Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3553
Peer-review started: February 15, 2019
First decision: May 31, 2019
Revised: June 19, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: November 6, 2019
Processing time: 269 Days and 19.5 Hours
Immunosuppression is effective in treating a number of diseases, but adverse effects such as bone marrow suppression, infection, and oncogenesis are of concern. Methotrexate is a key immunosuppressant used to treat rheumatoid arthritis. Although it is effective for many patients, various side effects have been reported, one of the most serious being methotrexate-related lymphoproliferative disorder. While this may occur in various organs, liver involvement is rare. Information on these liver lesions, including clinical characteristics, course, and imaging studies, has not been summarized to date.
We present a case of 70-year-old woman presented with a 2-wk history of fever and abdominal pain. She had had rheumatoid arthritis for 5 years and was being treated with medication including methotrexate. Contrast-enhanced computed tomography revealed multiple low density tumors in the liver and the histological analyses showed significant proliferation of lymphocytes in masses that were positive on immunohistochemical staining for CD3, CD4, CD8, and CD79a but negative for CD20 and CD56. Staining for Epstein-Barr virus-encoded RNA was negative. And based on these findings, the liver tumors were diagnosed as Methotrexate-related lymphoproliferative disorders. A time-dependent disappearance of the liver tumors after stopping methotrexate supported the diagnoses.
The information obtained from our case and a review of 9 additional cases reported thus far assist physicians who may face the challenge of diagnosing and managing this disorder.
Core tip: Methotrexate-related lymphoproliferative disorder is a severe adverse event of immunosuppression in the treatment of rheumatoid arthritis. Even if the methotrexate is discontinued leading to resolution of the side effect, the disorder may recur. Decisions based on the patient’s disease status in regard to stopping or continuing administration of methotrexate are challenging. Methotrexate-related lymphoproliferative disorder has rarely caused tumors in the liver. Therefore, little information has been reported on clinical findings, imaging characteristics, and treatment of this entity. To promote early diagnosis and appropriate treatment, we report a new case with multiple imaging studies and summarized previously reported cases.
- Citation: Mizusawa T, Kamimura K, Sato H, Suda T, Fukunari H, Hasegawa G, Shibata O, Morita S, Sakamaki A, Yokoyama J, Saito Y, Hori Y, Maruyama Y, Yoshimine F, Hoshi T, Morita S, Kanefuji T, Kobayashi M, Terai S. Methotrexate-related lymphoproliferative disorders in the liver: Case presentation and mini-review. World J Clin Cases 2019; 7(21): 3553-3561
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3553.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3553
Methotrexate (MTX) is a key drug used in the management of rheumatoid arthritis (RA)[1]. Although it is an effective treatment, it has also been noted to cause the severe adverse effect of MTX-related lymphoproliferative disorder (MTX-LPD)[2]. This condition is categorized as an iatrogenic immunodeficiency-related proliferative disease according to the World Health Organization (WHO)’s fourth edition of tissue classification of lymphoid tumors in 2008[3]. Reported MTX-LPD pathology patterns include monoclonal expansion of B cells leading to diffuse large B-cell lymphoma, Hodgkin lymphoma, and polyclonal expansion of T and B cells[4]. Although a possible contribution of Epstein-Barr virus (EBV) infection was reported in a case of B-cell MTX-LPD[5,6], it is unclear if the virus had a causative role. Currently, the therapeutic options for MTX-LPD include discontinuing MTX, which had led to improvement in 30% of all cases[7,8] and chemotherapy for lymphoma based on the proliferating cell type. To date, only 9 cases of MTX-LPD producing a tumor in the liver have been reported[9-17]. In this article, we report another case and then summarize all 10 cases, reviewing the clinical findings, imaging characteristics, response to therapy, and outcome. Imaging showed small, low density masses on computed tomography (CT); low signal intensity in T1 images and high signal intensity in T2 and diffusion images on magnetic resonance imaging (MRI); high uptake on positron emission tomography; and a hypoechoic ultrasound (US) appearance. Most cases had poor or only mild enhancement on CT, MRI, or US. These results suggest imaging findings similar to those seen in hepatic lymphoma. Particular modalities to detect the tumor and assess hypovascularity might thus be useful. However, differentiating this entity from other liver tumors is difficult, so histologic diagnosis is essential. The infor-mation summarized here from the 10 reported cases will be of help to physicians in managing patients taking MTX who are found to have liver tumors. It may alert them to consider the possibility of MTX-LPD.
A 70-year-old woman presented with a 2-wk history of fever and abdominal pain.
She had had RA for 5 years and was being treated with MTX (8 mg/wk), pred-nisolone (5 mg/d), and golimumab (50 mg/mo).
No remarkable personal and family histories were marked.
On physical examination of the abdomen, there was mild right upper quadrant tenderness.
Laboratory examination showed evidence of inflammation with peripheral leukocytosis (13900/µL); CRP (18.7 mg/dL); LDH (2844 IU/L); elevated levels of hepatobiliary enzymes with AST (316 IU/L), ALT (370 IU/L), ALP (2006 IU/L), and γ-GTP (1300 IU/L); T-Bil (2.51 mg/dL); and sIL-2R (2120 IU/mL). Levels of other tumor markers—CEA (1.5 ng/mL), CA19-9 (4.0 U/mL), AFP (2.0 ng/mL), and PIVKA-II (16 mAU/mL) were within normal limits (Table 1).
| Item | Data | Item | Data | Item | Data |
| White blood cells | 13900 /μL | Total protein | 7.1 g/dL | Sodium | 134 mEq/L |
| Neutrophils | 82.9% | Albumin | 3.6 g/dL | Potassium | 4.3 mEq/L |
| Lymphocytes | 8.6% | AST | 316 IU/L | Chloride | 97 mEq/L |
| Monocytes | 8.4% | ALT | 370 IU/L | CRP | 18.74 mg/dL |
| Eosinophils | 0% | ALP | 2006 IU/L | CEA | 1.5 ng/mL |
| Basophils | 0.1% | LDH | 2844 IU/L | CA19-9 | 4.0 U/mL |
| Red blood cells | 389 × 104 /μL | γ-GTP | 1300 IU/L | AFP | 2.0 ng/mL |
| Hemoglobin | 12.6 g/dL | T. Bil | 2.51 mg/dL | PIVKA-II | 16 mAU/mL |
| Platelet count | 25.8 × 104 /μL | D. Bil | 0.82 mg/dL | sIL2R | 2120 U/mL |
| PT | 84.7% | BUN | 25.6 mg/dL | ||
| APTT | 30.4 s | Creatinine | 0.63 mg/dL |
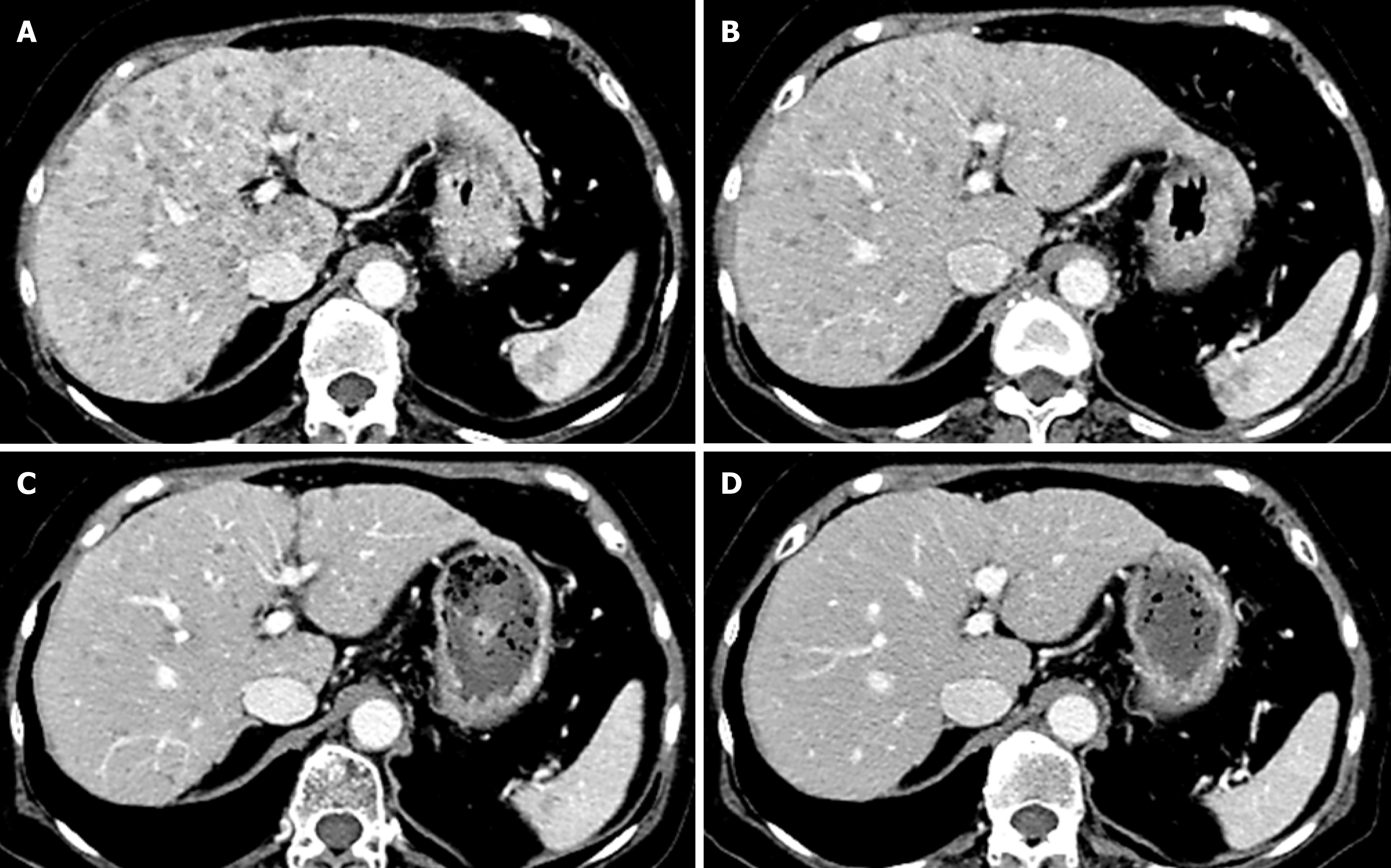
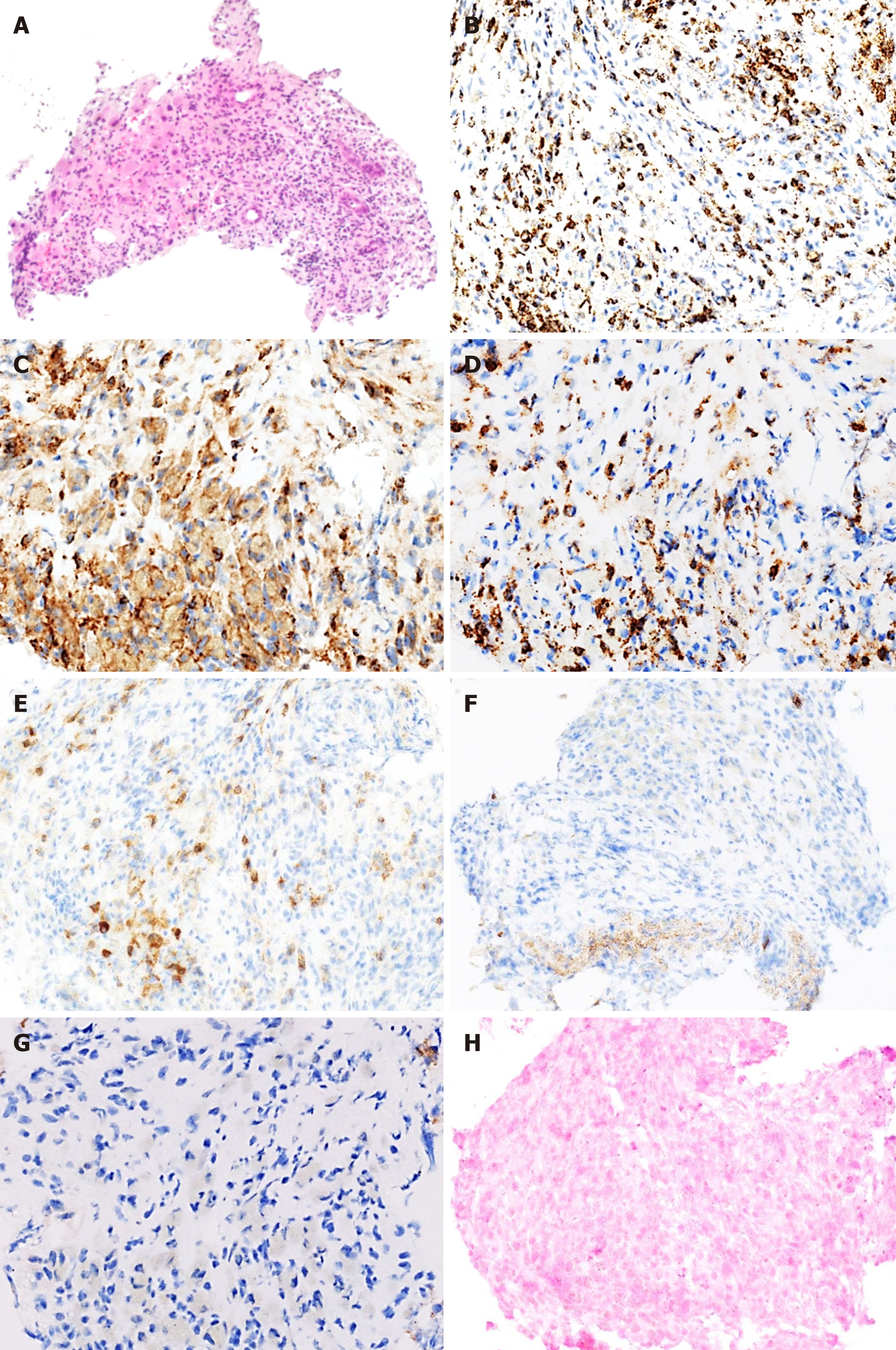
Contrast-enhanced CT revealed multiple low density tumors in the liver and spleen with no significant enhancement other than mild ring enhancement in the late phase (Figure 1). On MRI, the liver tumors had low signal intensity in T1-weighted images and high signal intensity in T2-weighted images. US revealed multiple hypoechoic tumors in the liver, and contrast-enhanced US (CEUS) revealed poor enhancement in the early phase followed by a hypoechoic pattern in the Kupffer phase. Given this clear view, CEUS-guided fine-needle biopsy of a liver tumor was performed for histologic analysis. Hematoxylin and eosin staining showed significant proliferation of lymphocytes in masses (Figure 2A) that were positive on immunohistochemical staining for CD3 (Figure 2B), CD4 (Figure 2C), CD8 (Figure 2D) and CD79a (Figure 2E) but negative for CD20 (Figure 2F) and CD56 (Figure 2G). Staining for EBV-encoded RNA was negative (Figure 2H).
Based on these findings above, the liver tumors were diagnosed as MTX-LPD, and MTX was discontinued.
The patient’s abdominal pain resolved within a few days after that, and the WBC, AST, ALT, LDH, and sIL-2R returned to the normal range within 3 mo. CT images 2 wk (Figure 1B), 1 mo (Figure 1C), and 2 mo (Figure 1D) after stopping MTX showed time-dependent disappearance of the liver and splenic tumors. There has been no sign of recurrence for 2 years.
Based on this sequence of events, the diagnosis of MTX-LPD in the liver and the spleen is confirmed. The patient’s RA has since been managed mainly with prednisolone.
Currently, MTX is a key drug for treating RA, with approximately 70 million people using it worldwide[1]. Although it is effective, it has substantial possible side effects, including liver dysfunction, renal dysfunction, myelosuppression, infection, and interstitial pneumonia. The number of patients diagnosed with MTX-LPD has been increasing since its first report in 1991[2]. It is categorized as an iatrogenic immunodeficiency-related proliferative disease according to WHO’s fourth edition of tissue classification of lymphoid tumors in 2008[3]. There are 3 major types of MTX-LPD, malignant lymphoma, benign reactive hyperplasia, and cases intermediate between the two. Generally, the average age of patients is 67 (34–87) years, and the disease occurs in twice as many women as men. MTX-LPD reportedly occurs after about 5 years of treatment with the drug[4]. Although the pathology patterns vary, monoclonal expansion of B cells leading to diffuse large B-cell lymphoma is the most common type, accounting for 35% to 60% of all MTX-LPDs, followed by Hodgkin lymphoma in 12% to 25%, and mixed T and B-cell lesions[4]. The pathogenesis had not been clearly demonstrated to date, although a possible contribution of EBV infection was reported in a case of B-cell MTX-LPD[5]. Molecular mechanisms seen in other LPDs associated with EBV include the activation of growth factors, transcription factors, and apoptosis inhibitory factors leading to lymphoblastic changes in B cells and tumorigenesis[6]. Currently, MTX-LPD is treated by stopping the MTX, which leads to improvement in 30% of all cases[7] and when no response is seen, chemotherapy is begun. Careful follow-up is essential even resulted in a good response[4], as 50% of patients have been found to have recurrence of the MTX-LPD. MTX-LPD is further classified as regressive if the tumor disappears after discontinuing MTX, persistent if there is no response to stopping the MTX and administering chemotherapy, and other in cases where MTX had been administered previously but the LPD developed during treatment with other antirheumatic drugs. LDH, CRP, and sIL-2 are all markers of the malignant potential for LPD. These have reportedly been higher in patients with MTX-LPD classified as persistent or other rather than regressive[8].
MTX-LPD in the liver is quite rare, with only a few reported cases with clinical information available. This includes some cases where the disease entity is called MTX-related primary hepatic lymphoma (MTX-PHL). We have summarized 10 cases of MTX-LPD in the liver, including our own case along with 9 that were previously reported[9-17] (Table 2). Patients had a median age of 67.5 (56–76) years and included 3 men and 7 women. This ratio is similar to the ratio reported for all the cases of MTX-LPD. MTX had been given for 2 to 10 years. Symptoms at onset included abdominal pain (in 5 patients), fever (in 4), malaise (in 1), and anorexia (in 1). These are likely all attributable to the local progression of the disorder in the liver. Multiple lesions were present in 7 patients. On plain CT, the lesions appeared as low density areas. The tumors were hypovascular in contrast-enhanced CT images in 8 cases, while the other 2 had mild ring enhancement. On MRI, the lesions had low signal intensity in T1 images and high signal intensity in both T2 and diffusion images, just like the appearance in our case. The tumors had high uptake on positron emission to-mography, and they were hypoechoic on B-mode US. CEUS[18] revealed tumor hypovascularity in our patient. These imaging characteristics are similar to those seen in hepatic lymphoma, confirming the utility of hypovascularity in suggesting the diagnosis. However, histologic diagnosis is still essential. Tissue for histology was collected percutaneously in 7 cases and surgically in the other 3 (Table 2). There was proliferation of B cells in 6 cases, T cells in 1, T and B cells in 2, and Hodgkin lymphoma cells in 1. Based on the histologic analysis, cases 1 to 6 are considered to be MTX-PHL. Other than the patient with Hodgkin lymphoma, all others responded to treatment. These included response to discontinuation of MTX in 3, surgery and stopping of MTX in 3, and chemotherapy including R-CHOP and R-THP-COP in 3. No recurrence had been seen in these cases at the time they were reported (Table 2). Further analysis and follow-up is necessary to clarify the differences in the monoclonal cell proliferation pattern (MTX-PHL) and other cases of MTX-LPD in the liver. The characteristics of MTX-LPD (Table 3) and characteristic of the image of MTX-LPD in the liver (Table 4) have been summarized in Tables. As the MTX is effective to treat the posttransplant lymphoproliferative disorder which is generally associated with Epstein-Barr virus infection[19], further molecular analyses to clarify the mechanisms of carcinogenesis and the immunologic interactions in the conditions.
| Ca-se | Ref. | Age | Sex | Sym-ptom | MTX peri-od (yr) | Num-ber of tu-mors | CT | MRI | PET | US | CE-US | Ti-ssue Colle-ction | Path-ology | Immunohisto-chemistry | Treat-ment | Re-spon-se | Re-currence | On-set pa-ttern | Prog-nosis |
| 1 | 9 | 68 | F | Ma-laise | 8 | Mul-tiple | Low den-sity hypovas-cular | N/A | N/A | Hypo-echoic | N/A | US-gui-ded | Hodg-kin lym-pho-ma | CD15+, CD20-, CD30+ | Un-trea-ted | No re-spon-se | None | Persis-tent | Death |
| 2 | 10 | 76 | M | None | 4.5 | Single | Low den-sity hypovas-cular | N/A | Hypo-echoic | N/A | Sur-gery | B cell | CD10-, CD20+, CD79a+ | Resec-tion, cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (10 mo) | |
| 3 | 11 | 67 | F | Abdo-minal pain, fever | 6 | Mul-tiple | Low den-sity hypovas-cular | N/A | High up-take | N/A | N/A | US-gui-ded | B cell | CD10-, CD20+, CD5-, EBER+ | R-THP-COP | Effec-tive | None | Persis-tent | Alive (1 yr) |
| 4 | 12 | 56 | F | Weig-ht loss, fever | 7 | Mul-tiple | Iso den-sity hypovas-cular | N/A | High up-take | N/A | N/A | US-gui-ded | B cell | CD10+, CD20+, CD5- | R-CH-OP | Effec-tive | None | Persis-tent | Alive (6 mo) |
| 5 | 13 | 64 | M | Abdo-minal pain, fever | 2 | Mul-tiple | Low den-sity hypovas-cular | N/A | N/A | N/A | N/A | US-gui-ded | B cell | CD10+, CD79a+, CD20+; Bcl-2-, CD3-, EBER- | R-CH-OP | Effec-tive | None | Persis-tent | Alive (2 yr) |
| 6 | 14 | 65 | F | None | 7 | Single | Low den-sity mild enhance-ment | T1: low, T2: high, diffu-sion: high | N/A | Hypo-echoic | N/A | Sur-gery | B cell | CD10-, CD15-, CD20+, CD30+, CD79a+, EBER+ | Resec-tion, cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (1 yr) |
| 7 | 15 | 70 | F | Abdo-minal pain | 10 | Single | Low den-sity hypovas-cular | T1: low, T2: high, diffu-sion: high | N/A | N/A | N/A | Surgery | T cell | CD10-, CD20-, CD79a-, CD3+, CD45RO+, CD5+, bcl-2-, EBER- | Resec-tion, cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (1 yr) |
| 8 | 16 | 76 | F | Ano-rexia | 9 | Mul-tiple | Low den-sity | N/A | N/A | N/A | N/A | US-gui-ded | T and B cell | N/A | Cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (N/A) |
| 9 | 17 | 63 | M | Abdo-minal pain | 10 | Mul-tiple | Low den-sity | N/A | High up-take | Hypo-echoic | Hypovas-cular | US-gui-ded | B cell | N/A | Cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (7 mo) |
| 10 | Our case | 70 | F | Abdo-minal pain, fever | 5 | Mul-tiple | Low den-sity hypovas-cular | T1: low, T2: high | N/A | Hypo-echoic | Hypovas-cular | US-gui-ded | T and B cell | CD3+, CD4+, CD8+, CD56-, CD20-, CD79a+, EBER- | Cessa-tion | Effec-tive | None | Re-gre-ssive | Alive (2 yr) |
| Characteristic of MTX-LPD |
| Potential association of EB virus |
| Treatment includes stopping the MTX and administering chemotherapy or performing surgery in some cases |
| By stopping MTX, about 30% of cases show improvement within a month |
| Chemotherapy includes R-CHP, R-THP-COP, etc. depending on the histological pattern |
| Characteristic of the image of MTX-LPD in the liver |
| CT: Low density, poor enhancement effect |
| MRI: Low signal intensity in T1-weighted image |
| High signal intensity in T2-weighted image |
| US: Low echoic pattern |
| CE-US: Poor enhancement, low echoic area in the Kupffer phase |
As a key drug used to treat RA, MTX has been very effective, but proper diagnosis and management of adverse events associated with it are essential. We have reported a rare case of MTX-LPD in the liver and spleen and summarized the clinical information of the 9 other cases reported to date. Although imaging modalities are useful in suggesting the diagnosis, histologic analysis is required to confirm it. Treatment includes stopping the MTX and administering chemotherapy or performing surgery in some cases. The information summarized here will help physicians who are assessing liver tumors in patients treated with MTX and will alert them to considering a diagnosis of MTX-LPD.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bramhall S, Cerwenka H, Chiu KW, Sergi C S-Editor: Wang JL L-Editor:A E-Editor: Xing YX
| 1. | Braun J. Methotrexate: optimizing the efficacy in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2011;3:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ellman MH, Hurwitz H, Thomas C, Kozloff M. Lymphoma developing in a patient with rheumatoid arthritis taking low dose weekly methotrexate. J Rheumatol. 1991;18:1741-1743. [PubMed] |
| 3. | Gaulard P. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, editor. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC Press 2008; 350-351. |
| 4. | Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, Tomita Y, Nakatsuka S, Tamaru J, Iizuka A, Takeuchi T, Aozasa K. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34:322-331. [PubMed] |
| 5. | Kikuchi K, Miyazaki Y, Tanaka A, Shigematu H, Kojima M, Sakashita H, Kusama K. Methotrexate-related Epstein-Barr Virus (EBV)-associated lymphoproliferative disorder--so-called "Hodgkin-like lesion"--of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010;4:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Grywalska E, Markowicz J, Grabarczyk P, Pasiarski M, Roliński J. Epstein-Barr virus-associated lymphoproliferative disorders. Postepy Hig Med Dosw (Online). 2013;67:481-490. [PubMed] |
| 7. | Yamakawa N, Fujimoto M, Kawabata D, Terao C, Nishikori M, Nakashima R, Imura Y, Yukawa N, Yoshifuji H, Ohmura K, Fujii T, Kitano T, Kondo T, Yurugi K, Miura Y, Maekawa T, Saji H, Takaori-Kondo A, Matsuda F, Haga H, Mimori T. A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol. 2014;41:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Tokuhira M, Watanabe R, Nemoto T, Sagawa M, Tomikawa T, Tamaru J, Itoyama S, Nagasawa H, Amano K, Kameda H, Takeuchi T, Mori S, Kizaki M. Clinicopathological analyses in patients with other iatrogenic immunodeficiency-associated lymphoproliferative diseases and rheumatoid arthritis. Leuk Lymphoma. 2012;53:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Takasumi M, Okai K, Asano T, Kanno Y, Abe K, Takahashi A, Kobayashi H, Hashimoto Y, Watanabe H, Ohira H. [A case of methotrexate-associated lymphoproliferative disorder diagnosed by liver biopsy]. Nihon Shokakibyo Gakkai Zasshi. 2015;112:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Matsuura Y, Mizuno S, Hibino M, Hirata A, Tamauchi T. Liver tumor associated with amethotrexate-associated lympholiferative disorder. Nihon Rinsho Geka Gakkai Zasshi. 2015;70:1015-1020. |
| 11. | Tatsumi G, Ukyo N, Hirata H, Tsudo M. Primary hepatic lymphoma in a patient with rheumatoid arthritis treated with methotrexate. Case Rep Hematol. 2014;2014:460574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Miyagawa K, Shibata M, Noguchi H, Hayashi T, Oe S, Hiura M, Abe S, Harada M. Methotrexate-related primary hepatic lymphoma in a patient with rheumatoid arthritis. Intern Med. 2015;54:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kawahara A, Tsukada J, Yamaguchi T, Katsuragi T, Higashi T. Reversible methotrexate-associated lymphoma of the liver in rheumatoid arthritis: a unique case of primary hepatic lymphoma. Biomark Res. 2015;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Takei D, Abe T, Amano H, Hirano N, Kobayashi T, Ohdan H, Kondo T, Nakahara M, Noriyuki T. Methotrexate-associated primary hepatic malignant lymphoma following hepatectomy: A case report. Int J Surg Case Rep. 2017;31:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Omori T, Takigawa M, Taguchi C, Hirakawa K, Irie K. A case report of MTX-LPD (methotrexate-associated lymphoproliferative disorders) of the liver. Japan J Clin Radiol. 2017;62:803-809. |
| 16. | Kawakami M, Ikeda N. Case presented in JAPANESE. J Shimane Medical Association. 2018;38:39-42. |
| 17. | Matsumoto R, Numata K, Doba N, Hara K, Chuma M, Fukuda H, Nozaki A, Tanaka K, Ishii Y, Maeda S. A case of multiple hepatic lesions associated with methotrexate-associated lymphoproliferative disorder. J Med Ultrason (2001). 2016;43:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:40-45. [PubMed] |
| 19. | Nabors LB, Palmer CA, Julian BA, Przekwas AM, Kew CE. Isolated central nervous system posttransplant lymphoproliferative disorder treated with high-dose intravenous methotrexate. Am J Transplant. 2009;9:1243-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |