Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3757
Peer-review started: May 23, 2019
First decision: August 1, 2019
Revised: September 16, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 192 Days and 20.5 Hours
Acute recurrent pancreatitis (ARP) is characterized by episodes of acute pancreatitis in an otherwise normal gland. When no cause of ARP is identifiable, the diagnosis of "idiopathic" ARP is given. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene increase the risk of ARP by 3- to 4-times compared to the general population, while cystic fibrosis (CF) patients present with a 40- to 80-times higher risk of developing pancreatitis.
In non-classical CF or CFTR-related disorders, CFTR functional tests can help to ensure a proper diagnosis. We applied an individualized combination of standardized and new CFTR functional bioassays for a patient referred to the Verona CF Center for evaluation after several episodes of acute pancreatitis. The CFTR genotype was G542X+/- with IVS8Tn:T7/9 polymorphism. The sweat (Cl-) values were borderline. Intestinal current measurements were performed according to the European Cystic Fibrosis Society Standardized Operating Procedure. Recent nasal surgery for deviated septum did not allow for nasal potential difference measurements. Lung function and sputum cultures were normal; azoospermia was excluded. Pancreas divisum was excluded by imaging but hypoplasia of the left hepatic lobe was detected. Innovative tests applied in this case include sweat rate measurement by image analysis, CFTR function in monocytes evaluated using a membrane potential-sensitive fluorescent probe, and the intestinal organoids forskolin-induced swelling assay.
Combination of innovative CFTR functional assays might support a controversial diagnosis when CFTR-related disorders and/or non-classical CF are suspected.
Core tip: When the diagnosis of "idiopathic" recurrent acute pancreatitis is given, non-classical cystic fibrosis or disorders related to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene are sometimes suspected when the phenotype is consistent with cystic fibrosis. Combination of innovative CFTR functional assays might support a controversial diagnosis, contributing to better definitions of these clinical entities.
- Citation: Caldrer S, Bergamini G, Sandri A, Vercellone S, Rodella L, Cerofolini A, Tomba F, Catalano F, Frulloni L, Buffelli M, Tridello G, de Jonge H, Assael BM, Sorio C, Melotti P. Cystic fibrosis transmembrane conductance regulator functional evaluations in a G542X+/- IVS8Tn:T7/9 patient with acute recurrent pancreatitis. World J Clin Cases 2019; 7(22): 3757-3764
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3757.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3757
Cystic fibrosis (CF) is the most common lethal genetic disease in Caucasians, affecting around 1 in 3000 live births. Its underlying etiology is disease-causing genetic mutations occurring on both alleles of the cystic fibrosis transmembrane conductance regulator (CFTR) gene[1]. Within the spectrum of disorders associated with CFTR dysfunction is recurrent-acute (APR) and chronic pancreatitis that can harbor CFTR mutations within classic CF or CFTR-related pancreatitis[2].
ARP and chronic pancreatitis due to CFTR dysfunction can be indistinguishable from idiopathic (I) ARP in its initial clinical presentation[3].
The most recent consensus statement from the United States Cystic Fibrosis Foundation[4] provides for a diagnosis of CF to be made in individuals who present with a characteristic clinical phenotype or a history of CF in a sibling, in the presence of an abnormal sweat chloride value ≥ 60 mmol/L and/or two CF-causing mutations. Identification of CFTR mutations on both alleles is not sufficient to establish the diagnosis of CF especially when one or both are not designated disease-causing mutations[5]. While the management of pancreatitis associated with CF or CFTR mutations per se is currently not different from that of other forms of pancreatitis, the main concerns in these patients are a progression from pancreatic sufficiency to pancreatic insufficiency and increased risk of developing CF disease and/or disease in other CF-affected organs (e.g., bronchiectasis)[2,6], as such these patients should be referred to specialized gastroenterologists or centers with expertise in CF for proper management.
A careful evaluation of CFTR function is therefore important for proper management of these patients. Herein, we describe a patient who was evaluated by different assays targeting different tissues/cell types known to express CFTR, some of which are standardized for clinical application, such as the intestinal current measurement (ICM)[7] and nasal potential difference (NPD) measurement[8], or are considered as emerging approaches. The latter include the primary forskolin-induced swelling (FIS) assay on intestinal organoids[9], assessment of CFTR function in leukocytes[10], as well as the beta-adrenergic/cholinergic imaged sweat test[11].
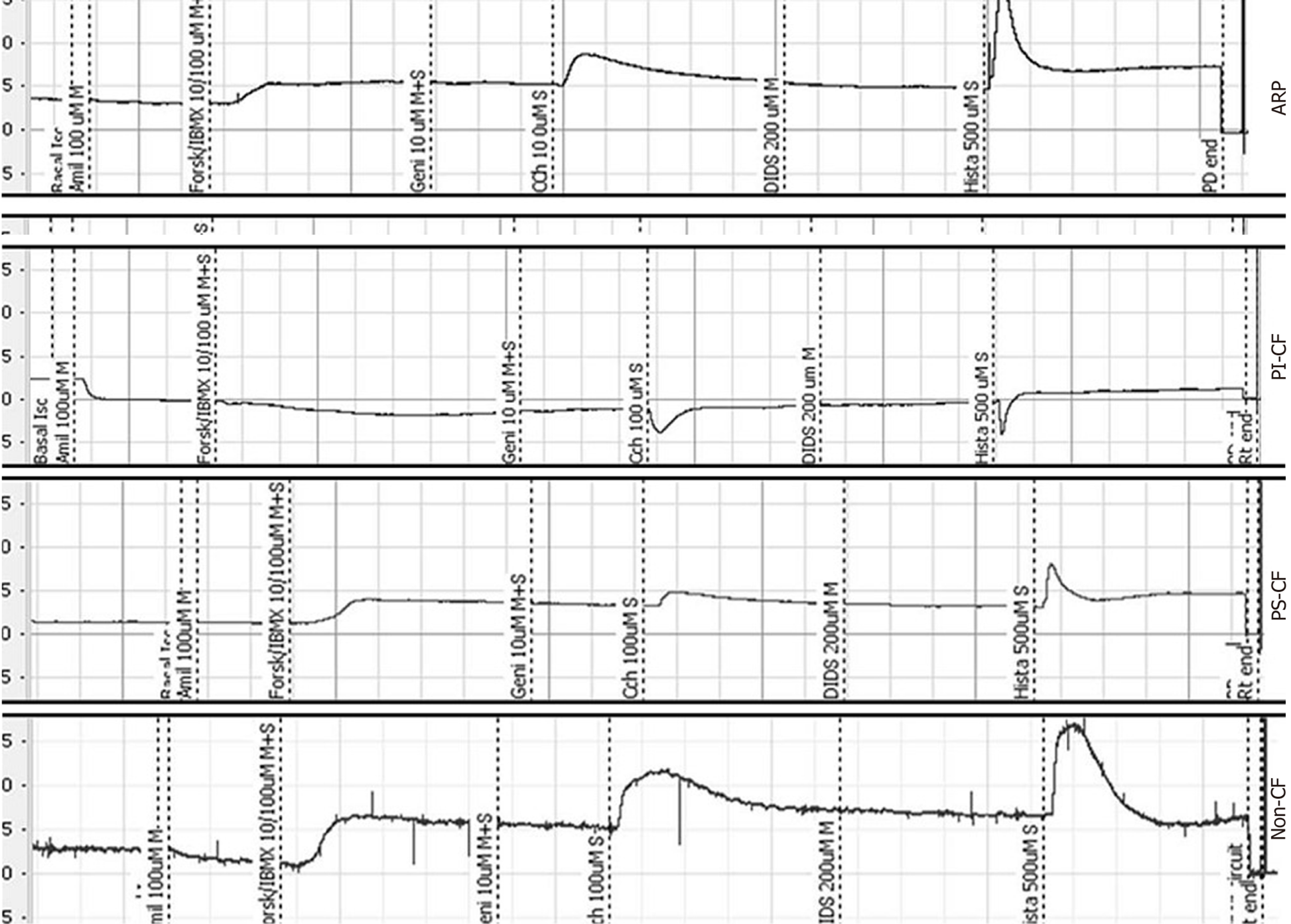
Intestinal current measurement: For the case described herein, ion transport was studied by ICM in rectal biopsies according to the ICM European CF Society Standard Operating Procedure. The transepithelial short-circuit current (Isc) across the tissue was registered in recirculating Ussing chambers, as described previously[7]. Briefly, superficial rectal biopsies were taken by forceps, mounted in Ussing chambers, and incubated with buffer solution at 37 °C. Basal potential difference, Isc, and transepithelial resistance were determined. The Isc, as a direct measure for the net movement of ions across the epithelium, was recorded for 60-75 min after adding amiloride, indomethacin, carbachol, 3-isobutyl-1-methylxanthine (IBMX), and forskolin, 4,4-diisothiocyanostilbene-2,2-disulfonic acid, and histamine to the mucosal and/or serosal side (referred to here as M and S, respectively). Responses to these compounds were detected and analyzed using PowerLab software version 7, and the outcome was calculated using the cumulative Isc response to carbachol, forskolin/IBMX and histamine[7].
Crypt isolation and organoids culture from human biopsies: Human intestinal biopsies were washed with cold complete chelation solution and incubated with 10 mmol/L EDTA for 30-60 min at 4 °C. Supernatant was harvested and EDTA was washed away. Crypts were isolated by centrifugation and embedded in Matrigel (growth factor reduced, phenol-free; BD Biosciences, Franklin Lakes, NJ, United States) and seeded (50-200 crypts per 50 μL Matrigel per well) in 24-well plates. The Matrigel was polymerized for 10 min at 37 °C and immersed in complete culture medium supplemented with 1% penicillin/streptomycin, 10 mmol/L HEPES, Glutamax, N2, B27 (all from Invitrogen, Carlsbad, CA, United States), 1 μM N-acetylcysteine (Sigma-Aldrich, St Louis, MO, United States) and growth factors of 50 ng/mL mEGF, 50% Wnt3a conditioned medium and 10% Noggin conditioned medium, 20% Rspo1 conditioned medium, 10 μmol/L nicotinamide (Sigma), 10 nmol/L gastrin (Sigma), 500 nmol/L A83-01 (Tocris Bioscience, Bristol, United Kingdom) and 10 μmol/L SB202190 (Sigma). The medium was refreshed every 2–3 d and organoids were passaged at about 1:5 ratio every 7–10 d. For the FIS assay, a standard culture medium was used, composed of Advanced DMEM-F12 (Invitrogen), 1% GlutaMAX-1 (Invitrogen), 1% HEPES (Invitrogen), 1% penicillin/streptomycin (Lonza, Basel, Switzerland) and 1% Primocin (Invitrogen).
Quantification of FIS: The procedure used was adapted slightly from the method described by Dekkers et al[9]. Human organoids at the second split were seeded on a μ-Slide 8 well chamber (80826; Ibidi, Martinsried, Germany) in 5 µL of 50% Matrigel containing about 20 organoids in 200 µL culture medium. Two days after the seeding, the organoids were incubated for 30 min with 100 µL standard culture medium containing 3 µmol/L Calcein-green (Invitrogen), and stimulated with forskolin (5 µM) for direct analysis by confocal live cell microscopy (TCS-SP5 inverted microscope; Leica, Wetzlar, Germany). For CFTR inhibition, organoids were pre-incubated (60 min) with 50 µmol/L CFTR-inh172 (Sigma). The organoid volume (xyz plane) increase, relative to t = 0 of forskolin treatment, was normalized to baseline (= 100%) and quantified using IMMARIS 7.2.1 software (Bitplane, Zurich, Switzerland). Occasionally, cell debris and unviable structures were manually excluded from the imaging analysis by use of defined settings.
Cell membrane depolarization assay: The potential-sensitive probe bis-(1,3-diethylthiobarbituric acid) trimethine oxonol (DiSBAC2(3); Life Technologies, Carlsbad, CA, United States) was used to monitor the CFTR-dependent membrane potential (Vm) changes in monocytes as previously described[10]. The ΔFt curves, computed as the difference between mean ΔFt (stimulus) and mean ΔFt (vehicle) in the last 5 min of recording, was termed “CF index” and was positive in healthy subjects and negative in CF patients, with intermediate values in healthy carriers[10].
Bubble sweat test (BST) protocol: For the drug delivery and imaging of M- and CFTR-dependent secretion (C)-sweating, injection area on the subject’s right forearm was chosen immediately before the test. An oil reservoir was placed and fixed on the arm, and the circular area (of the reservoir) was marked with three black dots of equal distance along the circumference. The measurement procedure for M- and C-sweat is described in the Supplementary Material.
In the measurement of sweat secretion for both phases, pictures were taken manually using ImageJ software. Starting from the image taken at the end of the M-phase, sweat bubbles were numbered progressively according to the following criteria[11]: (1) Bubbles must have clear and round outlines; (2) Their volumes must increase during the measurement period; and (3) The location of a C-sweat bubble must correspond to an M-sweat bubble.
Mapped glands were then superimposed on the image obtained at the end of the C-phase. Both images were saved as JPEG format and analyzed by ImageJ software that allows measurement of the diameter of each bubble and automatically calculates the volume. The average sweat rates of M- and C-sweating were finally determined by calculating the sweat volume secreted per unit of time.
A 44-year old male patient heterozygous for the nonsense CFTR mutation G542X was referred to our Center for evaluation. Denaturing Gradient Gel Electrophoresis and Reverse Dot Blot (kit INNOLiPA CFTR Deletions +6) were performed without detection of any other mutation (detection rate 98%). The status of the other family members was referred “healthy” by the patient except her sister with CFTR genotype G542X+/- who had recurrent bronchitis, normal sweat test and NPD, not available for all the tests performed for his brother.
The patient had experienced several episodes of acute pancreatitis since the age of 26 years.
The patient reported having been a smoker for 20 years (15 cigarettes per day), until 2010. During the years of smoking, he had occasional bronchitis (< 1 episode per year), sometimes requiring oral antibiotic treatment. He also declared occasional alcohol intake on the weekends, before 2000 (Table 1). His sister had recurrent bronchitis that disappeared after stopping smoking; she was a carrier of the G542X CFTR mutation.
| Year | Clinical condition |
| 1999 | Pancreatitis |
| Before 2000 | Heartburn and gastric reflux; occasional alcohol intake on the weekends |
| 1991-2010 | Smoker (15 cigarettes per day) |
| 2006 | Sinusitis (1 episode) |
| 2007-2014 | Bronchitis once a year |
Physical examination revealed positive Ewing’s sign but other examinations produced negative findings, in particular for thoracic auscultation and abdominal palpation. There was no digital clubbing. Lung function was normal.
Sweat chloride values, obtained by the Gibson and Cooke method[12], were 41-45 mEq/L. Sputum cultures were negative for Haemophilus parainfluenzae. Congenital bilateral absence of the vas deferens, which is the CF phenotype associated with the mildest impairment of CFTR function, was excluded based on absence of azoospermia[5]. Recent nasal surgery for deviation of the nasal septum and its complications of scars in both nostrils precluded the performance of NPD measurements or of nasal brushing. Occasionally (from 2000 to 2014 once or twice/year, first episode in 2000), high serum levels of amylase and lipase were detected.
X-ray examination showed modest hypertrophy of the inferior turbinates. Thoracic computed tomography scan showed evidence of colecystectomy and hypoplastic left hepatic lobe.
Genetic testing for mutations associated with pancreatitis in SPINK1 and PRSS1 genes provided negative results. Sequencing of entire coding regions of both genes was performed with diagnostic efficiency about 80%.
CFTR-related pancreatitis.
ICMs were performed on four rectal biopsies, following the European Cystic Fibrosis Society ICM SOP (http://qa.ecfs.eu/ecfs_dnwg), and showed tracings consistent with a non-CF pattern (Figure 1). In this case, the cumulative Isc value of carbachol + forskolin/IBMX + histamine, proposed by Derichs et al[7] as a diagnostic parameter, was 87.4 µA/cm², with a cut-off of 34 µA/cm² between non-CF and CF with pancreatic sufficiency (i.e., being inconsistent with the diagnosis of CF).
In order to cross-validate alternative assays using alternative primary cell samples, we tested CFTR function in monocytes. The CFTR agonists used in testing were 500 µmol/L 8-Br-cAMP (B5386; Sigma), 10 µmol/L forskolin and 100 µmol/L IBMX (I7018; Sigma), added 5 min after the start of recording. We had previously defined the CF index (CFI) according to the outcome of this assay and showed positive values in non-CF subjects, in contrast to negative values in CF patients[10]. In that study, CFTR activation was found in the ARP patient, a healthy carrier, and in a non-CF donor but not in the CF patient used as reference. In cases of ARP, the positive CFI (+44) indicates a response to CFTR stimulation within the positive range, as obtained in healthy carriers and non-CF donors (Figure 2A).
We also developed intestinal organoids from the same biopsies utilized for ICM and performed the FIS assay, as described by Dekkers et al[9] (Figure 2B). Organoids obtained from our ARP patient carrying the G542X+/- mutation, a healthy donor, and a CF patient (F508del+/+), were stimulated with 5 μM forskolin and analyzed by confocal live cell microscopy. The organoids’ volume (xyz plane) was increased relative to t = 0 of forskolin treatment and was normalized to the baseline (= 100%); volume changes were evaluated for 90 min and quantified automatically (by the Bitplane microscopy image analysis software). The normalized volume increase was 117.44 ± 5.7 (mean ± SE), which is consistent with a non-CF phenotype. Moreover, before stimulation, the ARP organoids showed a spherical appearance similar to non-CF organoids, suggesting the presence of functional CFTR.
We finally tested the patient’s CFTR function in vivo by measuring individual sweat gland cells through computation of the ratio between CFTR-dependent (C-sweat, evoked by a beta-adrenergic cocktail) and CFTR-independent (M-sweat, stimulated by methacholine) sweat secretion rates from multiple individual sweat glands. We obtained average ratios showing an approximately linear readout of CFTR function, as previously described[11,13]. The mean ratio was 0.20 in the non-CF samples and 0.10 in the healthy carrier samples but 0.00 in the CF patient, as seen in all the CF patients tested at our Center. These collective results obtained on the same day were consistent with values previously obtained at our site[13]. For our G542X+/- patient with ARP, the C/M ratio value was 0.10, overlapping our historical results of healthy carriers.
This patient was recommended to attend regularly the follow-up scheduled by the gastroenterologist and once-twice/year the Cystic Fibrosis Center in order to be monitored for possible new clinical signs, in particular those related to CFTR dysfunction.
This case report describes, for the first time, the combination of standardized assays with new, relatively simple and robust CFTR functional assays applied to several tissue types expressing CFTR; the results suggest how the combination of innovative techniques may support diagnosis at an individual level. ICM and/or NPD are suggested according to the algorithm already published[5]. Therefore a diagnostic algorithm for similar cases, in our opinion, should include ICM and (when possible) generation of intestinal organoids in order to obtain samples for supporting/confirming diagnosis and (possibly) perform theratyping. The importance of testing different tissue types lies on the tissue specificity of exon skipping, furthermore, by testing only airway tissue, we could miss to identify CFTR related pancreatitis caused by CFTR mutations selectively affecting bicarbonate transport[14]. In fact NPD test is unable to detect such selective bicarbonate impairment while ICM and 2D organoids are methods suitable for testing both anions transport. If standardized CFTR function test are inconclusive or not possible, as NPD and sweat chloride results in this patient, the other functional tests described can be proposed to confirm/exclude CFTR related pancreatitis. Recent data from the literature[11,13,15] suggest that BST can be more sensitive than sweat chloride measurements for monitoring CFTR improvement during CFTR targeted therapies, making this assay particularly suitable to this aim. Exclusion of CF and CRD is very important when other causes of pancreatitis are suspected (i.e., drug, gallstones, or sphincter of Oddi dysfunction), which may result in therapeutic approaches with limited/no effects (i.e., drug withdrawal, cholecystectomy, or endoscopic sphincterotomy respectively) in patients with recurrent pancreatitis.
For the case presented herein, the results of a number of different tests, standardized as well as experimental, excluded the diagnosis of CF. All the recently developed assays described in this study-involving the intestinal organoids, leukocytes, and individual sweat glands-provided results consistent with those of ICM which, according to the currently suggested diagnostic algorithm, was considered the only standardized CFTR functional test available for this patient[5,16]. Contraindications for NPD measurements are not rare when non-classical CF and CRD are suspected[5,16].
In our opinion, the outcomes of some of these approaches should be further evaluated in a larger number of subjects in order to collect reference values. This might help for an individualized diagnostic approach considering sensibility, specificity, costs, and feasibility. When used alone or in combination, selected on the basis of specialized centres availability and patient’s conditions, these tests might be valuable not only for diagnostic applications but also for theratyping approaches[17,18].
This case report was presented at the following conferences: 39th ECFS Conference (8-11 June 2016 in Basel, Switzerland; as “Supporting Diagnosis With a Combination of Standardized And New CFTR Functional Tests”) and at the 13th ECFS Basic Science Conference 2016 (30 March-02 April 2016 in Pisa, Italy; as “Combining Standardized and New CFTR Functional Tests for Improving Diagnosis”).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reddy DN S-Editor: Wang JL L-Editor: A E-Editor: Liu JH
| 1. | Maitra A, Kumar V, Kumar V, Abbas A, Fausto N. Diseases of infancy and childhood. Robbins and cotran pathologic basis of disease. Kumar V, Abbas A, Fausto N. Philadelphia: Elsevier Saunders 2005; 469-508. |
| 2. | Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, Freedman SD, Zielenski J, Berthiaume Y, Corey M, Schibli S, Tullis E, Durie PR. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Testoni PA. Acute recurrent pancreatitis: Etiopathogenesis, diagnosis and treatment. World J Gastroenterol. 2014;20:16891-16901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, Howenstine M, McColley SA, Rock M, Rosenfeld M, Sermet-Gaudelus I, Southern KW, Marshall BC, Sosnay PR. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181S:S4-S15.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 541] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 5. | Bombieri C, Claustres M, De Boeck K, Derichs N, Dodge J, Girodon E, Sermet I, Schwarz M, Tzetis M, Wilschanski M, Bareil C, Bilton D, Castellani C, Cuppens H, Cutting GR, Drevínek P, Farrell P, Elborn JS, Jarvi K, Kerem B, Kerem E, Knowles M, Macek M, Munck A, Radojkovic D, Seia M, Sheppard DN, Southern KW, Stuhrmann M, Tullis E, Zielenski J, Pignatti PF, Ferec C. Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros. 2011;10 Suppl 2:S86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 6. | Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest. 2004;126:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Derichs N, Sanz J, Von Kanel T, Stolpe C, Zapf A, Tümmler B, Gallati S, Ballmann M. Intestinal current measurement for diagnostic classification of patients with questionable cystic fibrosis: validation and reference data. Thorax. 2010;65:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Naehrlich L, Ballmann M, Davies J, Derichs N, Gonska T, Hjelte L, van Konigsbruggen-Rietschel S, Leal T, Melotti P, Middleton P, Tümmler B, Vermeulen F, Wilschanski M; ECFS Diagnostic Network Working Group. Nasal potential difference measurements in diagnosis of cystic fibrosis: an international survey. J Cyst Fibros. 2014;13:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 756] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 10. | Sorio C, Buffelli M, Angiari C, Ettorre M, Johansson J, Vezzalini M, Viviani L, Ricciardi M, Verzè G, Assael BM, Melotti P. Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One. 2011;6:e22212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Wine JJ, Char JE, Chen J, Cho HJ, Dunn C, Frisbee E, Joo NS, Milla C, Modlin SE, Park IH, Thomas EA, Tran KV, Verma R, Wolfe MH. In vivo readout of CFTR function: ratiometric measurement of CFTR-dependent secretion by individual, identifiable human sweat glands. PLoS One. 2013;8:e77114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | GIBSON LE, COOKE RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545-549. [PubMed] |
| 13. | Bergamini G, Tridello G, Calcaterra E, Ceri S, Tagliasacchi M, Bianchi F, Monti F, Masciadri A, Laudanna E, Peserico D, Sorio E, Esposito V, Leal T, Assael BM, Sorio C, Melotti P. Ratiometric sweat secretion optical test in cystic fibrosis, carriers and healthy subjects. J Cyst Fibros. 2018;17:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | LaRusch J, Jung J, General IJ, Lewis MD, Park HW, Brand RE, Gelrud A, Anderson MA, Banks PA, Conwell D, Lawrence C, Romagnuolo J, Baillie J, Alkaade S, Cote G, Gardner TB, Amann ST, Slivka A, Sandhu B, Aloe A, Kienholz ML, Yadav D, Barmada MM, Bahar I, Lee MG, Whitcomb DC; North American Pancreatitis Study Group. Mechanisms of CFTR functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genet. 2014;10:e1004376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Salinas DB, Peng YH, Horwich B, Wee CP, Frisbee E, Maarek JM. Image-based β-adrenergic sweat rate assay captures minimal cystic fibrosis transmembrane conductance regulator function. Pediatr Res. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J, Sinaasappel M; Diagnostic Working Group. Cystic fibrosis: terminology and diagnostic algorithms. Thorax. 2006;61:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Beekman JM, Sermet-Gaudelus I, de Boeck K, Gonska T, Derichs N, Mall MA, Mehta A, Martin U, Drumm M, Amaral MD. CFTR functional measurements in human models for diagnosis, prognosis and personalized therapy: Report on the pre-conference meeting to the 11th ECFS Basic Science Conference, Malta, 26-29 March 2014. J Cyst Fibros. 2014;13:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Char JE, Wolfe MH, Cho HJ, Park IH, Jeong JH, Frisbee E, Dunn C, Davies Z, Milla C, Moss RB, Thomas EA, Wine JJ. A little CFTR goes a long way: CFTR-dependent sweat secretion from G551D and R117H-5T cystic fibrosis subjects taking ivacaftor. PLoS One. 2014;9:e88564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |