Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3812
Peer-review started: May 23, 2019
First decision: September 9, 2019
Revised: September 30, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 193 Days and 23.9 Hours
Klebsiella pneumoniae (K. pneumoniae) used to affect mainly people with compromised immunity or weakened by other infections, but recent emergence of hypervirulent strains has increased infections even in healthy individuals. These infections include liver abscess, pneumonia, bacteremia, meningitis, necrotizing fasciitis, and endophthalmitis. Although metastatic infection by hypervirulent K. pneumoniae (hvKP) is increasingly recognized, co-infection with Cryptococcus neoformans (C. neoformans) meningitis in immunocompetent hosts is rare but fatal. So, it is necessary to determine the risk factors, complications, and comorbidity of this disease.
This report describes a 58-year-old man with hvKP pulmonary abscess, bacteremia, and meningitis, accompanied by fatal Cryptococcus meningitis. This patient presented with fever for 1 wk and drowsiness for 3 d. Laboratory findings revealed pulmonary abscess and bacteremia of K. pneumoniae. He was given intravenous antibiotic therapy, and the infection was under control for about 1 wk. However, his condition deteriorated rapidly because of metastatic purulent meningitis. Although hvKP and C. neoformans were isolated and confirmed, the patient died of spontaneous respiratory and cardiac arrest caused by cerebral hernia.
HvKP has emerged as a cause of metastatic infections in immunocompetent hosts. polymicrobial co-infections should be taken into consideration when metastatic infection is present.
Core tip: Hypervirulent Klebsiella pneumoniae (hvKP) has emerged as a cause of metastatic infections in immunocompetent hosts and presents mainly as a monomicrobial infection. We present a rare case of metastatic infection caused by hvKP and co-infection with Cryptococcus neoformans meningitis. The patient received antibiotics that were sensitive to pathogens in time, and the infection was under control for 1 wk. However, his condition deteriorated rapidly because of metastatic purulent meningitis, and he died of spontaneous respiratory and cardiac arrest caused by cerebral hernia. This case highlights the risk of complications and polymicrobial co-infections in hvKP infected patients. Timely ventricle drainage is strongly recommended for polymicrobial co-infected meningitis.
- Citation: Shi YF, Wang YK, Wang YH, Liu H, Shi XH, Li XJ, Wu BQ. Metastatic infection caused by hypervirulent Klebsiella pneumonia and co-infection with Cryptococcus meningitis: A case report. World J Clin Cases 2019; 7(22): 3812-3820
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3812.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3812
Klebsiella pneumoniae (K. pneumoniae) is a Gram-negative, nonmotile, capsulated, aerobic bacterium. It is widely found in nature, as well as in hospital settings, causing community-acquired and nosocomial infections. Invasive infections resulting from K. pneumoniae dissemination include liver abscess, pneumonia, bacteremia, meningitis, necrotizing fasciitis, endophthalmitis, and even sepsis[1]. In the past decade, more attention has been given to cases of hypervirulent K. pneumoniae (hvKP) infection in eastern countries. The death rate from hvKP infection has been as high as 60% as a result of antibiotic-resistant strains, despite the use of broad-spectrum antibiotics such as carbapenems[2,3].
Metastatic infection caused by hvKP is generally community-acquired and presents mainly as a monomicrobial disease. Here, we present and discuss a rare case of metastatic infection due to hvKP with pulmonary abscess, bacteremia, purulent meningitis, and co-infection with fatal Cryptococcus meningitis. To the best of our knowledge, this is the first case of such a metastatic infection caused by hvKP and C. neoformans, which should raise concern among clinicians for the risk of such infection.
Fever for 1 wk and drowsiness for 3 d.
The patient, a 58-year-old man, presented with high fever (> 40.0 °C) for 1 wk before admission, with simultaneous fatigue, occasional cough without sputum, and swelling of the left upper limb. The patient was referred to Sun Yat-sen University Cancer Center (SYSUCC) at first because routine blood tests in his hometown showed white blood cell (WBC) count 34.9 × 109/L and hemoglobin level 67 g/L. In the next 6 d at SYSUCC, laboratory findings confirmed pulmonary abscess and bacteremia caused by K. pneumoniae. He was given antibiotic therapy of imipenem–cilastatin combined with voriconazole at normal dose. However, he still had high fever and gradually developed altered mental state, which presented as abepithymia and drowsiness. There was no headache, nausea, or vomiting. Based on a rapid increase in creatinine (from normal to 264 µmol/L) and bacteremia, he was diagnosed with acute renal insufficiency and sepsis and admitted to the Medical Intensive Care Unit (MICU) of the Third Affiliated Hospital of Sun Yat-Sen University.
The patient had been well prior to onset of the present illness. He had no chronic illness such as diabetes mellitus, immunodeficiency, or psychiatric or psychological disease. He had no tuberculosis or lung cancer.
The patient was a non-smoker. He had no history of alcohol or illicit drug abuse. He resided in Guangdong, southern China. He had no recent travel, tick bites, or contact with sick people.
When the patient was admitted to MICU (day 1), his vital signs were as follows: Temperature 36.4 °C, pulse rate 95 beats/min, respiratory rate 20 breaths/min, blood pressure 126/85 mmHg, and pulse oxygen saturation 98% on 5 L/min oxygen flow rate. Low respiratory tone and fine crackles in the right upper lung were noted on pulmonary auscultation. There was no audible murmur on cardiac auscultation. Tenderness and hepatosplenomegaly were not detected. Tenderness in his left upper limb was noted. Both lower limbs had mild edema. He had abepithymia but was still conscious, oriented with Glasgow Coma Scale score 15, and had no neck stiffness. No rash was observed.
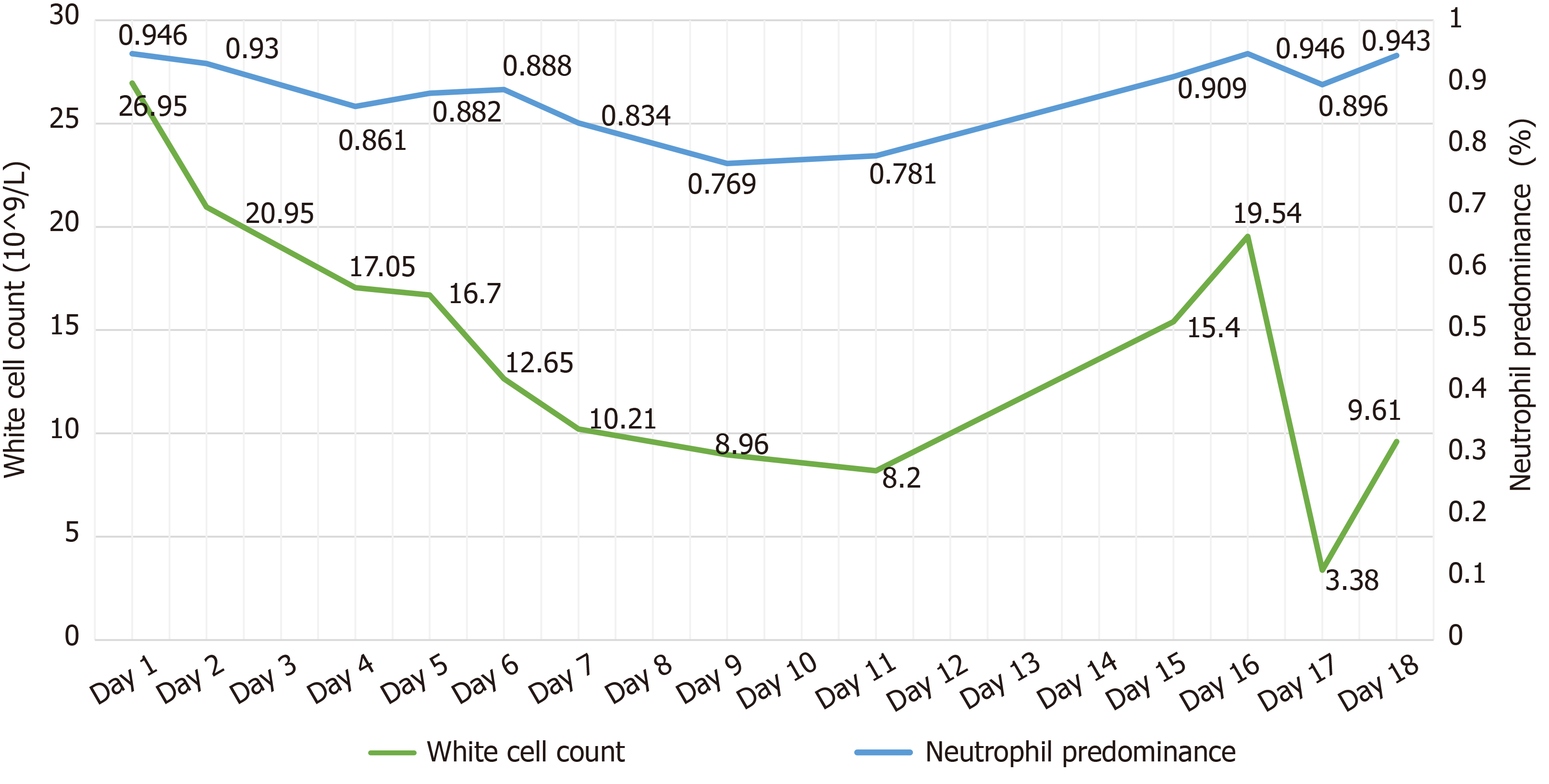
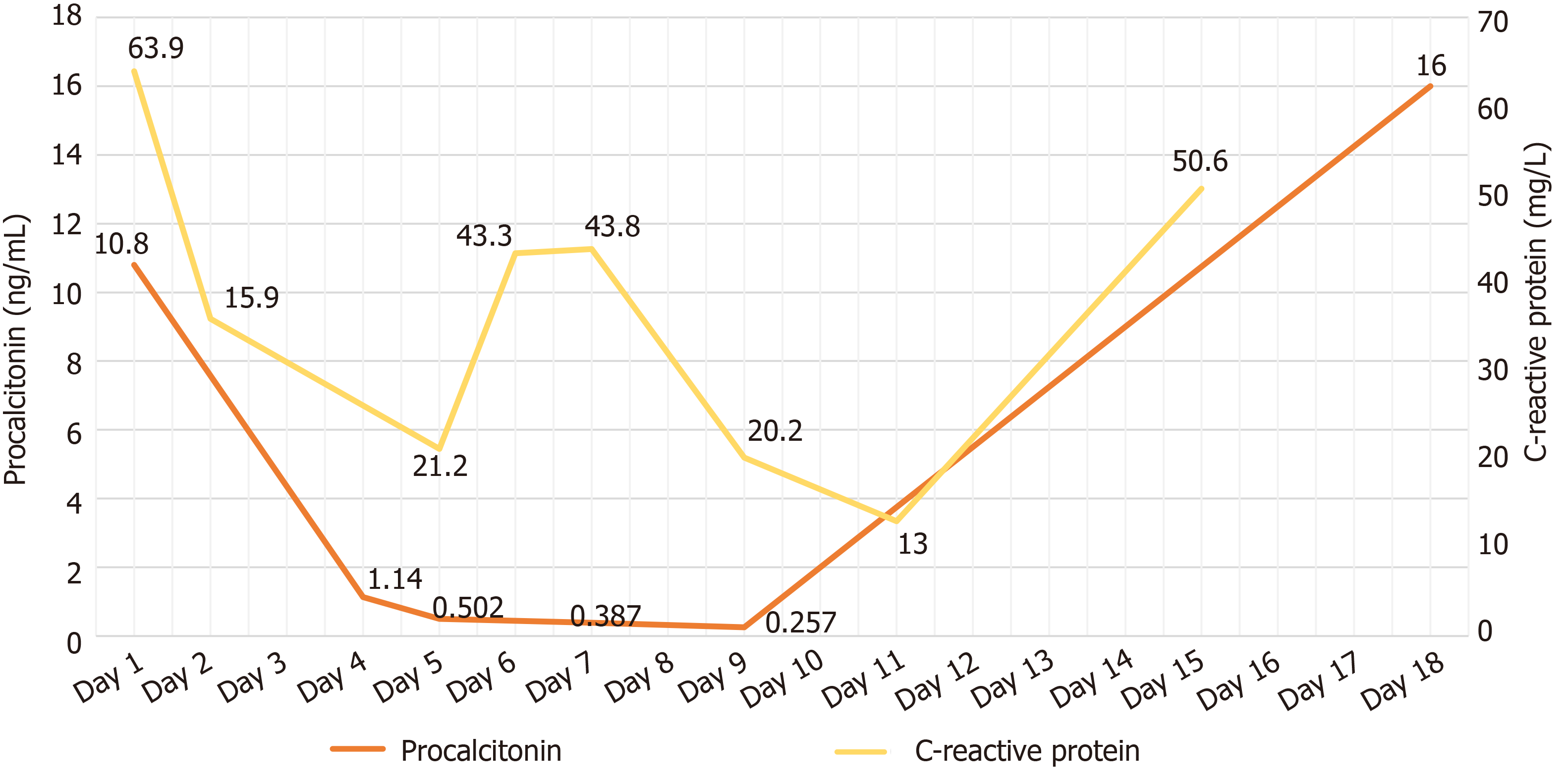
Laboratory tests from SYSUCC revealed WBC count 27.0 × 109/L with an elevated neutrophil ratio of 95.4%. The concentration of C-reactive protein (CRP) and procalcitonin (PCT) were 233.17 mg/L and 1.7 ng/mL, respectively. Glucose concentration was 7.11 mmol/L. Full blood culture grew K. pneumoniae (Table 1). Cryptococcal antigen was negative. Galacto Mannan test and β-D-Glucan test both were negative. Concentration of G-lipopolysaccharides was 0.13 EU/mL. Bone marrow smear and biopsy confirmed no malignant tumor cells. Chest computed tomography (CT) scan found a lump that could not be identified as a tumor or infection in the upper right lung. Head CT scan showed no acute findings. CT scan of the left upper limb revealed no abnormality.
| Antibiotic | Drug sensitivity | MIC |
| Ampicillin | R | ≥ 32 |
| Cefazolin | S | ≤ 4 |
| Ceftazidime | S | ≤ 1 |
| Ceftriaxone | S | ≤ 1 |
| Cefepime | S | ≤ 1 |
| Aztreonam | S | ≤ 1 |
| Imipenem | S | ≤ 1 |
| Ertapenem | S | ≤ 0.5 |
| Amoxicillin/clavulanate | R | UD |
| Piperacillin/tazobactam | S | ≤ 4 |
| Ciprofloxacin | S | ≤ 0.25 |
| Levofloxacin | S | ≤ 0.25 |
| Tobramycin | S | ≤ 1 |
| Gentamicin | S | ≤ 1 |
| Amikacin | S | ≤ 2 |
| Cefotiam | S | UD |
| Cefotetan | S | ≤ 4 |
| Latamoxef | S | UD |
| Cefoperazone/sulbactam | S | ≤ 4 |
| Ampicillin/sulbactam | S | 4 |
| SMZCo | S | ≤ 20 |
| Macrodantin | I | 64 |
| Moxifloxacin | S | ≤ 0.25 |
| ESBL detection | Negative | |
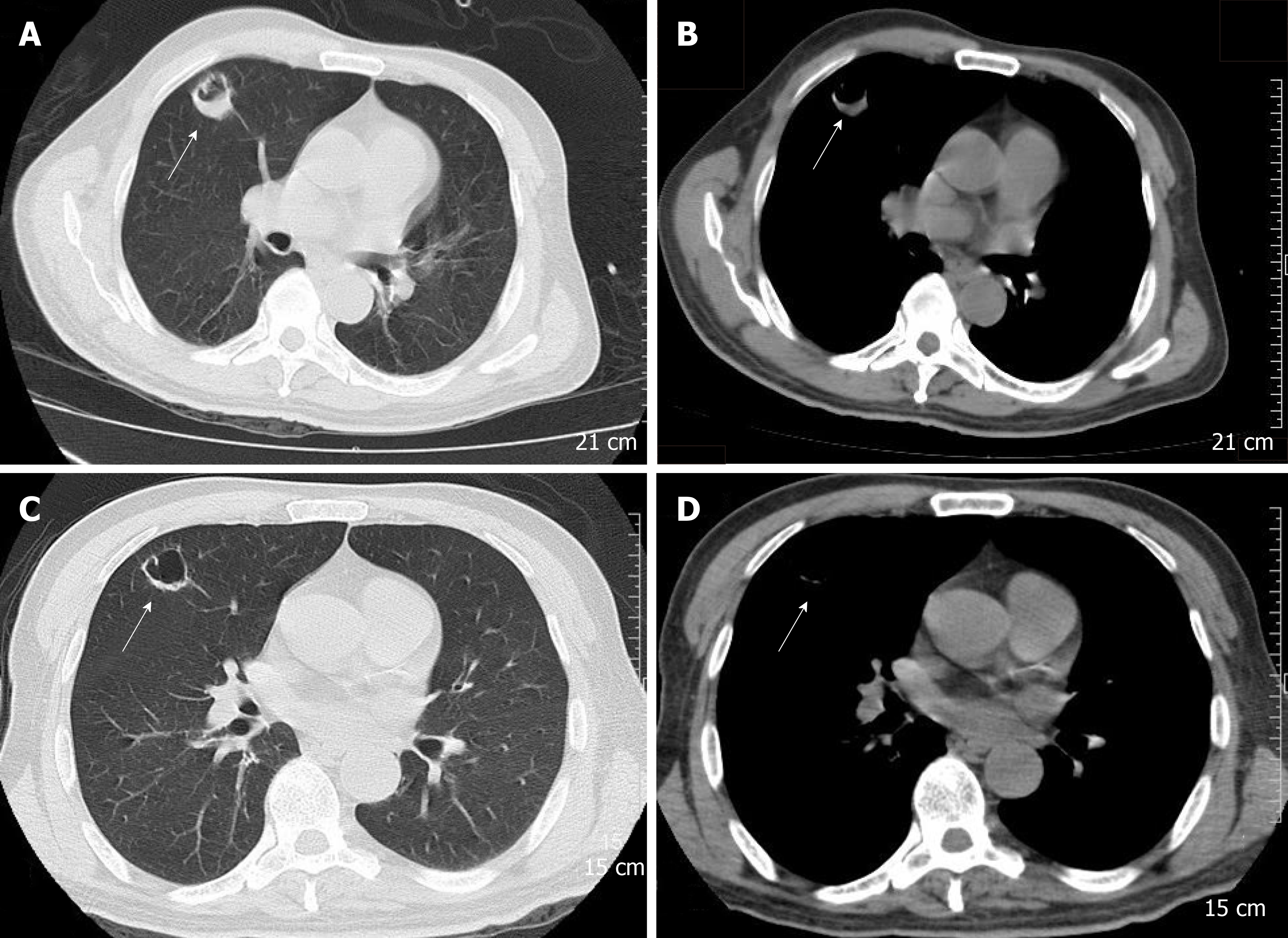
Laboratory data upon admission in MICU demonstrated WBC count 26.95 × 109/L with an elevated neutrophil ratio of 94.6%. CRP was 63.9 mg/L, PCT was 10.8 ng/mL, and erythrocyte sedimentation rate was 126 mm/h. Electrolytes were within normal limits. The concentration of glucose was 7.0 mmol/L with hemoglobin A1c in the normal range. Albumin level was 28.4 g/L. Creatinine was 341 μmol/L. Bedside cardiac ultrasound found left ventricular ejection fraction of 0.63 and no pericardial effusion. No intra-abdominal pathology was observed by bedside ultrasound. Chest CT scan found a 25 mm × 27mm, round, thick-walled cavity in the right upper lobe (Figure 1A and Figure 1B).
Based on the clinical data, the final diagnoses were confirmed as pulmonary abscess, bacteremia, metastatic purulent meningitis, and sepsis due to hvKP, with co-infection with C. neoformans.
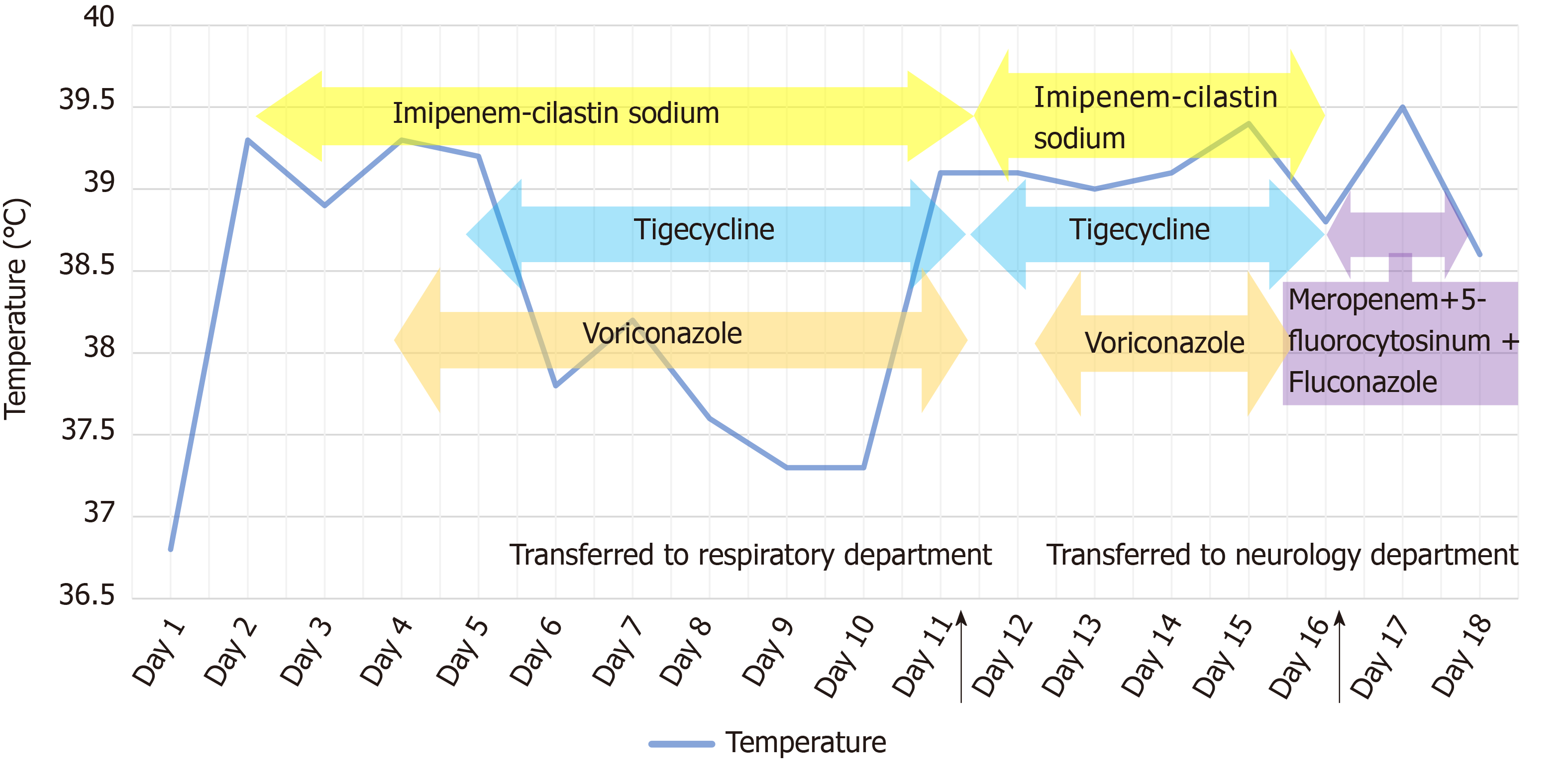
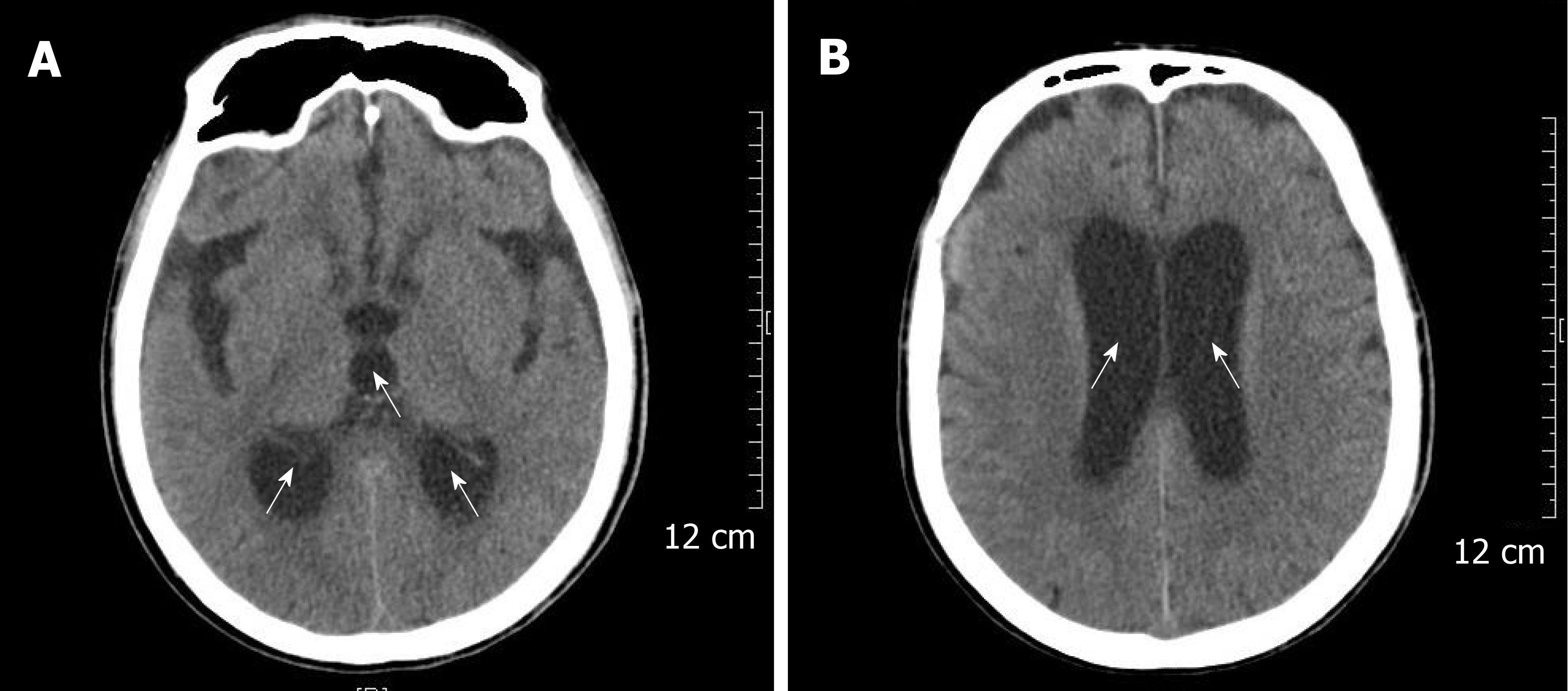
The patient received imipenem–cilastatin, tigecycline combined with voriconazole (Figure 2), together with supportive treatment. The patient continued to have high fever (maximum 39.3 °C), which finally began to relieve 4 d after treatment. After 11 d of treatment, his WBC count dropped continuously to 8.2 × 109/L, with a normal neutrophil predominance of 78%, as well as decrease of CRP and PCT (Figure 3 and Figure 4). Creatinine dropped from 341 to 57 µmol/L. The lung abscess was lessened upon CT scan (Figure 1). Two sets of peripheral blood cultures were ordered during episodes of fever, but all results were negative. The patient was transferred to the Respiratory Department (a general ward) on day 11. However, 3 d later, the patient had projectile vomiting and altered mental state. Physical examination demonstrated intermittent somnolence and neck stiffness. Head CT scan and lumbar puncture were arranged. CT images showed marked dilatation of the bilateral and third ventricles caused by hydrocephalus (Figure 5). Lumbar puncture showed intracranial pressure of 120 mmH2O, and cerebrospinal fluid (CSF) was transparent and colorless. CSF was collected for routine examination, biochemistry, Gram staining, and bacterial culture. CSF cytology and biochemistry showed 1560 WBCs/µL with 90% neutrophils, 18 red blood cells/µL, protein 2.03 g/L, glucose 0.03 mmol/L, and chloride ions 110.3 mmol/L. It was noticeable that C. neoformans was found by CSF staining, with a count of 85566/mL.
The patient was transferred to the Neurology Department on day 16. He was intubated and transferred to the neurologic intensive care unit because of unstable vital signs on the same night. Considering the high risk of surgery, the patient’s family refused bilateral ventricular drainage that was proposed by a neurosurgeon. The patient was given anti-infective therapy of meropenem combined with 5-fluorocytosine and fluconazole. On day 18, the patient’s vital signs and homeostasis deteriorated rapidly. He died of cerebral hernia caused by co-infection with purulent meningitis. K. pneumoniae was subsequently isolated from CSF (Table 2), closely followed by isolation from sputum collected by bronchoscopy (Table 3). Both were string test positive, which reflected the hypermucoviscous phenotype and were considered as hypervirulent strains.
| Antibiotics | Drug sensitivity | MIC |
| Ampicillin | R | 16 |
| Piperacillin | S | ≤ 16 |
| Cefazolin | S | ≤ 8 |
| Cefoxitin | S | ≤ 8 |
| Cefuroxime | S | ≤ 4 |
| Cefotaxime | S | ≤ 2 |
| Ceftazidime | S | ≤ 1 |
| Ceftriaxone | S | ≤ 8 |
| Cefepime | S | ≤ 8 |
| Aztreonam | S | ≤ 8 |
| Imipenem | S | ≤ 1 |
| Meropenem | S | ≤ 1 |
| Ertapenem | S | ≤ 2 |
| Amoxicillin/clavulanate | S | ≤ 8/4 |
| Piperacillin/tazobactam | S | ≤ 16 |
| Ticarcillin/clavulanate | S | ≤ 16 |
| Ciprofloxacin | S | ≤ 1 |
| Levofloxacin | S | ≤ 2 |
| Tobramycin | S | ≤ 4 |
| Gentamicin | S | ≤ 4 |
| Amikacin | S | ≤ 8 |
| Tetracycline | S | ≤ 4 |
| Trimethoprim/sulfanilamide | S | ≤ 2/38 |
| Cefotaxime/clavulanate | S | ≤ 0.5 |
| Ceftazidime/clavulanate | S | ≤ 0.25 |
| Antibiotic | Drug sensitivity | MIC |
| Ampicillin | R | > 32 |
| Cefazolin | S | 2 |
| Cefoxitin | S | ≤ 2 |
| Cefuroxime | S | 4 |
| Cefotaxime | S | ≤ 0.5 |
| Ceftazidime | S | ≤ 1 |
| Cefepime | S | ≤ 1 |
| Imipenem | S | ≤ 0.5 |
| Piperacillin/tazobactam | I | 32 |
The infection was under control for 1 wk, but his condition deteriorated rapidly because of co-infective purulent meningitis. Unfortunately, the patient died of spontaneous respiratory and cardiac arrest caused by cerebral hernia.
A number of serious infections caused by K. pneumoniae that may lack response to anti-infective therapy have been increasingly recorded over the past decade. These strains acquire extra genetic traits and become hypervirulent or antibiotic resistant[4]. Capsular polysaccharide is the most important virulence factor of K. pneumoniae, and others include fimbriae, lipopolysaccharide, determinants of iron acquisition, outer membrane proteins, and nitrogen source utilization[5]. Some underlying risk factors such as diabetes mellitus may damage host defense by causing inhibition of phagocytosis and bactericidal activity, which contributes to the initiation and spread of infection[6]. Bacterial virulence is still the primary and most important factor. Since the 1980s, hvKP from the community has attracted increased attention due to its ability to cause metastatic and aggressive infections called “invasive klebsiella syndrome” in healthy hosts[7-9]. There is an urgent need to highlight clinical awareness and management of hvKP infection.
Over the past 10 years, hvKP has become more pervasive with a varied geographic distribution and more likely to cause serious infection in China. Some strains present with both hypervirulence and antibiotic resistance[10,11]. Compared with classic K. pneumoniae, hvKP is more effective at capsule production, forming a high mucous phenotype and causing severe invasive multi organ infection[9]. However, there is no internationally agreed definition for hvKP and its virulence level. Eight capsular serotypes, such as K1 and K2, have been associated with hypervirulent strains[12,13]. Besides, mucoid phenotype A (rmpA) and mucoviscosity-associated gene A (magA) are two major genes linked with hypervirulence. Hypermucoviscosity correlates with higher virulence gene content[14], which supports the value of string tests. The string test, with a viscous string > 5 mm in length, reflects the hypermucoviscous phenotype, and is still the most common laboratory-based method presently available[8]. The string test for K. pneumoniae in the present case was positive. This hypervirulent strain caused pulmonary abscess, bacteremia, and metastatic purulent meningitis.
K. pneumoniae remains one of the most prevalent pathogens in bacterial meningitis[15,16]. C. neoformans is the most common cause of fungal meningitis in immunocompetent hosts[17]. Meningitis caused by mixed infection of K. pneumoniae and C. neoformans is rare. Despite the use of potent antibiotics, the mortality of K. pneumoniae meningitis remains high. In such cases, invasive treatment, such as lateral ventricular drainage or irrigation, is recommended to reduce intracranial pressure and relieve inflammatory reaction in the brain tissue[18], especially for those cases with fatal polymicrobial co-infection[19].
In the present case, the middle-aged male patient neither became infected in hospital, nor did he have putative risk factors such as history of diabetes mellitus or hypoimmunity. Until to his death, the clinical data strongly supported the diagnosis of a severe outbreak of sepsis caused by K. pneumoniae, which caused metastatic infection accompanied by C. neoformans meningitis. Besides, the patient initially complained of swelling of the left upper limb, which reminded us of complicated skin and soft tissue infections. However, there is no evidence to confirm that, and the swelling was relieved during hospitalization. K. pneumoniae was not isolated again until the patient’s death, nor was C. neoformans identified. The K. pneumoniae strain was not further investigated genetically to confirm the capsular serotype or the virulence genes. A positive string test indicated a hypervirulent strain, which explained the aggressive progression of infection. Considering the difficulties for identification of potential pathogens, which maybe polymicrobial, high-throughput approaches such as biomarkers have been increasingly used in scientific research and clinical studies in recent years[20].
Early recognition of meningitis is not always easy, particularly when the patient presents with nonspecific symptoms. Detailed history of illness, systematic examination, and multidisciplinary consultation are required. For example, for cases with obviously altered mental state, clinicians should consider the possibility of cryptococcal meningitis[21]. Besides anti-infective therapy, timely cerebroventricular drainage is equally or even more important when purulent meningitis is confirmed to improve clinical outcomes[19].
In conclusion, hvKP has emerged as a not uncommon pathogen that could cause metastatic and aggressive infections. Meningitis should be taken into consideration when a metastatic infection is present. Timely anti-infection therapy and ventricle drainage are strongly recommended, especially for polymicrobial co-infection cases.
At last, although it is not possible to explore thoroughly such a complicated infection because of the limitations of case reports, it is our duty to report and accumulate such cases.
HvKP has emerged as a cause of metastatic infections in immunocompetent hosts. Polymicrobial co-infections should be taken into consideration when metastatic infection is present.
We are grateful to Professor Jie Qin, Dr. Xiu-Zhen Chen, and Dr. Jun-Hui Ba for patient consultation and expert advice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu MY
| 1. | Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80:629-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 1168] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Zeng J, Liu W, Zhao F, Hu Z, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Cao B, Wang H. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Palacios M, Miner TA, Frederick DR, Sepulveda VE, Quinn JD, Walker KA, Miller VL. Identification of Two Regulators of Virulence That Are Conserved in Klebsiella pneumoniae Classical and Hypervirulent Strains. MBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Li B, Zhao Y, Liu C, Chen Z, Zhou D. Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2014;9:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Lin YT, Wang FD, Wu PF, Fung CP. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis. 2013;13:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 841] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 9. | Marr CM, Russo TA. Hypervirulent Klebsiella pneumoniae: a new public health threat. Expert Rev Anti Infect Ther. 2019;17:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Wang R, Wang H. High Prevalence of Hypervirulent Klebsiella pneumoniae Infection in China: Geographic Distribution, Clinical Characteristics, and Antimicrobial Resistance. Antimicrob Agents Chemother. 2016;60:6115-6120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 11. | Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Shi Q, Lan P, Huang D, Hua X, Jiang Y, Zhou J, Yu Y. Diversity of virulence level phenotype of hypervirulent Klebsiella pneumoniae from different sequence type lineage. BMC Microbiol. 2018;18:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Yan JJ, Zheng PX, Wang MC, Tsai SH, Wang LR, Wu JJ. Allocation of Klebsiella pneumoniae Bloodstream Isolates into Four Distinct Groups by ompK36 Typing in a Taiwanese University Hospital. J Clin Microbiol. 2015;53:3256-3263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Long F, Chen QL, Wu SY, Liu Y, Zhang WL, Liao QF, Wang MJ, Lu XJ, He C, Kang M. [Etiology, Prognosis and Risk Factors of 181 Adult Community-acquired Acute Bacterial Meningitis]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49:808-811. [PubMed] |
| 16. | Ku YH, Chuang YC, Chen CC, Lee MF, Yang YC, Tang HJ, Yu WL. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, Virulence and Antibiotic Resistance. Sci Rep. 2017;7:6634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Shih RY, Koeller KK. Bacterial, Fungal, and Parasitic Infections of the Central Nervous System: Radiologic-Pathologic Correlation and Historical Perspectives. Radiographics. 2015;35:1141-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Xu M, Fu Y, Fang Y, Xu H, Kong H, Liu Y, Chen Y, Li L. High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect Drug Resist. 2019;12:641-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Conde-Pereira C, Rodas-Rodríguez L, Díaz-Paz M, Palacios-Rivera H, Firacative C, Meyer W, Alcázar-Castillo M. Fatal Case of Polymicrobial Meningitis Caused by Cryptococcus liquefaciens and Mycobacterium tuberculosis Complex in a Human Immunodeficiency Virus-Infected Patient. J Clin Microbiol. 2015;53:2753-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Russo TA, Olson R, Fang CT, Stoesser N, Miller M, MacDonald U, Hutson A, Barker JH, La Hoz RM, Johnson JR. Identification of Biomarkers for Differentiation of Hypervirulent Klebsiella pneumoniae from Classical K. pneumoniae. J Clin Microbiol. 2018;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 472] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 21. | Qu J, Zhou T, Zhong C, Deng R, Lü X. Comparison of clinical features and prognostic factors in HIV-negative adults with cryptococcal meningitis and tuberculous meningitis: a retrospective study. BMC Infect Dis. 2017;17:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |