Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3711
Peer-review started: June 4, 2019
First decision: August 1, 2019
Revised: August 30, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 26, 2019
Processing time: 181 Days and 19.7 Hours
The relationship between hyperkalaemia and metabolic acidosis is well described in the critical care setting; however, the relationship between acute respiratory acidosis and plasma potassium concentration is less well understood. In a controlled model of increasing levels of hypercarbia, we tested the hypothesis of whether increasing levels of hypercarbia are associated with changes in plasma potassium concentrations.
To determine whether increasing levels of hypercarbia are associated with changes in plasma potassium concentrations.
We performed a post-hoc study examining changes in serum potassium in 24 patients who received increased levels of hypercarbia during cardiac surgery. Arterial blood gases and plasma concentrations of potassium were measured at baseline, 3 min prior to, and then every 3 min for 15 min during the intervention of hypercarbia. The primary endpoint was the absolute change in serum K+ at 15 min compared to the baseline K+ value. The following secondary endpoints were evaluated: (1) The association between CO2 and serum K+ concentration; and (2) The correlation between plasma pH and serum K+ concentrations.
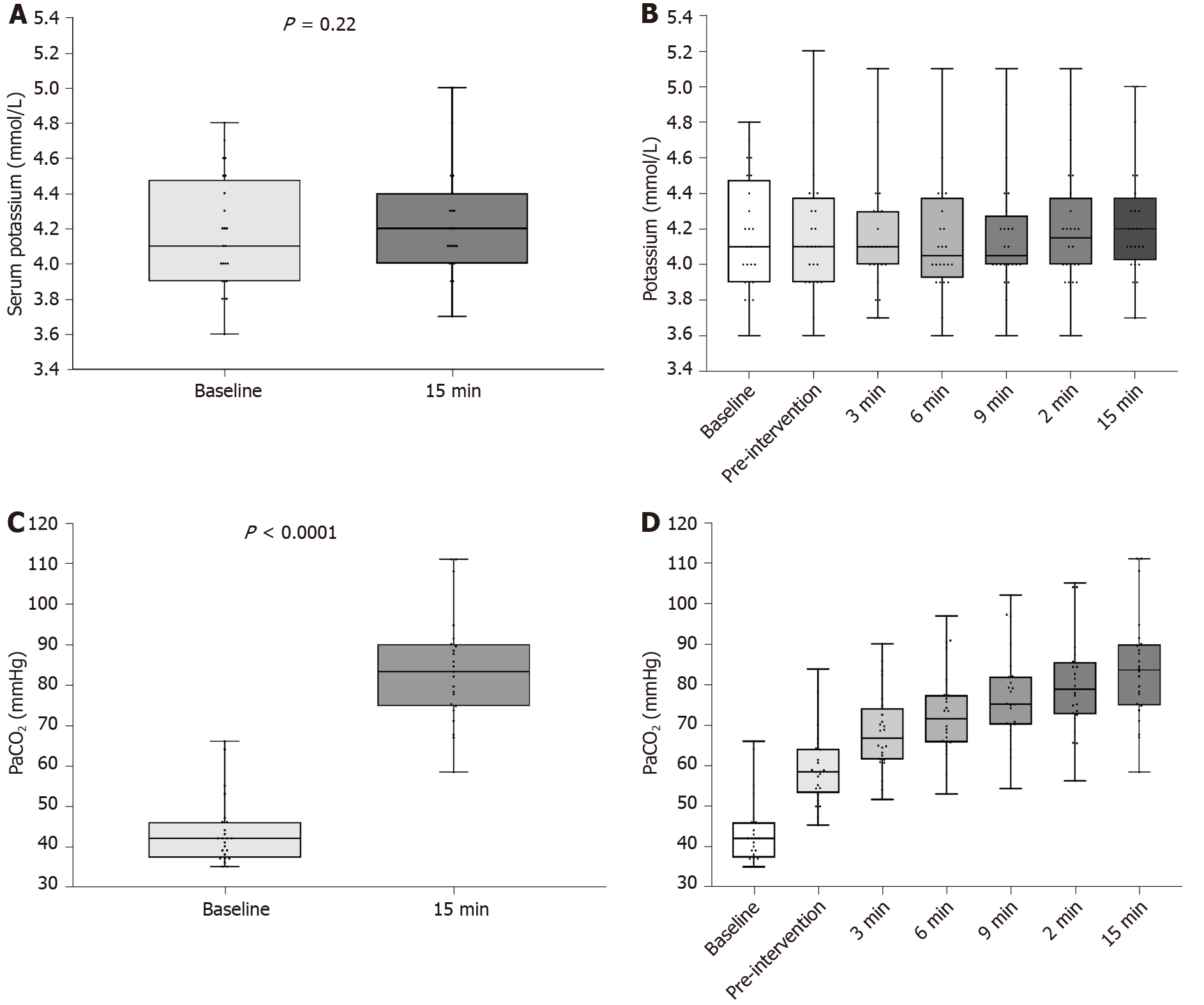
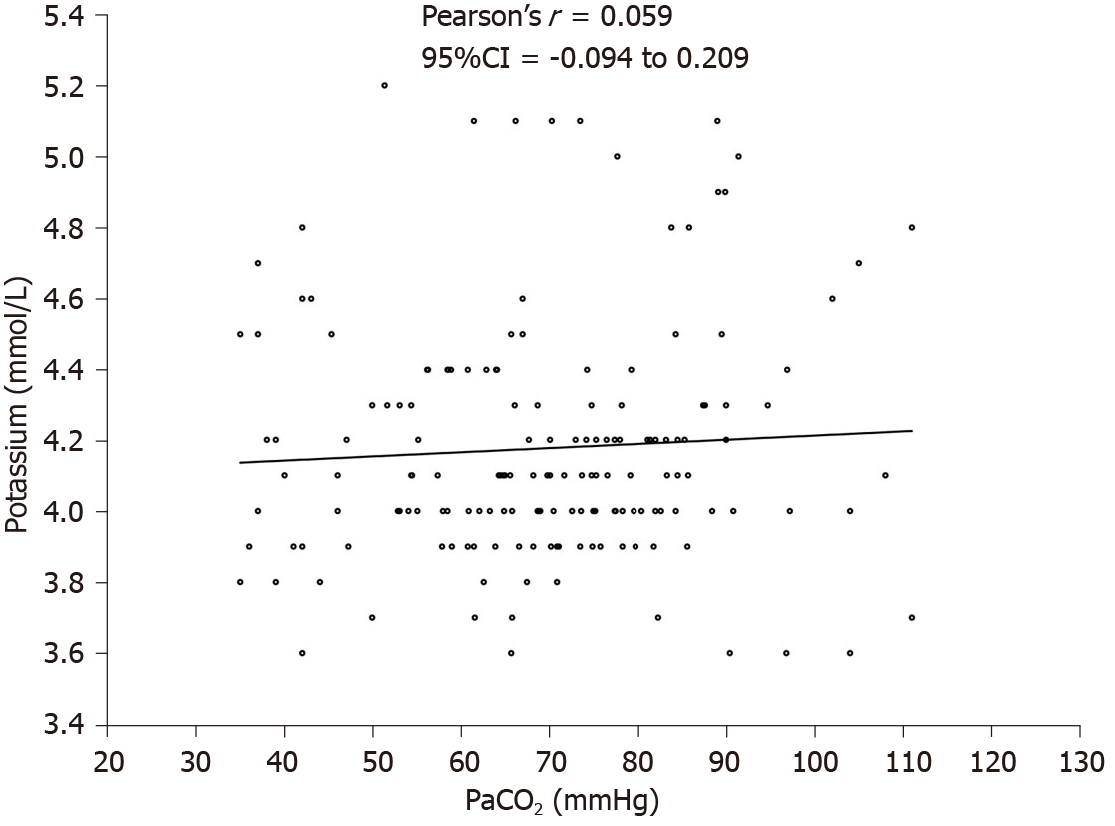
During the intervention, PaCO2 increased from 43.6 mmHg (95%CI: 40.1 to 47.1) at pre-intervention to 83.9 mmHg (95%CI: 78.0 to 89.8) at 15 min after intervention; P < 0.0001. The mean (SD) serum potassium increased from 4.16 (0.35) mmol/L at baseline to 4.28 (0.33) mmol/L at 15 min (effect size 0.09 mol/L; P = 0.22). There was no significant correlation between PaCO2 and potassium (Pearson’s coefficient 0.06; 95%CI: -0.09 to 0.21) or between pH and potassium (Pearson’s coefficient -0.07; 95%CI: -0.22 to 0.09).
Acute hypercarbia and subsequent respiratory acidaemia were not associated with hyperkalaemia in patients undergoing major surgery.
Core tip: Acute hypercarbia results in respiratory acidosis and subsequent respiratory acidaemia. Acute hypercarbia does not affect serum potassium concentrations in the setting of anaesthesia and major surgery. Respiratory acidaemia does not affect serum potassium concentrations in patients undergoing major surgery.
- Citation: Weinberg L, Russell A, Mackley L, Dunnachie C, Meyerov J, Tan C, Li M, Hu R, Karalapillai D. Relationship between acute hypercarbia and hyperkalaemia during surgery. World J Clin Cases 2019; 7(22): 3711-3717
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3711.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3711
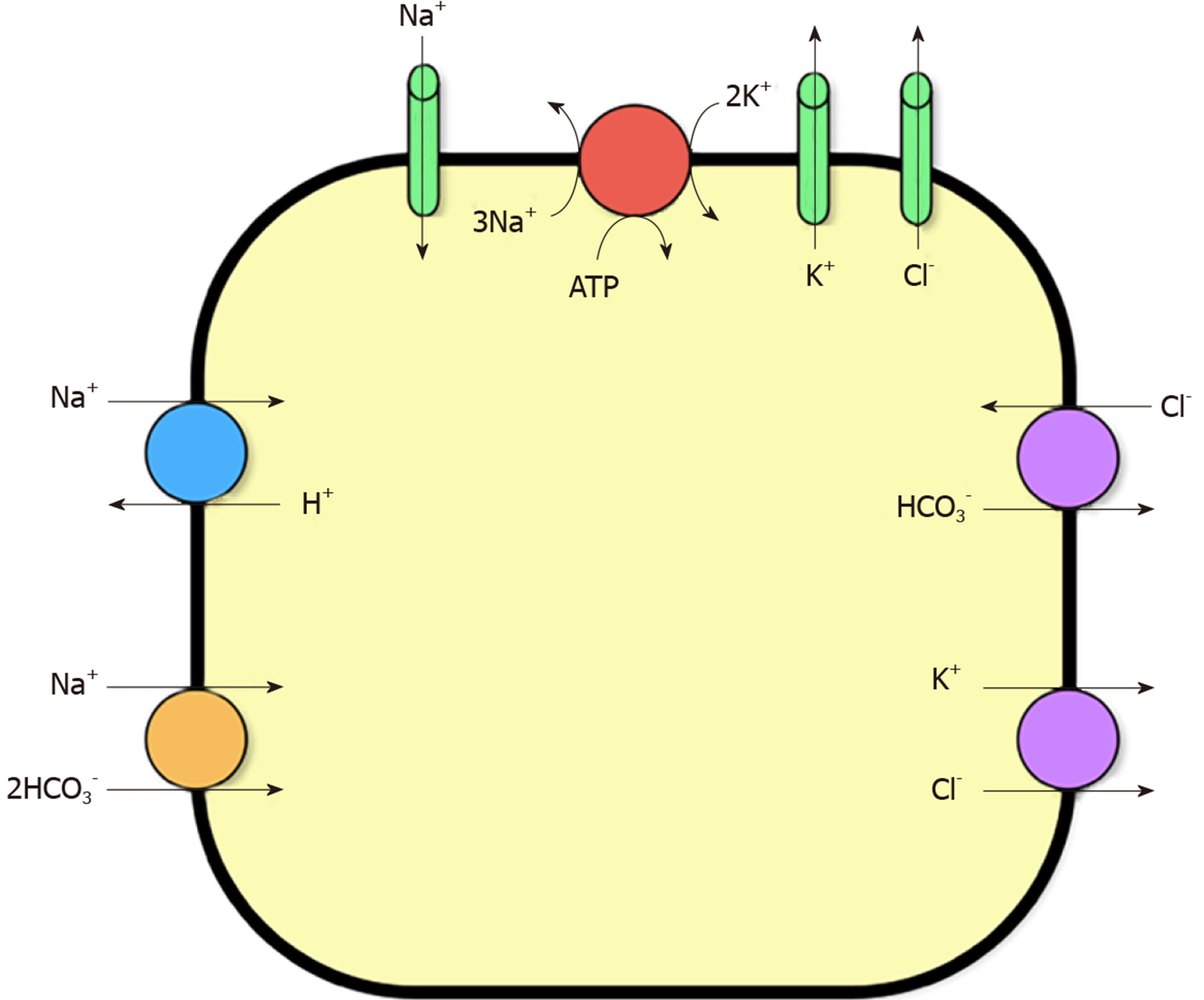
The linear correlation between arterial carbon dioxide and plasma pH is well described[1,2] with acute hypercarbia frequently used as a surrogate marker of respiratory acidosis. However, the effects of hypercarbia or respiratory acidaemia on plasma potassium levels are unknown. In the critical care context, K+ homeostasis in plasma is influenced by redistribution of K+ ions between the intra- and extracellular fluid (ECF) space, allowing concentrations to remain stable. The majority of the body’s K+ content is found in the intracellular space of skeletal muscle[3]. pH-dependent control of potassium homeostasis occurs in the distal tubule of the nephron via the H+-K+-ATPase transporter[3,4]. These channels are directly affected by acute acidosis. When plasma pH drops, K+ is released from the intracellular space and K+ movement into the intracellular space results in a shift of potassium into the ECF space. These effects are mediated by three main mechanisms[3]: Firstly, the sodium-hydrogen ion exchanger is inhibited, causing decreased sodium-K+-adenosine triphosphatase (Na+K+ATPase) activity; secondly, Na+ influx via the Na+HCO3 co-transporter is inhibited by a fall in extracellular HCO3, again decreasing Na+K+ATPase activity; finally, chloride influx via the chloride-bicarbonate ion Cl exchange is reduced, due to the fall in extracellular HCO3, promoting K+ efflux into ECF via the K+Cl- co-transporter. The key ion channels involved in K+ homeostasis in skeletal muscle cells are shown in Figure 1.
Metabolic acidosis caused by mineral acids, such as hyperchloraemic acidosis, causes a larger shift of K+ when compared to organic metabolic acidosis, such as lactic and ketoacidosis[3,4]. This is due to the organic acids entering the cell causing an intracellular acidosis. This would have the opposite effect to an extracellular acidosis on the channels shown in Figure 1, causing a net decrease in K+ efflux into the plasma[2-4]. Given that hypercarbia is ubiquitous in both the critical care and anaesthesia settings, it is imperative to better understand the relationship between acute hypercarbia and hyperkalaemia. Therefore, we hypothesised that in a model of acute hypercarbia and subsequent acidaemia, there would be a strong association between increasing arterial carbon dioxide levels and serum K+ concentrations.
We performed a post-hoc analysis utilising data from a previously published randomised controlled trial[5]. In the original single centre, randomised controlled study, adult patients over the age of 18 years, undergoing elective CABG requiring harvesting of the left internal mammary artery were included. Exclusion criteria included emergency CABG surgery, non-CABG surgery, chronic lung disease, moderate pulmonary hypertension (mean pulmonary artery pressure > 40 mmHg), super obesity (body mass index > 50 kg/m2), and American Society of Anaesthesiologists physical status V. The original trial compared the absolute change in partial pressure of arterial carbon dioxide (PaCO2) in patients who underwent either apnoeic oxygenation (complete suspension of ventilation for 15 min, n = 12) or low tidal volume ventilation (VT) (2.5 mL/kg ideal body weight, n = 12) for 15 min, during harvesting of the left internal mammary artery. As part of the trial protocol, PaCO2 was measured at baseline after induction of anaesthesia, immediately prior to the intervention, and then over a 15-min duration of apnoea or low TV ventilation, generating a model of acute hypercarbia and subsequent respiratory acidosis. Serial plasma K+ concentrations were measured; however, the association between acute hypercarbia and hyperkalaemia was not investigated.
The primary aim of this study was to evaluate the change in serum K+ at 15 min compared to the baseline K+ value. Secondary endpoints evaluated were: (1) The association between CO2 and serum K+ concentrations; and (2) Any correlation between plasma pH and serum K+ concentrations. The initial trial was approved by the Austin Health Research and Ethics Committee (HREC/15/Austin/412) and prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN: 12616000146493). All participants provided informed and written consent.
In the original study, all participants underwent a standardised anaesthesia protocol. Participants had an arterial line inserted for sequential blood gas sampling. After induction of anaesthesia and tracheal intubation, total intravenous anaesthesia with propofol was established using a target-controlled technique, ensuring adequate depth of anaesthesia by maintaining a bispectral index value of 40-60. Deep neuromuscular blockade (train of four count of ≤ 2) was maintained throughout the study and transoesophageal echocardiography (TOE) was utilised to monitor cardiac function in real time. Insulin or bicarbonate was not administered at any time point throughout the study period. Baseline potassium and CO2 values were sampled 10 min after induction of anaesthesia. To ensure similar baseline CO2 values prior to initiation of the 15-min intervention period, all participants received low VT ventilation (2.5 mL/kg ideal body weight) at a respiratory rate of 20 for 3 min prior to commencing apnoeic oxygenation or low tidal volume ventilation. Throughout this time, 100% oxygen at 10 L/min was delivered via the cuffed endotracheal tube and no positive end-expiratory pressure was given. Participants randomized to apnoeic oxygenation subsequently had total suspension of ventilation with 100% oxygen at 10 L/min delivered through a cuffed endotracheal tube. Participants randomized to low VT ventilation continued to receive a tidal volume of 2.5 mL/kg ideal body weight at the same baseline settings. Each group received the allocated ventilation technique for 15 min during harvesting of the left internal mammary artery. No other intravenous fluids were administered during the 15-min study period. The study was ceased, and normal ventilation initiated if any of the following occurred: PaO2 < 95 mmHg; SpO2 <93%; pH < 7.1; decrease in right or left cardiac contractility on TOE; malignant arrhythmias/ST segment changes; and if the surgeon or attending anaesthetist requested termination of the protocol.
Arterial blood gas sampling was performed at the following time points: Baseline (10 min after induction of anaesthesia); 3 min prior to initiation of the intervention; then at 3, 6, 9, 12 and 15-min after the allocated intervention. Measurement of pH, CO2 and plasma potassium levels in arterial blood were completed using an ABL 800 Blood Gas Analyser (Radiometer, Copenhagen, Denmark) with a fully automated micromode eliminating the risk of user-induced bias or loss of accuracy with very small samples. Other data collected included baseline patient characteristics such as age, gender, weight, height, EuroScore II, indications for surgery, routine haematological and biochemical function, cardiac structure and function, and respiratory function.
The statistical analysis was performed by a biomedical statistician. Continuous data was tested for normality and measures of central tendency were compared as means and SD using the Student t-test for normally distributed variables; and as medians with interquartile range using the Mann-Whitney U test for non-parametric variables. A two-tailed P value less than 0.05 was considered statistically significant. Categorical variables were compared using Tukey’s multiple comparisons test. Repeated measures ANOVA was used to compare means in potassium and CO2 values from baseline to 15 min. Corresponding associations are summarized as appropriate effect size with 95%CIs. Statistical analysis was performed using PRISM 7.03 GraphPad software (La Jolla, CA, United States). This study is reported following the STROBE statement checklist for observational studies.
Twenty-four participants were included. There were no missing data and no violations in the study protocol. The mean (SD) age of the participants was 63.1 (12.1) years. Twenty-one (88%) participants were male. The mean (SD) body mass index was 29.8 (5.5) mg/m2. The ASA Physical Status was 3 in 11 (45.8%) participants, and 4 (54.2%) in 13 participants. During the intervention, mean (SD) PaCO2 increased from 43.58 (8.3) mmHg at pre-intervention to 83.90 (13.6) 15 min post-intervention (effect size 40.1 mmHg; P < 0.0001) (Figure 2C). PaCO2 trends with time are presented graphically in Figure 2D. The mean (SD) serum bicarbonate increased from 26.8 (2.0) mmol/L at baseline to 28.2 (1.9) mmol/L at 15 min; P = 0.02. There were no significant changes in other electrolytes including plasma glucose concentrations.
Mean (SD) serum potassium increased from 4.16 (0.35) mmol/L at baseline to 4.28 (0.33) mmol/L at 15 min (effect size 0.09 mol/L; P = 0.22) (Figure 2A). There were no changes in mean potassium values over the study period (repeated measures ANOVA, P = 0.95) (Figure 2B). There was no significant correlation between PaCO2 and potassium; Pearson’s coefficient 0.06 (95%CI: -0.09 to 0.21) (Figure 3). Similarly, there was no significant correlation between pH and potassium (Pearson’s coefficient -0.07; 95%CI: -0.22 to 0.09).
In a controlled model of increasing hypercarbia, we found no significant correlation between increasing arterial carbon dioxide and serum potassium concentration. Our findings contrast with those reported in models of metabolic acidosis, where there is a stronger relationship between metabolic acidaemia and hyperkalaemia. This finding may reflect an increase in extracellular HCO3 in respiratory acidosis[3,4], a finding also observed in our study. Several studies have previously examined the relationship between acute respiratory acidosis and serum potassium with some conflicting findings. A 1978 prospective trial[1] concluded that acute respiratory acidosis during anaesthesia was associated with increased plasma potassium concentrations. However, these findings should be interpreted with caution due to several important methodological limitations. First, blood glucose concentrations were not controlled, with pre-operative values differing between treatment arms, whilst 5% glucose fluid therapy was allowed to be administered intraoperatively. It is well understood that glucose, together with its influence on insulin, plays a contributory role in regulating potassium distribution into and out of cells[3,6]. Therefore, any change in potassium concentration seen in this study cannot be attributed simply to PaCO2, but instead reflects a combination of the effects of PaCO2 and glucose/insulin. Furthermore, the correlation between PaCO2 and potassium was only analysed in one treatment arm. Moreover, in patients with extreme changes in H+, no tendency for altered potassium was apparent. Additionally, in contrast to the present study, anaesthetic protocols were not standardised, with normocapnic patients receiving up to 150% of the fentanyl administered to hypercapnic patients. Increased opioid dosing may have introduced another source of bias as potassium distribution is affected by catecholamine activity, which is in turn influenced by opioid administration[7].
A more recent prospective randomised controlled trial by Natalini et al[8] examined plasma potassium in 17 normokalaemic anaesthetised patients who underwent either spontaneous-assisted ventilation or intermittent negative-pressure ventilation during interventional rigid bronchoscopy. Similar to our findings, the authors observed no association between increased extracellular H+ and plasma potassium concentrations. Of note, this study compared the preoperative H+ and K+ levels with values obtained after 20 min of anaesthesia, which was similar to the 15 min total intervention time that was used in our trial. It has been suggested that the majority (65%) of any change in potassium occurs in the first 30 min of acute respiratory acidosis[1] and both these studies fall within this time frame. However, our study differed in regard to surgical and anaesthetic procedures, patient populations and analysis. In particular, patients with coronary artery disease were specifically excluded in the Natalini et al[8] study, which is an important comorbidity in modern anaesthetic practice. In addition, only the relationship between potassium and H+ was discussed in the Natalini et al[8] paper, whereas our study also looked at the influence of PaCO2 on potassium. This is important as PaCO2 is commonly manipulated during anaesthesia.
It is possible that our study underestimated any change in potassium concentration, as potassium levels were measured only during the 15-min hypercarbic period, rather than after a longer period of time. Thus, our study may not adequately reflect the spectrum of changes due to renal handling of potassium, a control mechanism that takes longer to manifest. However, further research evaluating the effects of prolonged periods of hypercarbia (particularly at profound levels of hypercarbia) in a human model under general anaesthesia and controlled ventilation may be considered unethical due to the possible effects of prolonged hypercarbia and acidaemia on myocardial dysfunction, arrhythmias, pulmonary hypertension, and raised intracranial pressure. It has been suggested that general anaesthesia may marginally reduce plasma potassium concentration[9], so these findings may also not apply to awake participants.
In conclusion, our post-hoc analysis demonstrated no significant association between acute hypercarbia and serum potassium in the setting of anaesthesia and major surgery. This corroborates the most recent prospective randomised controlled trial in this area and suggests that acute hypercarbia can be tolerated in critically ill patients and those undergoing anaesthesia, without significant risk of hyperkalaemia and its associated adverse outcomes.
The relationship between acute respiratory acidosis and plasma potassium concentration is poorly understood.
In a controlled model of increasing levels of hypercarbia, the authors tested the hypothesis of whether increasing levels of hypercarbia are associated with changes in plasma potassium concentrations.
In this study, the authors aimed to determine whether increasing levels of hypercarbia are associated with changes in plasma potassium concentrations.
The authors performed a post-hoc study examining changes in serum potassium in 24 patients who received increased levels of hypercarbia during cardiac surgery.
The authors found no significant correlation between increasing arterial carbon dioxide and serum potassium concentration.
Those findings suggest that acute hypercarbia can be tolerated in critically ill patients and those undergoing anaesthesia, without significant risk of hyperkalaemia and its associated adverse outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng JB S-Editor: Wang JL L-Editor: Webster JR E-Editor: Liu JH
| 1. | Finsterer U, Lühr HG, Wirth AE. Effects of acute hypercapnia and hypocapnia on plasma and red cell potassium, blood lactate and base excess in man during anesthesia. Acta Anaesthesiol Scand. 1978;22:353-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Adrogué HJ, Madias NE. Management of life-threatening acid-base disorders. First of two parts. N Engl J Med. 1998;338:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Adrogué HJ, Madias NE. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981;71:456-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 196] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Machan L, Churilov L, Hu R, Peyton P, Tan C, Pillai P, Ellard L, Harley I, Story D, Hayward P, Matalanis G, Roubos N, Seevanayagam S, Weinberg L. Apneic Oxygenation Versus Low-Tidal-Volume Ventilation in Anesthetized Cardiac Surgical Patients: A Prospective, Single-Center, Randomized Controlled Trial. J Cardiothorac Vasc Anesth. 2017;31:2000-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hishida A, Suzuki H, Ohishi K, Honda N. Roles of hormones in plasma potassium alteration in acute respiratory acidosis in dogs. Miner Electrolyte Metab. 1992;18:56-60. [PubMed] |
| 7. | DeFronzo RA, Sherwin RS, Dillingham M, Hendler R, Tamborlane WV, Felig P. Influence of basal insulin and glucagon secretion on potassium and sodium metabolism. Studies with somatostatin in normal dogs and in normal and diabetic human beings. J Clin Invest. 1978;61:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Natalini G, Seramondi V, Fassini P, Foccoli P, Toninelli C, Cavaliere S, Candiani A. Acute respiratory acidosis does not increase plasma potassium in normokalaemic anaesthetized patients. A controlled randomized trial. Eur J Anaesthesiol. 2001;18:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Westfall TC, Westfall DP, Brunton L, Chabner B, Knollman B. Goodman and Gilman's Pharmacological Basis of Therapeutics. Brunton L, Chabner B, Knollman B. Neurotransmission: The autonomic and somatic motor nervous systems. 12th edition. New York: McGraw Hill 2011; 171-218. |