Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3662
Peer-review started: April 19, 2019
First decision: September 9, 2019
Revised: September 24, 2019
Accepted: October 5, 2019
Article in press: October 5, 2019
Published online: November 6, 2019
Processing time: 206 Days and 14 Hours
Hypoparathyroidism with basal ganglia calcification is clinically rare. Here, we report a case of Fahr’s syndrome due to hypoparathyroidism and review the literature in terms of etiology, clinical manifestation, diagnosis, and treatment.
A 62-year-old man experienced repeated twitching of both hands in recent 10 years. On July 28, 2017, the patient was admitted to our hospital due to slow response and speech difficulties. On medical examinations, he had a positive Chvostek sign, while no Albright’s hereditary osteodystrophy signs or history of neck surgery or radiation, and his family members had no similar medical history. Laboratory examinations revealed hypocalcemia, hyperphosphatemia, and low parathyroid hormone (PTH) levels. Computed tomography revealed basal ganglia calcification. Based on these investigations, a diagnosis of Fahr’s syndrome due to hypoparathyroidism was suggested. After receiving intravenous calcium gluconate to relieve symptoms, the patient continued to take oral calcium carbonate and calcitriol for treatment.
The possibility of hypoparathyroidism should be considered in patients with chronic hypocalcemia, recurrent tetany, and even neuropsychiatric symptoms. Hypoparathyroidism is a common cause of basal ganglia calcification. Therefore, it is recommended that blood calcium, phosphorus, and PTH levels should be measured in all individuals with basal ganglia calcification to exclude hypoparathyroidism.
Core tip: The clinical manifestations of hypoparathyroidism are complex and varied. Fahr's syndrome is diagnosed when basal ganglia calcification occurs. Fahr's syndrome is clinically rare. Here, we report a case of Fahr’s syndrome due to hypoparathyroidism and review the literature from etiology, clinical manifestation, diagnosis, and treatment. On the one hand, this case reflects the importance of standardized treatment and follow-up in patients with hypoparathyroidism. On the other hand, it is recommended that clinicians first consider the possibility of hypoparathyroidism when looking for the cause of basal ganglia calcification.
- Citation: Zhou YY, Yang Y, Qiu HM. Hypoparathyroidism with Fahr’s syndrome: A case report and review of the literature. World J Clin Cases 2019; 7(21): 3662-3670
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3662.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3662
Hypoparathyroidism refers to an endocrine disorder caused by insufficient secretion and/or effect of parathyroid hormone (PTH)[1]. Its clinical manifestations are varied; however, the main manifestation is increased excitability of nerves and muscles caused by decreased blood calcium. Fahr’s syndrome is diagnosed when hypoparathyroidism is combined with basal ganglia calcification[2]. Although Fahr’s syndrome is not clinically frequent, hypoparathyroidism is the most common cause[3].
On July 28, 2017, a 62-year-old male farmer was admitted to the Emergency Department of People’s Hospital of Yuxi City (China) due to slow response and speech difficulties for half a day.
The patient has experienced repeated twitching of both hands in recent 10 years. He had been diagnosed as “hypocalcemia” in a primary hospital, and the symptoms can be alleviated after "calcium supplementation". However, the patient had poor compliance and did not regularly take calcium supplements. The symptoms mentioned above were repeated.
The patient had cataract, while no history of neck surgery or neck radiation.
He had no history of smoking or drinking. His family members had no similar medical history.
His vital signs were as follows: Blood pressure was 130/80 mmHg, pulse rate was 70 beat per minutes, respiratory rate was 20 breaths/min, and body temperature was 36.4 °C. His consciousness was clear. Neurological examination revealed a positive Chvostek sign, while no Albright’s hereditary osteodystrophy (AHO) signs, and cranial nerve abnormalities were not observed.
The laboratory examinations are shown in Table 1, Coagulation function and glucose were within normal limits. There were no significant changes in full blood count or blood gas analysis. Electrolyte analysis revealed hypocalcemia and hyperphosphatemia: Total calcium, 1.28 mmol/L (normal range: 2.04-2.39 mmol/L); free calcium, 0.64 mmol/L (normal range: 1.00-1.25 mmol/L); phosphorus, 2.08 mmol/L (normal range: 0.87-1.45 mmol/L).
| Value | Normal range | |
| Liver function | ||
| TBIL | 26.8 | ≤ 21.0 µmol/L |
| DBIL | 10 | 0.0-6.8 µmol/L |
| IBIL | 16.8 | 0.5-10.5 µmol/L |
| TP | 63.1 | 65.0-85.0 g/L |
| ALB | 40.8 | 40-55 g/L |
| AST | 34 | 15-40 IU/L |
| ALT | 42 | 9-50 IU/L |
| ALP | 83 | 45-125 IU/L |
| Thyroid function | ||
| FT3 | 2.67 | 3.22-6.47 pmol/L |
| FT4 | 20.51 | 10.18-21.36 pmol/L |
| T3 | 0.43 | 0.73-1.91 µg/L |
| T4 | 68 | 45-135 µg/L |
| TSH | 0.743 | 0.3-4.44 mIU/L |
| a-Tg | 6.06 | 0.0-100.0 IU/mL |
| Tg | 5.68 | 0.0-70.0 µg/L |
| a-TPO | 3.31 | 0.0-16.0 IU/ml |
| Coagulation function | ||
| PT | 13.7 | 11.0-14.5 s |
| aPTT | 35.6 | 26.0-42.0 s |
| TT | 16.4 | 14.0-21.0 s |
| FIB | 4.88 | 2.0-4.0 g/L |
| Blood gas analysis | ||
| pH | 7.46 | 7.35-7.45 |
| PO2 | 91.2 | 85.0-105.0 mmHg |
| PCO2 | 33.6 | 35.0-45.0 mmHg |
| HCO3- | 23.6 | 22.0-29.0 mmol/L |
| Pituitary hormone | ||
| FSH | 14.98 | 1.3-11.8 IU/L |
| LH | 12.76 | 2.8-6.8 IU/L |
| PRL | 13.25 | 4.1-18.5 µg/L |
| Sex hormone | ||
| E2Gen | 39.3 | 0.0-44.5 ng/L |
| PROG | 0.58 | 0.0-0.61 g/L |
| TEST | 1.38 | 1.95-8.95 µg/L |
| Blood cell count | ||
| WBC | 10.06 × 109 | 3.5-9.5 × 109/L |
| RBC | 4.99 × 1012 | 4.3-5.8 × 1012/L |
| PLT | 206 × 109 | 125.0-350.0 × 109/L |
| Others | ||
| PTH | 2.46 | 6.0-80.0 ng/L |
| CT | 6.9 | 0.0-18.0 ng/L |
| 25OHD | 85.75 | 76.0–250.0 nmol/L |
| 24 h urinary calcium | 4.07 | 2.7-7.5 mmol/24 h |
| 24 h urine phosphorus | 3.63 | 12.9-42.0 mmol/24 h |
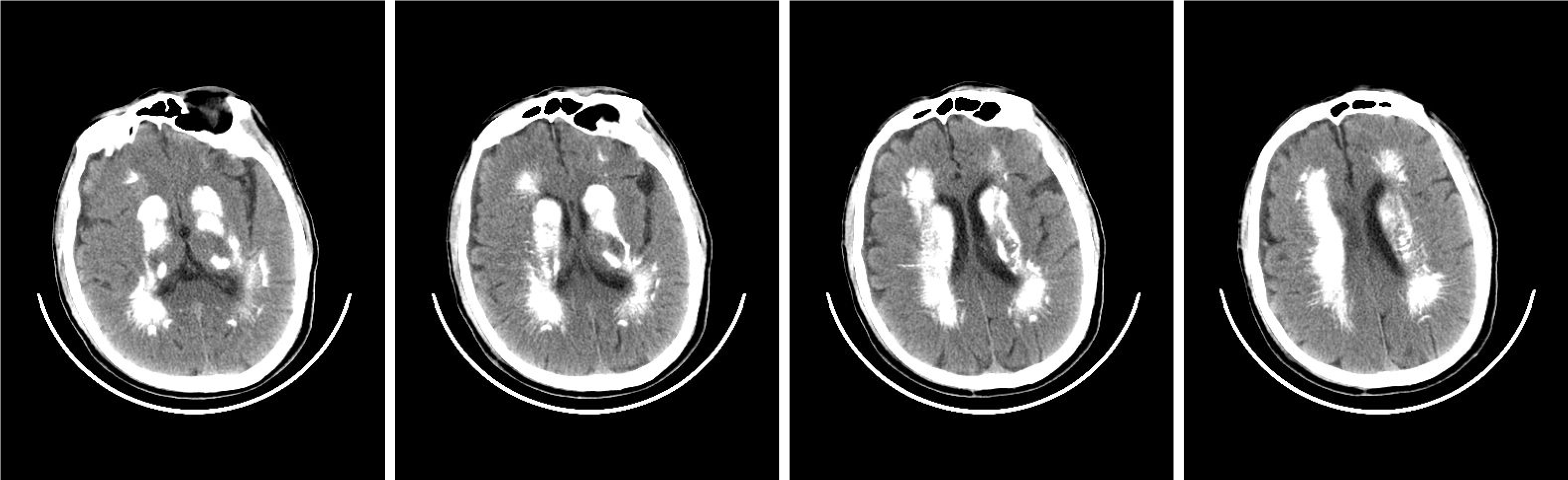
Brain, chest, and abdomen computed tomography showed: (1) Multiple calcifications in the dentate nucleus and basal ganglia of bilateral cerebellum hemispheres (Figure 1); (2) Inflammation in bilateral lower lobes of the lungs; (3) Pulmonary balloon shadow in bilateral lower lobes near the pleura; (4) A small amount of pleural effusion in the right pleura; (5) Right renal cyst; and (6) No abnormality was found in plain scans of the liver, gallbladder, pancreas, spleen, left kidney, and lower abdomen.
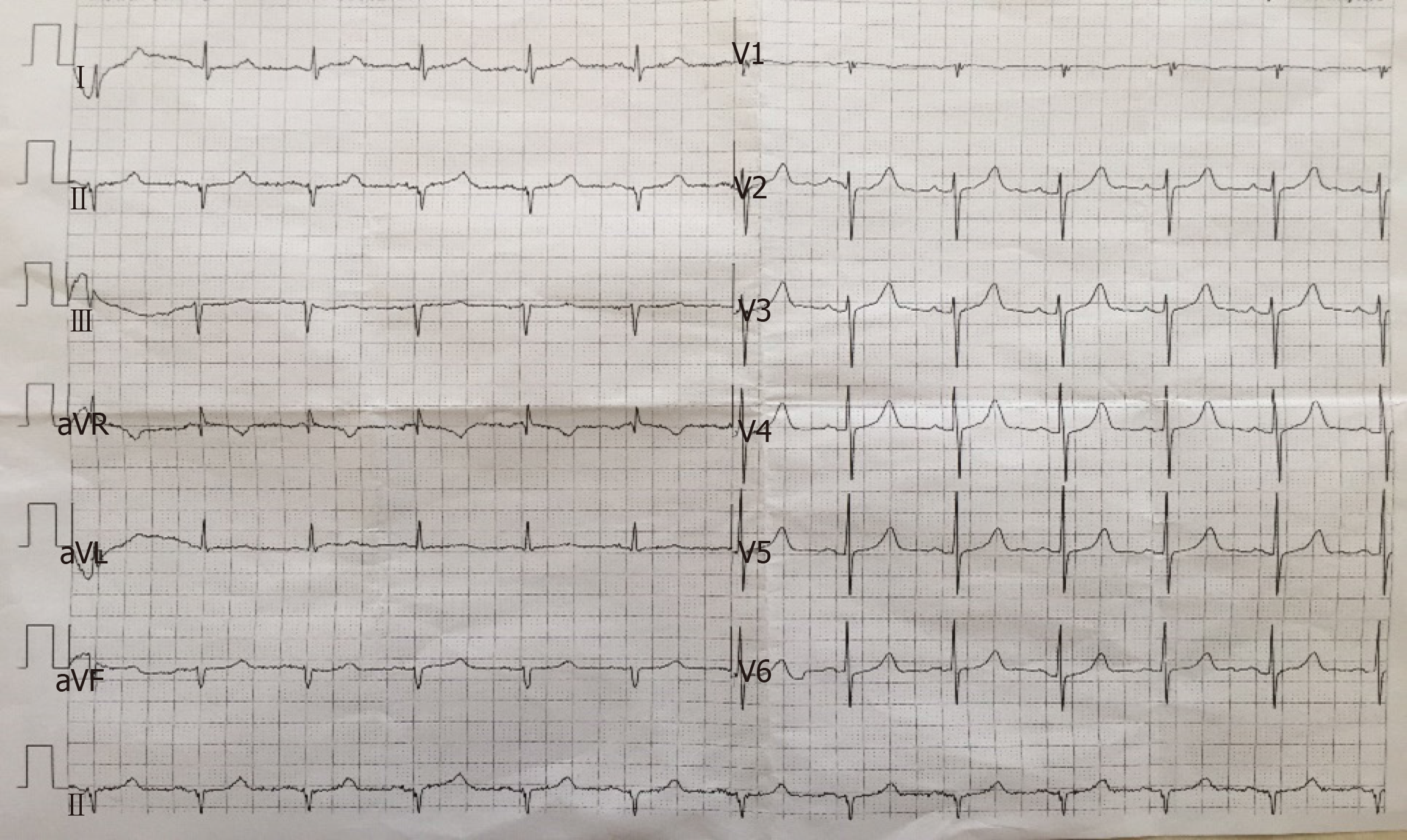
Combined with hypocalcemia, hyperphosphatemia, and basal ganglia calcification, suspected hypoparathyroidism was preliminarily diagnosed. The patient was admitted to the Department of Endocrinology for further diagnosis and treatment. The relevant auxiliary examinations are shown in Table 1. Regulatory hormones involved in the metabolism of calcium and phosphorus had changed: PTH, 2.46 ng/L (normal range: 6.0-80.0 ng/L); calcitonin (CT), 6.9 ng/L (normal range: 0.0-18.0 ng/L); 25-hydroxy (OH) vitamin D, 85.75 nmol/L (normal range: 76.0-250.0nmol/L). Thyroid function test revealed low triiodothyronine syndrome: free triiodothyronine and triiodothyronine were lower than normal ranges. The 24 h urine phosphorus was decreased to 3.63 mmol/24 h (normal range: 12.9-42.0 mmol/24 h). There was no remarkable changes in liver function or pituitary hormones. The renal function, sex hormones, cortisol rhythm, tumor markers, and antinuclear antibody spectrum were within the normal ranges. Electrocardiography (ECG) revealed (Figure 2): (1) Sinus rhythm; (2) ECG axis shifting -61° to the left; and (3) QS type in the II, III, and avF leads. Thyroid sonography revealed no abnormalities in bilateral thyroid glands and their periphery. Electroencephalogram and echocardiography results were not available because the patient refused.
Fahr’s syndrome due to hypoparathyroidism was taken as a final diagnosis into account.
Combined with the clinical manifestations and laboratory examinations of the patient, it was revealed that there was acute hypocalcemia. Calcium gluconate (10%; 10 mL) was slowly intravenously injected for treatment. Because of repeated symptoms, the patient was given continuous intravenous drip of calcium (10% calcium gluconate [100 mL] diluted to 5% glucose solution [1000 mL]). Acute symptoms were relieved after 3 d, and calcium carbonate (600 mg calcium carbonate was administered 3 times/d) and calcitriol (0.25 µg calcitriol was administered twice/d) were given routinely. Blood electrolyte changes during treatment are shown in Table 2. Due to the patient’s economic constraints, the patient required to be discharged automatically after 11 days of hospitalization.
| Date | Potassium | Sodium | Chlorine | Total calcium | Free calcium | Phosphorus | Magnesium | Lactic acid |
| 7.28 | 3.39 | 138 | 100 | 1.41 | 0.70 | 2.07 | 0.72 | 1.4 |
| 7.29 (06:30) | 3.74 | 136 | 100 | 1.79 | 0.9 | 2.21 | 0.72 | 1.7 |
| 7.29 (15:00) | 3.87 | 138 | 100 | 1.83 | 0.92 | 2.05 | 0.73 | 2.2 |
| 7.30 (06:00) | 3.88 | 136 | 97 | 1.68 | 0.84 | 1.92 | 0.75 | 2.2 |
| 7.30 (16:00) | 4.02 | 139 | 101 | 2.13 | 1.06 | 1.96 | 0.76 | 2.5 |
| 7.31 | 4.08 | 135 | 99 | 1.87 | 0.94 | 1.82 | 0.81 | 2.2 |
| 8.1 | 4.01 | 137 | 97 | 1.87 | 0.94 | 1.44 | 0.81 | 2.9 |
| 8.2 | 3.52 | 139 | 99 | 1.76 | 0.88 | 1.73 | 0.82 | 1.6 |
| 8.3 | 3.94 | 137 | 96 | 1.87 | 0.94 | 1.61 | 0.91 | 2.0 |
| 8.7 | 3.94 | 135 | 96 | 1.91 | 0.95 | 1.87 | 0.89 | 2.4 |
The patients did not attend follow-up visits regularly at our hospital, thus the follow-up data could not be obtained.
Hypoparathyroidism is a clinical syndrome caused by insufficient secretion and/or influence of PTH. Its clinical characteristics are mainly manifested as hypocalcemia, hyperphosphatemia, increased neuromuscular excitability as well as heterotopic calcification of soft tissues[1]. It is noteworthy that the clinical syndrome of hypocalcemia, hyperphosphatemia, and hyper PTH are called pseudo-hypoparathyroidism (PHP) because of the resistance of target organs (e.g., the kidneys and bone) to PTH. Some patients have been reported to have special physical deformities, such as short stature, obesity, round face, short neck, short finger (toe), and even mental retardation, i.e., typical AHO[1,4]. An epidemiological survey conducted in Japan showed that 58% of PHP patients were female[5]. A patient who has a singular sign of AHO while lacks a corresponding biochemical and metabolic abnormality is regarded to have pseudo-PHP[1].
The causes of hypoparathyroidism are varied. Neck surgery is the most frequent cause of hypoparathyroidism, accounting for about 75%[1]. Second, autoimmune diseases and genetic factors can affect parathyroid glands alone or simultaneously influence several other endocrine glands[6-8]. Gene detection is an important auxiliary diagnostic method. For example, type 1 autoimmune polyglandular syndrome, in addition to hypoparathyroidism, is also associated with Edison’s disease, candidiasis, malignant anemia, type 1 diabetes mellitus, primary hypothyroidism, autoimmune thyroid disease and so on[9]. Moreover, abnormal magnesium metabolism, both hypermagnesemia and severe hypomagnesemia, could inhibit the secretion and function of PTH[6,10]. If factors, such as surgery, inheritance, and abnormal magnesium metabolism are excluded, rare causes, involving invasive diseases, tumor metastasis, and ionizing radiation, should be considered[6].
In this case, the clinical manifestation of the patient was recurrent tetany in the past 10 years, and his personality gradually became isolated. The patient visited hospital because of slow reaction and speech difficulties. He had a positive Chvostek sign, while no AHO signs; he had no history of neck surgery or radiation; however, a history of cataract (suggesting ectodermal malnutrition) was found. Laboratory tests showed that total blood calcium and free calcium were significantly lower than normal, blood phosphorus was elevated, urine phosphorus was reduced, blood magnesium was normal, PTH was lower than normal, and bone turnover index was normal. There was no evidence of hypocalcemia caused by vitamin D deficiency, liver and kidney dysfunction, alkalosis, malnutrition, etc. No evidence of autoimmune diseases (e.g., hypofunction of adrenal cortex, autoimmune-related hypothyroidism, and diabetes) or tumors was noted as well. However, genetic testing could not be undertaken due to the limitations, thus genetic factors could not be excluded. Therefore, the etiology of hypoparathyroidism was undetermined. The patient’s brain computed tomography showed manifestations in the basal ganglia, simultaneously suggesting the diagnosis of Fahr’s syndrome.
Basal ganglia calcification, also known as Fahr’s syndrome or Fahr’s disease, is a neurological disorder, in which there are calcium deposits abnormally in areas of the brain that control motor activity, leading to neuropsychiatric symptoms[11]. However, it should be clearly pointed out that Fahr’s syndrome is not exactly Fahr’s disease, although they have similar clinical manifestations, and there are obvious differences in etiology, prognosis, and treatment. Fahr’s syndrome is often associated with other diseases, and parathyroid dysfunction (especially hypoparathyroidism) is the most common cause of Fahr’s syndrome[3,12]. With the improvement of the means of diagnosis, cases of Fahr’s syndrome caused by hypoparathyroidism had gradually increased in recent years. Most patients had neuropsychiatric symptoms or motor disorder, including seizure[13], early onset demensia[14], chorea[15], etc. Furthermore, some rare complications have also been reported, such as cerebral hemorrhage[16] and thoracic ossification of the posterior longitudinal ligament[17]. In addition to hypoparathyroidism, Fahr’s syndrome can also be observed in neuroferritinopathy, Kenny-Caffey syndrome type 1, intrauterine or perinatal infection (e.g., toxoplasma gondii, rubella), tuberous sclerosis complex, brucella infection, etc[2,18]. Fahr’s disease refers to familial idiopathic basal ganglia calcification, which is a rare hereditary neurodegenerative disease. It has the following characteristics: Autosomal dominant inheritance; age of onset is concentrated in 40-60 years old; large, progressive, bilateral symmetrical calcification of the basal ganglia; exclusive of infection, trauma, or poisoning; exclusive of mitochondrial or metabolic diseases or other systemic diseases[2,18]. For the past years, some mutations in genes that are related to Fahr’s disease had been identified, including SLC20A2, PDGFRB, PDGFB, and XPR1, together with novel mutations in the myogenic regulating glycosylase gene[19].
Fahr’s syndrome and Fahr's disease share similar clinical manifestations, mainly neurological signs (e.g., loss of consciousness, tetany, and epilepsy)[3], motor disorder (e.g., clumsiness, involuntary movement, and muscle cramping)[20,21], as well as neuropsychiatric features (e.g., mild difficulty with concentration and memory, changes in personality or behaviors, psychosis, and dementia)[22,23]. In contrast to Fahr’s disease, which has no effective treatment, Fahr’s syndrome can be remarkably improved after correcting the primary etiology.
Calcium and vitamin D preparations are the basic treatment for hypo-parathyroidism[24,25]. Therapeutic principle is based on the fact that deficiency or dysfunction of PTH may lead to a decrease in production of 1-25 (OH), an active metabolite of vitamin D, leading to a decrease in intestinal calcium uptake[26]. Calcium carbonate is the most commonly used calcium agent, containing 40% of elemental calcium[27]. It has been recommended to supplement calcium 500-1000 mg, 2-3 times/d. Active vitamin D preparations recommended are calcitriol (0.25-2 µg/d) and 1 alpha-hydroxy vitamin D (0.5-3 µg/d)[28-30]. If the patient has recurrent tetany, acute hypocalcemia is predicted. Slow intravenous infusion of 10% calcium gluconate 10-20 mL (90-180 mg elemental calcium, 10-20 min) usually relieves symptoms immediately. If the symptoms are repeated and difficult to alleviate, continuous intravenous drip of calcium is necessary, i.e., 10% calcium gluconate 100 mL (930 mg elemental calcium) should be diluted to 5% calcium gluconate 1000 mL[31-32]. Routine supplementation of calcium and active vitamin D should be given after remission of acute symptoms. In this case, because of acute hypocalcemia and repeated symptoms at the beginning of admission, the patient was given continuous intravenous drip of calcium. Calcium carbonate and calcitriol were given routinely after the acute symptoms were relieved three days later. It is worth noting that if long-term supplementation of large doses of calcium and active vitamin D fails to meet the treatment target, PTH replacement therapy, such as rhPTH1-34 (Teriparatide)[7,33,34] or rhPTH1-84 (Natpara)[35-37], may be a promising option. In addition, health education is also an important part of treatment. Patients and family members should acquire basic knowledge on clinical manifestations and treatment, and understand the importance of regular follow-up to prevent or delay the occurrence of long-term complications.
In this case report, because the primary hospital is limited to temporary treatment of calcium supplement symptoms, the cause of the disease has not been further investigated and the relevant laboratory tests have not been improved, resulting in the patient tossing and turning for 10 years before the final diagnosis. Therefore, for patients with long-term hypocalcemia, recurrent tetany, or even epileptic-like psychiatric symptoms, clinicians should associate with the possibility of hypoparathyroidism in order to make early diagnosis and treatment. Hypoparathyroidism is a common cause of basal ganglia calcification in the majority of individuals. It is recommended to determine serum calcium, phosphorus, and PTH levels in all individuals with basal ganglia calcification and exclude hypoparathyroidism. In addition, hypoparathyroidism is often accompanied by other complications and comorbidities. It is important for clinicians, especially endocrinologists, to understand the clinical manifestations, diagnosis, and treatment of the disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen GX S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Bilezikian JP, Khan A, Potts JT, Brandi ML, Clarke BL, Shoback D, Jüppner H, D'Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 2. | Perugula ML, Lippmann S. Fahr's Disease or Fahr's Syndrome? Innov Clin Neurosci. 2016;13:45-46. [PubMed] |
| 3. | Saleem S, Aslam HM, Anwar M, Anwar S, Saleem M, Saleem A, Rehmani MA. Fahr's syndrome: literature review of current evidence. Orphanet J Rare Dis. 2013;8:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Levine MA. An update on the clinical and molecular characteristics of pseudohypoparathyroidism. Curr Opin Endocrinol Diabetes Obes. 2012;19:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Nakamura Y, Matsumoto T, Tamakoshi A, Kawamura T, Seino Y, Kasuga M, Yanagawa H, Ohno Y. Prevalence of idiopathic hypoparathyroidism and pseudohypoparathyroidism in Japan. J Epidemiol. 2000;10:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Shoback DM, Bilezikian JP, Costa AG, Dempster D, Dralle H, Khan AA, Peacock M, Raffaelli M, Silva BC, Thakker RV, Vokes T, Bouillon R. Presentation of Hypoparathyroidism: Etiologies and Clinical Features. J Clin Endocrinol Metab. 2016;101:2300-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Bilezikian JP, Brandi ML, Cusano NE, Mannstadt M, Rejnmark L, Rizzoli R, Rubin MR, Winer KK, Liberman UA, Potts JT. Management of Hypoparathyroidism: Present and Future. J Clin Endocrinol Metab. 2016;101:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Clarke BL, Brown EM, Collins MT, Jüppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV. Epidemiology and Diagnosis of Hypoparathyroidism. J Clin Endocrinol Metab. 2016;101:2284-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Betterle C, Garelli S, Presotto F. Diagnosis and classification of autoimmune parathyroid disease. Autoimmun Rev. 2014;13:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Koratala A, Morales Lappot J. Fahr's syndrome. Intern Emerg Med. 2019;14:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Illum F, Dupont E. Prevalences of CT-detected calcification in the basal ganglia in idiopathic hypoparathyroidism and pseudohypoparathyroidism. Neuroradiology. 1985;27:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 13. | Lee YJ, Park S, Kim YW, Park KM, Kim IH, Park JH, Park BS. A Case of Seizure Revealing Fahr's Syndrome with Primary Hypoparathyroidism. Am J Case Rep. 2018;19:1430-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kechaou I, Boukhris I. [Hypoparathyroidism and early onset dementia: Fahr syndrome should be suspected]. Pan Afr Med J. 2018;30:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Ramos-Lopes J, Brás A, Morgadinho A, Moreira F. [Acute Generalized Chorea, Dystonia and Brain Calcifications: A Case Report]. Acta Med Port. 2019;32:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Jang BH, Son SW, Kim CR. Fahr's Disease With Intracerebral Hemorrhage at the Uncommon Location: A Case Report. Ann Rehabil Med. 2019;43:230-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Jeon I, Cho KH, Kim SW. Concomitant Fahr's syndrome and thoracic ossification of the posterior longitudinal ligament caused by idiopathic hypoparathyroidism - case report. BMC Musculoskelet Disord. 2019;20:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ramos EM, Oliveira J, Sobrido MJ, Coppola G. Primary Familial Brain Calcification. GeneReviews. Seattle (WA): University of Washington, Seattle 1993; . |
| 19. | Donzuso G, Mostile G, Nicoletti A, Zappia M. Basal ganglia calcifications (Fahr's syndrome): related conditions and clinical features. Neurol Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Manyam BV, Walters AS, Narla KR. Bilateral striopallidodentate calcinosis: clinical characteristics of patients seen in a registry. Mov Disord. 2001;16:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Manyam BV, Bhatt MH, Moore WD, Devleschoward AB, Anderson DR, Calne DB. Bilateral striopallidodentate calcinosis: cerebrospinal fluid, imaging, and electrophysiological studies. Ann Neurol. 1992;31:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Benke T, Karner E, Seppi K, Delazer M, Marksteiner J, Donnemiller E. Subacute dementia and imaging correlates in a case of Fahr's disease. J Neurol Neurosurg Psychiatry. 2004;75:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Geschwind DH, Loginov M, Stern JM. Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease). Am J Hum Genet. 1999;65:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, van Biesen W, Dekkers OM; European Society of Endocrinology. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173:G1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, Thakker RV, Khan AA, Potts JT. Management of Hypoparathyroidism: Summary Statement and Guidelines. J Clin Endocrinol Metab. 2016;101:2273-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 26. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9407] [Article Influence: 522.6] [Reference Citation Analysis (1)] |
| 27. | Heaney RP, Dowell MS, Bierman J, Hale CA, Bendich A. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr. 2001;20:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Kooh SW, Fraser D, DeLuca HF, Holick MF, Belsey RE, Clark MB, Murray TM. Treatment of hypoparathyroidism and pseudohypoparathyroidism with metabolites of vitamin D: evidence for impaired conversion of 25-hydroxyvitamin D to 1 alpha,25-dihydroxyvitamin D. N Engl J Med. 1975;293:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Neer RM, Holick MF, DeLuca HF, Potts JT. Effects of 1alpha-hydroxy-vitamin D3 and 1,25-dihydroxy-vitamin D3 on calcium and phosphorus metabolism in hypoparathyroidism. Metabolism. 1975;24:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Mortensen L, Hyldstrup L, Charles P. Effect of vitamin D treatment in hypoparathyroid patients: a study on calcium, phosphate and magnesium homeostasis. Eur J Endocrinol. 1997;136:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am. 1993;22:363-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB. Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J Clin Endocrinol Metab. 2010;95:2680-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Gafni RI, Brahim JS, Andreopoulou P, Bhattacharyya N, Kelly MH, Brillante BA, Reynolds JC, Zhou H, Dempster DW, Collins MT. Daily parathyroid hormone 1-34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J Bone Miner Res. 2012;27:1811-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Mannstadt M, Clarke BL, Vokes T, Brandi ML, Ranganath L, Fraser WD, Lakatos P, Bajnok L, Garceau R, Mosekilde L, Lagast H, Shoback D, Bilezikian JP. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Cusano NE, Rubin MR, McMahon DJ, Zhang C, Ives R, Tulley A, Sliney J, Cremers SC, Bilezikian JP. Therapy of hypoparathyroidism with PTH(1-84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Palermo A, Santonati A, Tabacco G, Bosco D, Spada A, Pedone C, Raggiunti B, Doris T, Maggi D, Grimaldi F, Manfrini S, Vescini F. PTH(1-34) for Surgical Hypoparathyroidism: A 2-Year Prospective, Open-Label Investigation of Efficacy and Quality of Life. J Clin Endocrinol Metab. 2018;103:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |