Alive, 29 d | ||||
| Delic et al[23] | 2016 | 73 | NO | Double | NR | Alive, NR |
Some patients may be asymptomatic initially; however, as the disease progresses, both lungs become fibrotic, which may lead to restrictive ventilatory disorder and respiratory failure[6]. Our patient coughed and expectorated since childhood, experienced shortness of breath for four years prior to his diagnosis, and gradually received medical treatment. The patient’s symptoms were consistent with the clinical manifestations of PAM.
The typical picture of PAM on a chest X-ray is sand-like, calcific micronodules that diffusely infiltrating both lungs, especially the middle and lower zones, and this is called “sandstorm lung”. The increased calcific density in lower zones is due to the larger surface area and greater thickness. While PAM is progressing, extensive microliths may cause obscuration of the mediastinal and diaphragmatic silhouette. Additionally, bullous emphysema may also be observed at the anterior margin or apex. Moreover, chest CT scans show thickening of the lobular septae with a distribution of microliths along the septae and around the centrilobular distal bronchioles. This is called “crazy paving” pattern[1,3,7]. Our patient had high-density micronodules in both lungs and repeated unhealed left pneumothorax, which were consistent with typical imaging manifestations.
Although the use of disphosphonates has been promoted for the treatment of PAM, there are mixed results associated with this treatment[3]. Currently, LuTx is an effective treatment for patients with end-stage PAM. However, owing to the small number of cases and lack of prognostic factors around the world, there are currently no guidelines for LuTx timing[8]. Based on our experience, LuTx is needed to be considered when respiratory failure, pneumothorax, or acute exacerbations occur and the patient requires long-term oxygen therapy. Table 1 summarizes the information from existing case reports of PAM. The mean age of 18 patients with PAM was 48.1 ± 11.9 years, which is similar to the age of the patient in the current study. Further, only four patients (Table 1) received SLuTx, for which the patient’s condition and lung imaging findings needed to be considered. Previous studies have reported that bilateral lung replacements are more effective than SLuTx because unilateral replacements may lead to shunting of blood through the underventilated native lung[9]. However, other studies have demonstrated that patients who received SLuTx had no evidence of recurrence in the transplanted lung[10,11,12]; therefore, a study that implements a longer follow-up period after SLuTX in patients with PAM should be conducted. Because our patient had acute pulmonary edema and acute left heart failure during the operation, and the left pneumothorax was unhealed before the surgery, the surgeon chose single LuTx finally. Further, recurrence was not observed during the first year after the operation.
Complications after LuTx are the main cause of death of patients undergoing transplantation. Infections are the second (18.7%) and main (36.3%) causes of death, respectively, from 30 days to 1 year after operation[13]. From the cases that we reviewed, two patients died from infections at 11 days (sepsis, n = 1/18) and 3 mo (n = 1/18) after operation, respectively. Other postoperative complications, including anastomotic stenosis, acute rejection, and reperfusion edema, occurred in 14 survival cases (Table 1). Some complications are associated with different imaging features. For example, infections are characterized by diffuse ground glass opacities, localized atelectasis or consolidation, small intrapulmonary nodules, peri-bronchovascular interstitial thickening, and pleural effusions. Acute rejection is characterized by diffuse ground glass opacities, consolidation, septal thickening, and pleural effusions. Bronchial stenosis refers to a narrowing of the bronchus in the CT. Based on these imaging features, our patient was diagnosed with local infection and stenosis.
The patient in this study was preoperatively diagnosed with PAM based on fiberoptic bronchoscopy biopsy, and imaging findings, such as the “sandstorm lung” from chest X-ray scans and the “crazy paving” pattern from chest CT scans, supported the diagnosis. In addition, our results demonstrated that LuTx is an effective treatment for patients with end-stage PAM. Further, the prevention of postoperative complications is important in order to improve the prognosis of patients who have received transplantations.
I would like to express gratitude to my colleagues who provided me with references and information on time.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvadori M S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Saito A, McCormack FX. Pulmonary Alveolar Microlithiasis. Clin Chest Med. 2016;37:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Castellana G, Carone D, Castellana M. Microlithiasis of Seminal Vesicles and Severe Oligoasthenospermia in Pulmonary Alveolar Microlithiasis (PAM): Report of An Unusual Sporadic Case. Int J Fertil Steril. 2015;9:137-140. [PubMed] |
| 3. | Castellana G, Castellana G, Gentile M, Castellana R, Resta O. Pulmonary alveolar microlithiasis: review of the 1022 cases reported worldwide. Eur Respir Rev. 2015;24:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Dogan OT, Ozsahin SL, Gul E, Arslan S, Koksal B, Berk S, Ozdemir O, Akkurt I. A frame-shift mutation in the SLC34A2 gene in three patients with pulmonary alveolar microlithiasis in an inbred family. Intern Med. 2010;49:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Jönsson ÅL, Simonsen U, Hilberg O, Bendstrup E. Pulmonary alveolar microlithiasis: two case reports and review of the literature. Eur Respir Rev. 2012;21:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Mariotta S, Ricci A, Papale M, De Clementi F, Sposato B, Guidi L, Mannino F. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:173-181. [PubMed] |
| 7. | Gasparetto EL, Tazoniero P, Escuissato DL, Marchiori E, Frare E Silva RL, Sakamoto D. Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT. Br J Radiol. 2004;77:974-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, Snell GI, Verleden GM, Zamora MR, Glanville AR. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 934] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 9. | Klikovits T, Slama A, Hoetzenecker K, Waseda R, Lambers C, Murakoezy G, Jaksch P, Aigner C, Taghavi S, Klepetko W, Lang G, Hoda MA. A rare indication for lung transplantation - pulmonary alveolar microlithiasis: institutional experience of five consecutive cases. Clin Transplant. 2016;30:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gomes Neto A, Monteiro Nogueira AS, Lopes De Medeiros I, Fernandes Viana De Araujo R, Carvalho Santos R, Sampaio Viana CM, Moreira Batista Aguiar F, Gomes Catunda L, Araújo Aragão L, Fava Alencar R. Single- and Double-Lung Transplantation: Results of an Initial Experience of 39 Cases in Ceará (Northeast Brazil). Transplant Proc. 2018;50:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Stamatis G, Zerkowski HR, Doetsch N, Greschuchna D, Konietzko N, Reidemeister JC. Sequential bilateral lung transplantation for pulmonary alveolar microlithiasis. Ann Thorac Surg. 1993;56:972-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Jackson KB, Modry DL, Halenar J, L'abbe J, Winton TL, Lien DC. Single lung transplantation for pulmonary alveolar microlithiasis. J Heart Lung Transplant. 2001;20:226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D. Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Stehlik J; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 14. | Bonnette P, Bisson A, el Kadi NB, Colchen A, Leroy M, Fischler M, Loirat P, Caubarere I. Bilateral single lung transplantation. Complications and results in 14 patients. Eur J Cardiothorac Surg. 1992;6:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Raffa H, El-Dakhakhny M, Al-Ibrahim K, Mansour MS. Single lung transplantation for alveolar micro-lithiasis: the first clinical report. Saudi J Kidney Dis Transpl. 1996;7:189-193. [PubMed] |
| 16. | Edelman JD, Bavaria J, Kaiser LR, Litzky LA, Palevsky HI, Kotloff RM. Bilateral sequential lung transplantation for pulmonary alveolar microlithiasis. Chest. 1997;112:1140-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Coulibaly B, Fernandez C, Reynaud-Gaubert M, D'Journo X, Doddoli C, Taséi AM. [Alveolar microlithiasis with severe interstitial fibrosis leading to lung transplantation]. Ann Pathol. 2009;29:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Shadmehr MB, Arab M, Pejhan S, Daneshvar A, Javaherzadeh N, Abbasi A, Ahmadi ZH, Radpay B, Dabir S, Parsa T, Mohammadi F, Mansoori SD, Tabarsi P, Amiri MV, Marjani M, Kashani BS, Najafizadeh K, Shafaghi S, Ghorbani F, Masjedi MR, Velayati AA. Eight years of lung transplantation: experience of the National Research Institute of Tuberculosis and Lung Diseases. Transplant Proc. 2009;41:2887-2889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Shigemura N, Bermudez C, Hattler BG, Johnson B, Crespo M, Pilewski J, Toyoda Y. Lung transplantation for pulmonary alveolar microlithiasis. J Thorac Cardiovasc Surg. 2010;139:e50-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Samano MN, Waisberg DR, Canzian M, Campos SV, Pêgo-Fernandes PM, Jatene FB. Lung transplantation for pulmonary alveolar microlithiasis: a case report. Clinics (Sao Paulo). 2010;65:233-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Borrelli R, Fossi A, Volterrani L, Voltolini L. Right single-lung transplantation for pulmonary alveolar microlithiasis. Eur J Cardiothorac Surg. 2014;45:e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Güçyetmez B, Ogan A, Cimet Ayyıldız A, Yalçın Güder B, Klepetko W. Lung transplantation in an intensive care patient with pulmonary alveolar microlithiasis - a case report. F1000Res. 2014;3:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Delic JA, Fuhrman CR, Trejo Bittar HE. Pulmonary Alveolar Microlithiasis: AIRP Best Cases in Radiologic-Pathologic Correlation. Radiographics. 2016;36:1334-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |