Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3832
Peer-review started: July 17, 2019
First decision: September 23, 2019
Revised: October 4, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 142 Days and 18.9 Hours
Hilar masses with stenosis of the bronchus occur mainly due to malignant diseases, such as lung cancer. Hilar masses resulting from invasive aspergillosis are extremely rare and occur mostly in severely immunosuppressed patients.
In the current case report, we have documented a unique case of invasive aspergillosis presenting as a mass in the hilum and bronchial stenosis under bronchoscopy mimicking lung cancer in a 54-year-old man with diabetes mellitus. The histological analysis of bronchial membrane biopsy demonstrated fungal elements of 45° branching hyphae with positive Periodic Acid-Schiff and Grocott staining. After 3 mo of antifungal therapy, the symptoms, computed tomography scan and bronchoscopy manifestations all showed improvement.
We highlight that clinicians should consider a diagnosis of invasive aspergillosis when radiological examination shows pseudotumor appearance in diabetes mellitus patients.
Core tip: Hilar masses with stenosis of the bronchus occur mainly due to malignant diseases. Hilar masses resulting from invasive aspergillosis are extremely rare and occur mostly in immunosuppressed patients. Herein, we have documented a case of invasive aspergillosis presenting as a mass in the hilum and bronchial stenosis under bronchoscopy in a 54-year-old man with diabetes mellitus. We have highlighted the importance of bronchoscopy with biopsy and culture for early diagnosis and treatment when radiological examination shows pseudotumor appearance in diabetes mellitus patients.
- Citation: Su SS, Zhou Y, Xu HY, Zhou LP, Chen CS, Li YP. Invasive aspergillosis presenting as hilar masses with stenosis of bronchus: A case report. World J Clin Cases 2019; 7(22): 3832-3837
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3832.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3832
Hilar masses with stenosis of the bronchus and enlarged lymph nodes are mainly due to malignant diseases, such as lung cancer. Invasive aspergillosis (IA) is a life-threatening opportunistic infection that primarily occurs in critically ill patients as well as in immunocompromised individuals. Hilar masses resulting from IA are extremely rare and occur predominantly in severely immunosuppressed patients. We herein report a unique case of IA presenting as a mass in the hilum and an abnormal appearance of bronchial membrane with bronchial stenosis under bronchoscopy mimicking lung cancer in a 54-year-old man with diabetes mellitus.
In April 2018, a 54-year-old man came to our hospital with pyrexia at onset along with the history of cough for 2 mo.
The patient’s symptoms started 2 mo ago with fever and cough. At a local hospital, he received intravenous antibiotics (unknown) that improved his fever; however, his cough was not relieved.
His medical history included diabetes mellitus for 17 years with poor glucose control. He had a smoking history of 360 pack-years.
His family history was negative.
On admission, vital signs were normal with no significant physical examination findings.
Laboratory tests showed an elevated level of fasting blood glucose (15.5 mmol/L, normal range 3.9-6.1 mmol/L) with normal liver and renal function tests. Other routine blood tests indicated that blood regular test, serum C-reactive protein, procalcitonin levels and serum tumor markers were within normal ranges. Sputum culture, sputum smear for acid-fast bacillus and T-SPOT®TB were negative. The titers of total IgG, IgM, IgA and T cell subsets were also normal and screening for antinuclear antibodies and antineutrophil cytoplasmic antibodies was negative. The results of the serologic tests for human immunodeficiency virus were negative.
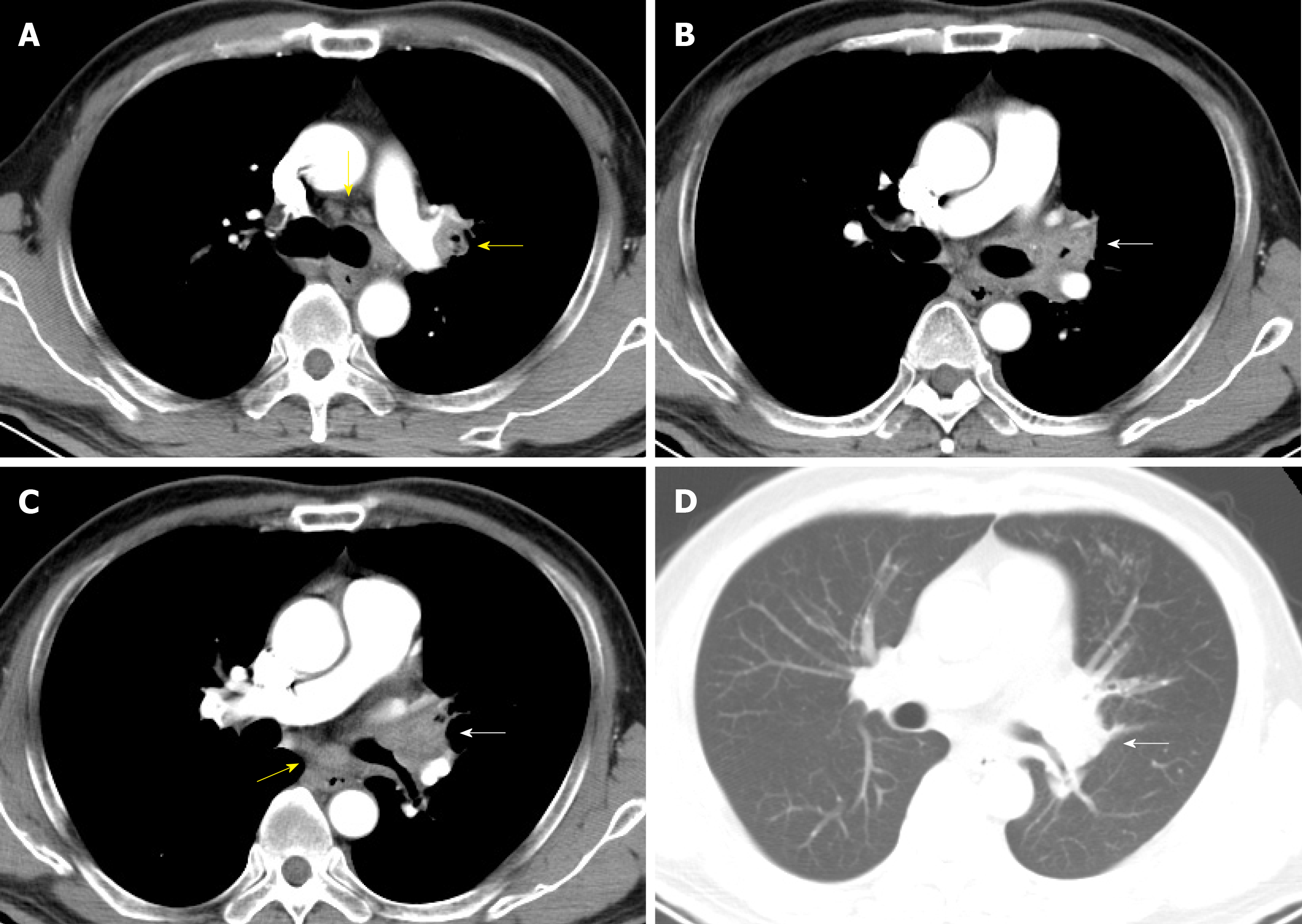
A contrast-enhanced chest computed tomography scan was conducted, which revealed enlarged mediastinum and hilum lymph nodes and an enlarged left hilum with a mass-like lesion leading to stenosis of the proximal part of the left upper bronchus (Figure 1).
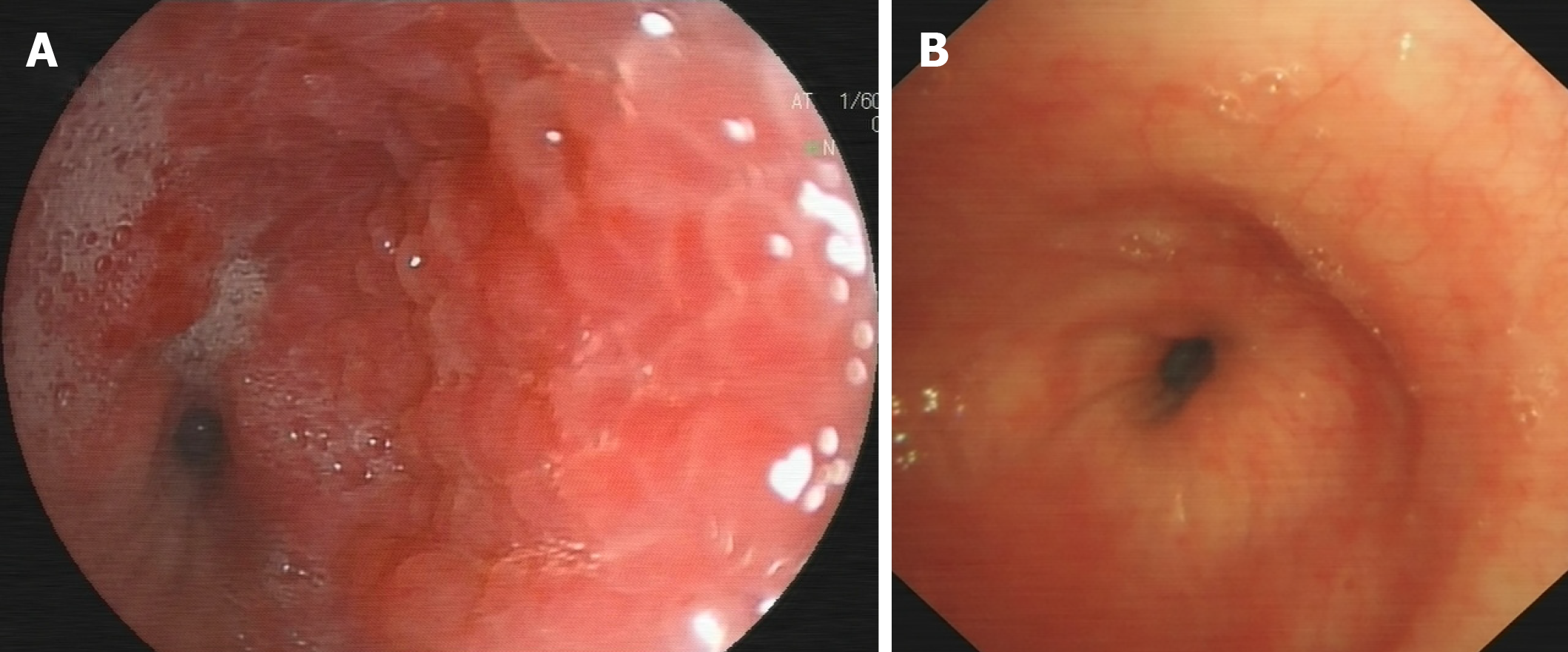
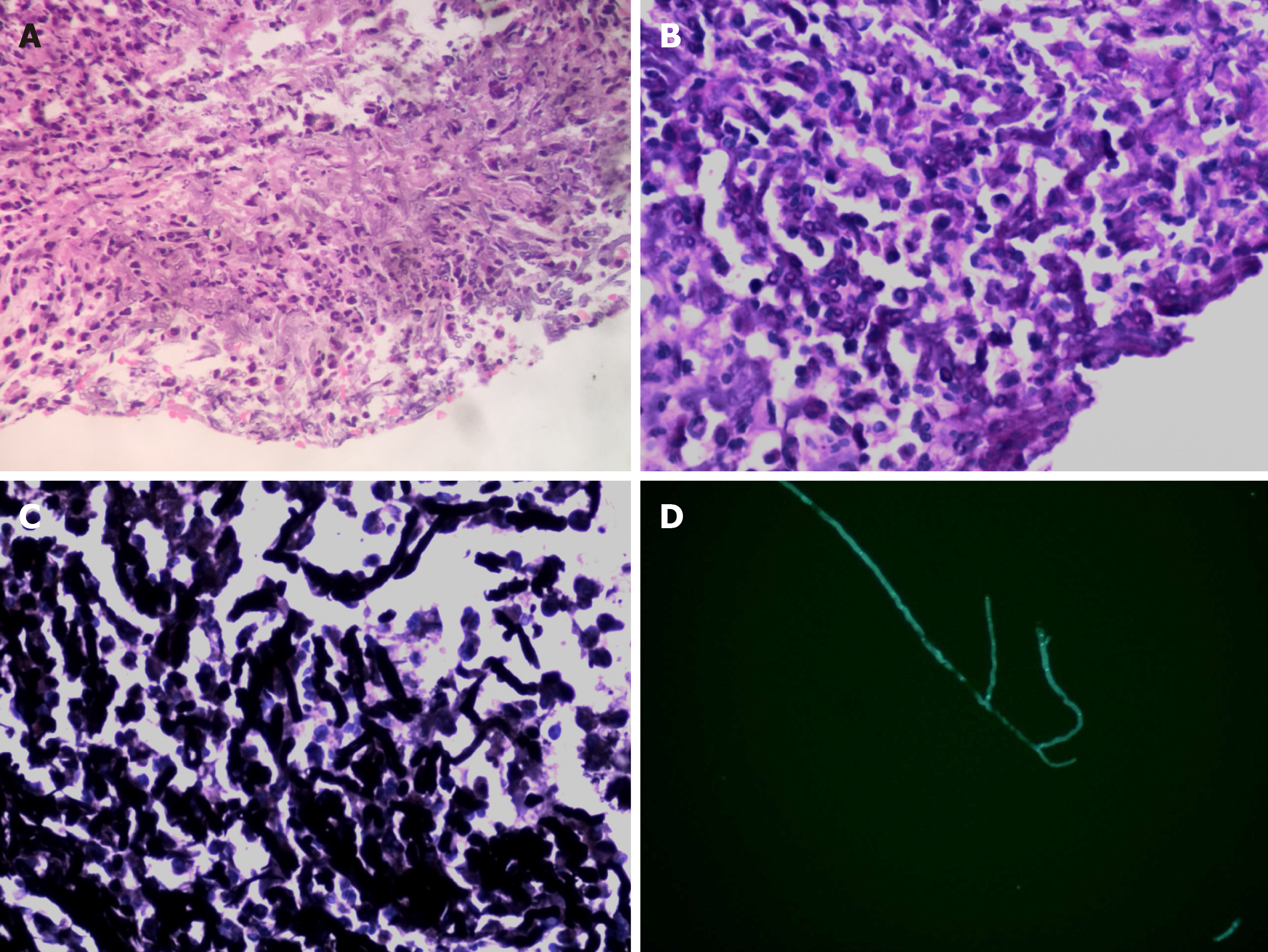
As the patient had normal levels of inflammatory biomarkers along with a mass-like lesion at the lung hilum, he was suspected to have lung cancer. Bronchoscopy revealed redness as well as thickening of bronchial membrane along with bronchial stenosis in the left main bronchus (Figure 2A). Bronchial membrane biopsy and histological analysis were performed, which unexpectedly demonstrated a formation of granulation tissue with 45° branching hyphae (Figure 3A). Moreover, the results of the Periodic Acid-Schiff staining and Grocott staining were positive (Figure 3B and C). But as infectious diseases were not suspected first at that moment, the culture for pathogens was not conducted. At the same time, the endobronchial ultrasound guided transbronchial needle aspiration of 7 and 11 L mediastinum/hilum lymph nodes and histological analysis were conducted, which just demonstrated as lymphocytes with no manifestations of cancer or fungal infections. Meanwhile, serum galactomannan assay indicated negative results (0.14, cut-off 0.5) along with elevated β-(1,3)-D-glucan assay (121.4 pg/mL, normal range < 100.5 pg/mL).
According to the pathology and elevated β-(1,3)-D-glucan assay, the diagnosis of invasive aspergillosis was confirmed.
An antifungal therapy with intravenous voriconazole (6 mg/kg every 12 h in the first 24 h, followed by 4 mg/kg every 12 h) was given for 2 wk and was followed by oral voriconazole.
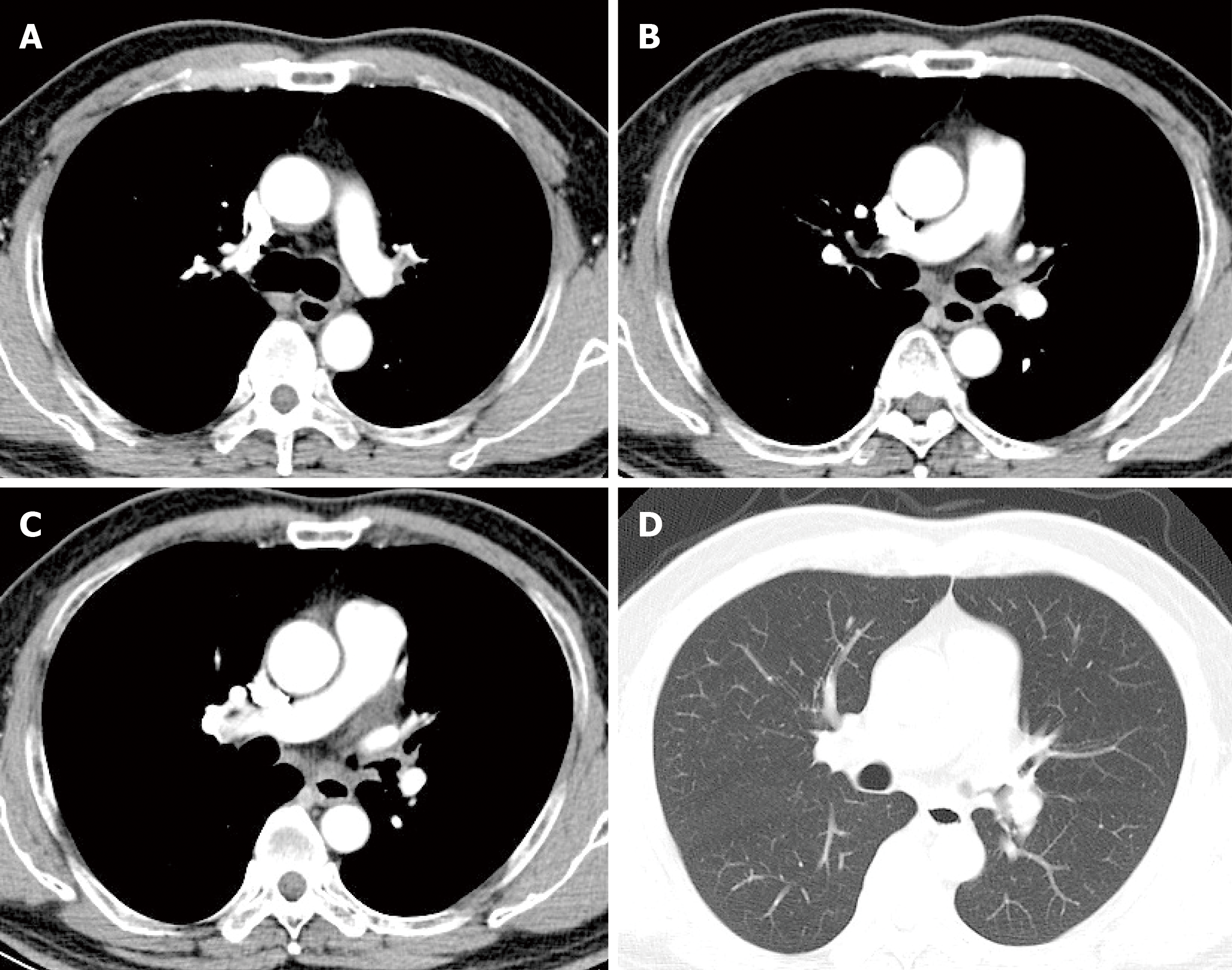
After 3 mo of antifungal therapy, the patient withdrew the drugs without consulting as his symptoms improved. A repeated computed tomography scan and bronchoscopy were done in March 2019, and both of these tests indicated improvement (Figure 4 and Figure 2B). However, fungal fluorescence staining of the bronchial membrane brushing sample still revealed branching septate hyphae (Figure 3D); nonetheless, the culture was negative. Meanwhile, the level of β-(1,3)-D-glucan assay returned to normal (50.7 pg/mL) with negative galactomannan assay (0.08). The patient was asymptomatic by far without any antifungal treatment and was followed closely.
This report describes a unique case of invasive fungal infection with histological evidence mimicking lung cancer in a 54-year-old man with diabetes mellitus. The histological analysis demonstrated fungal elements of 45° branching hyphae with positive Periodic Acid-Schiff and Grocott staining. In addition, fungal fluorescence staining also showed branching septate hyphae. It is well-known that the characteristic of Aspergillus hyphae is angular dichotomously branching septate hyphae, which is consistent with this case. However, other organisms, such as Fusariosis and Scedosporiosis may be morphologically indistinguishable from Aspergillus species. Therefore, culture confirmation is of much importance to differentiate aspergillosis from other filamentous fungal infections. However, there was no positive culture result in the present case. The negative culture results might be false negative due to systemic antifungal therapy. Nonetheless, according to the guideline, proven aspergillosis requires the recovery of an organism with one important exception, which includes the fairly frequent occurrence of histopathological demonstration of hyphae consistent with Aspergillus species in patients with negative culture results[1]. Besides, as culture has a poor sensitivity in the diagnosis of invasive aspergillosis, some experts[2] have supported the concept of a proven infection on the basis of the findings of histopathology or microscopy without necessarily requiring culture confirmation. Combined with the higher morbidity of Aspergillus compared with other filamentous fungi, we believe that the diagnosis of this case should be proven IA.
The radiological manifestations in this case are enlarged mediastinum and hilum lymph nodes and a mass-like lesion in the hilum narrowing the left upper bronchus. Bronchoscopy revealed stenosis of the left main bronchus. It was not clear in our patient whether invasive bronchial aspergillosis (IBA) resulted in lymph node enlargement and hilum mass or lymph nodal aspergillosis invading to the bronchus. As the most common route of entry of Aspergillus spores is by inhalation, we initially assumed the former one. The bronchoscopic manifestations of IBA include three different forms: Aspergillus tracheobronchitis, ulcerative Aspergillus tracheobronchitis, and pseudomembranous Aspergillus tracheobronchitis as described by Kramer et al[3] in 1991. Radiological abnormalities of IBA consist of pulmonary infiltrates, tracheobronchial wall thickening, nodules, endobronchial mass and atelectasis[4]. Nevertheless, in the present case, the manifestation of bronchoscopy and radiography demonstrated as none of the forms described above. Consequently, we presumed the latter one. There have been some extremely rare case reports regarding nodal aspergillosis[5-7]. Kumar et al[5] reported chest wall and mediastinal nodal aspergillosis in a 29-year-old woman with no underlying medical problems who presented with left hilar necrotic lymph nodes, enclosing the left main bronchus and mediastinal lymph nodes, direct extension to the left chest wall. Stern et al[6] reported bulky mediastinal aspergillosis manifested as mediastinal mass surrounding the artery and compressing the left main bronchus in an immunocompetent patient. In the current case, the hilar lesions and lymph nodes on computed tomography diminished after the administration of voriconazole. Therefore, it is highly credible that lymph nodal aspergillosis existed. The negative result of lymph nodal histological analysis may be due to smaller specimen of aspiration compared to biopsy.
As this case showed, Aspergillus infections can present as pseudotumors with radiological appearance and features similar and indistinguishable from lung cancer. Our case illustrates that Aspergillus infections should be considered in the differential diagnosis of mediastinal nodal and hilum involvement, even in immunocompetent patients. Bronchoscopy with mucous biopsies and culture is essential to make an early diagnosis and differential diagnosis in the clinical setting.
IA is a life-threatening opportunistic infection that primarily occurs in critically ill patients as well as in immunocompromised individuals. Major risk factors include neutropenia, prolonged immunosuppressive therapy, lung transplantation and hematological malignancy[8]. Recently, uncontrolled diabetes mellitus is considered as a low risk factor for aspergillosis. Diabetes mellitus can alter the normal immunologic response to infections, including reduced cytokine production, decreased granulocyte phagocytic ability and altered polymorphonuclear leucocyte response.
The latest Infectious Diseases Society of America guidelines[9] recommend voriconazole as the first line therapy. However, the optimal duration of antifungal therapy is not well defined. The guidelines generally recommend that treatment of IA be continued for a minimum of 6–12 wk, depending on the severity and continuation of immunosuppression as well as the extent of resolution of clinical disease. In this case, the patient underwent 3 mo of antifungal therapy with radiological and bronchoscopic improvement but not complete absorption. At 7 mo after the initial diagnosis, the IA had not recurred.
This case recommends that diagnosis of mediastinal and hilar lymphadenopathy does not always mean malignancy even in a heavy smoker. The clinicians should always consider a diagnosis of IA when radiological examination shows pseudotumor appearance in diabetes mellitus patients. Bronchoscopy with biopsy and culture is of great importance to confirm the diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Vosmik M, Vinh-Hung V, Wang HY S-Editor: Zhang L L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF; Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1903] [Cited by in RCA: 1877] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 2. | Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ; Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1840] [Cited by in RCA: 1797] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 3. | Kramer MR, Denning DW, Marshall SE, Ross DJ, Berry G, Lewiston NJ, Stevens DA, Theodore J. Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am Rev Respir Dis. 1991;144:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 177] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Fernández-Ruiz M, Silva JT, San-Juan R, de Dios B, García-Luján R, López-Medrano F, Lizasoain M, Aguado JM. Aspergillus tracheobronchitis: report of 8 cases and review of the literature. Medicine (Baltimore). 2012;91:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Kumar J, Seith A, Kumar A, Madan K, Guleria R. Chest wall and mediastinal nodal aspergillosis in an immunocompetent host. Diagn Interv Radiol. 2009;15:176-178. [PubMed] |
| 6. | Stern JB, Wyplosz B, Validire P, Angoulvant A, Fregeville A, Caliandro R, Gossot D. Bulky mediastinal aspergillosis mimicking cancer in an immunocompetent patient. Ann Thorac Surg. 2014;98:1472-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Mazzoni A, Ferrarese M, Manfredi R, Facchini A, Sturani C, Nanetti A. Primary lymph node invasive aspergillosis. Infection. 1996;24:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 9. | Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1851] [Article Influence: 205.7] [Reference Citation Analysis (0)] |