Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3463
Peer-review started: June 18, 2019
First decision: August 1, 2019
Revised: September 25, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 6, 2019
Processing time: 145 Days and 21.3 Hours
Several studies have demonstrated the feasibility and effectiveness of using ultrasound elastography to assess liver tissue stiffness. Virtual touch imaging quantification (VTIQ) based on acoustic radiation force impulse imaging has been developed as a latest and noninvasive method for assessing liver stiffness in children.
To determine the standard value in healthy children, and to identify possible factors that might influence the VTIQ measurement.
With the ethical approval, 202 children between 1 month and 15 years old were included in this study. None of them had any liver or systematic diseases. All children had a normal ultrasound scan and normal body mass index (BMI) range. The subjects were divided into four age and BMI groups. The effects of gender, age, liver lobe, measurement depth, and BMI on liver elasticity were investigated.
A significant correlation was found between age and shear wave velocity (SWV) value. At measurement depths of 1.5 cm and 2.0 cm in the left lobe, there were significant differences among the age groups. SWV values were significantly negatively correlated with the measurement depth. Gender, liver lobe, and BMI showed no significant effect on the SWV values. Age and BMI may influence the quality of the elastogram.
VTIQ is a noninvasive technique that is feasible to measure liver stiffness in children. The afore-mentioned velocity value obtained utilizing VTIQ method could be used as reference value for normal liver stiffness in children.
Core tip: Virtual touch imaging quantification (VTIQ) is the latest elastic technology based on acoustic radiation force impulse imaging. Previously, the high-frequency linear probe (9L4) with VTIQ technology was used for the evaluation of breast and thyroid lesions. We found that this probe might also be suitable for the abdominal organs in children. This study analyzed the normal liver stiffness measured by VTIQ method and assessed the potential influencing factors of VTIQ value and factors affecting the quality of the elastogram. This study suggested that VTIQ is a feasible technique to measure liver stiffness in children.
- Citation: Sun PX, Tong YY, Shi J, Zhang H, Liu SJ, Du J. Normal values of shear wave velocity in liver tissue of healthy children measured using the latest acoustic radiation force impulse technology. World J Clin Cases 2019; 7(21): 3463-3473
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3463.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3463
Children with chronic liver disease may be complicated by liver fibrosis due to repetitive hepatocyte injury, which leads to cirrhosis ultimately[1-3]. Therefore, it is of paramount significance to stage liver fibrosis to make early diagnosis and improve prognosis[4]. Although liver biopsy remains the gold standard for the diagnosis of fibrosis, it is invasive with risks of several complications[5,6]. Hence, an alternative method for assessing liver fibrosis is increasingly necessary. In the past few years, numerous studies have demonstrated the feasibility and effectiveness of using ultrasound elastography to assess liver tissue stiffness[7-11]. Virtual touch tissue quantification (VTQ) of acoustic radiation force impulse (ARFI) is a new ultrasound elasticity technology that could assess tissue hardness quantitatively and the results are shown as shear wave velocity (SWV) values. The stiffer the tissue, the higher the SWV value. Some studies have confirmed the value of VTQ for the evaluation of liver stiffness and the staging for liver fibrosis[12-14]. However, a fixed size of region of interest (ROI) of VTQ makes it not very suitable for the application in children liver. The ROI is too big for children liver to completely avoid blood vessels or biliary ducts; and for children who could not control their breath very well, the results may be affected by breath movement.
Virtual touch imaging quantification (VTIQ) is the latest elastic technology. Using the VTIQ method, the examiner can use a moveable Q box quantification tool to select a defined ROI. Different from VTQ which only provides the velocity value of a single point, the VTIQ technology instantly acquires comprehensive information in the ROI, which is displayed as a two-dimensional color-coded image of SWV[15,16]. The high-frequency linear probe (9L4) with VTIQ technology was used in evaluating the stiffness of breast and thyroid lesions. And this probe may also be suitable for the abdominal organs in children, with a better identification of vessels and biliary structures that should not be included in the ROI[10].
Recently, a few studies have investigated the clinical application of this method in differentiating biliary atresia from other causes of neonatal/infantile liver diseases[17], and assessing the degree of liver parenchymal fibrosis in children[3,11]. Given the differences between infants and teenagers and the diversity of children, it seems essential to establish a normal range of liver stiffness measurement in children to differentiate the normal liver from abnormalities[4,6] so that the method could be employed correctly in evaluation of liver diseases of children.
Therefore, the objective of the present study was to determine the standard values of SWV in healthy children using VTIQ technology, to assess the potential effects of factors such as gender, age, body mass index (BMI), and the depth and position of the measurement, and to analyze factors affecting the quality of the elastogram.
The study was approved by the institutional ethics committee of Shanghai Children’s Medical Center; parental informed consent was obtained in all cases. This study was carried out between July and December 2018 and included 220 healthy children between 1 mo and 15 year of age, all without known hepatic or systematic disease, altered liver tests, or chromosomal abnormality. Children who could not cooperate, with a bad quality of elastogram, or with abnormal findings upon abdominal ultrasound examination were excluded. BMI had to be between the 3rd and 97th percentiles. BMI was calculated according to the World Health Organization (WHO) Anthropometric Program (WHO Anthro, version 3.2.2, Jan 2011) for children younger than 5 years or according to WHO BMI-for-age percentile reference table for children older than 5 years.
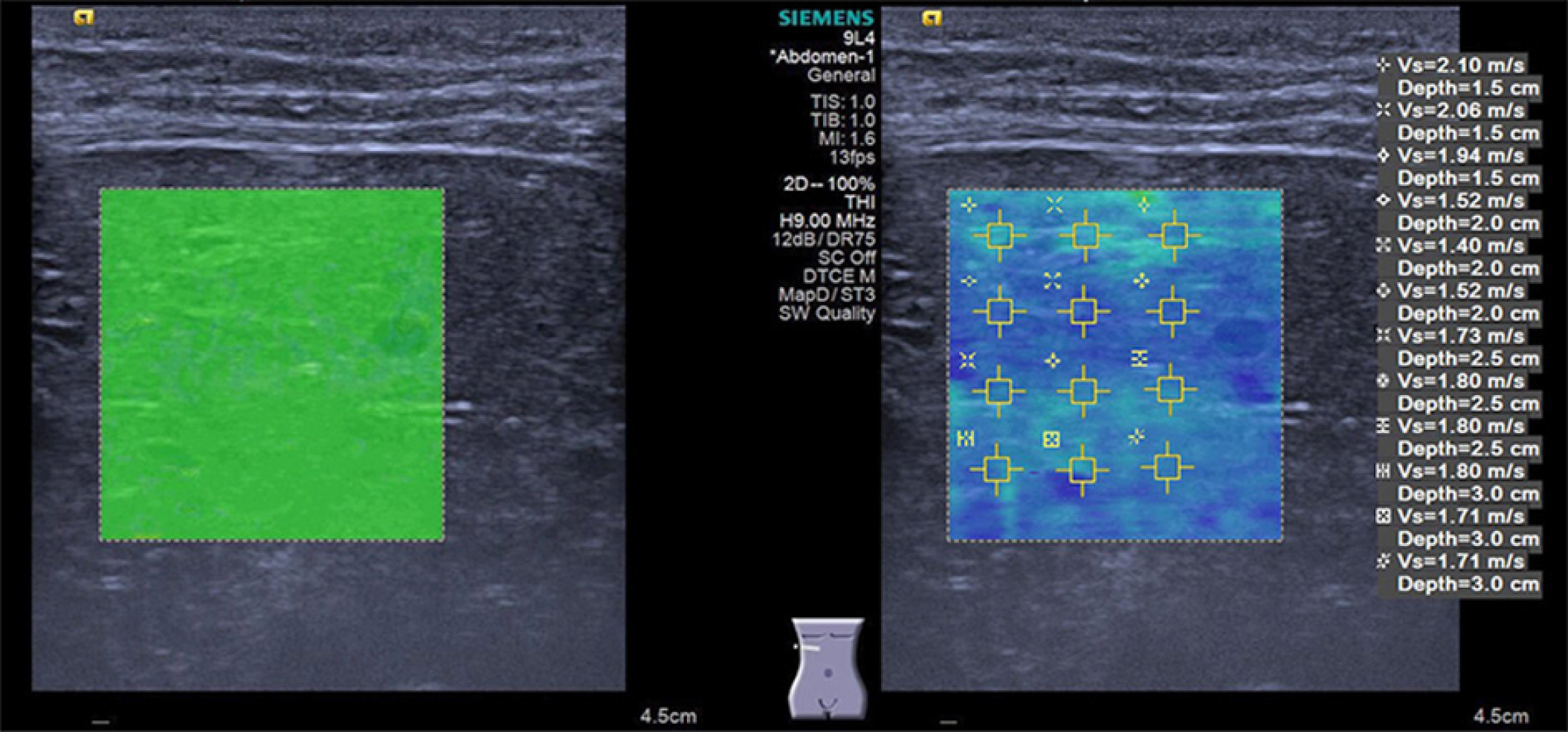
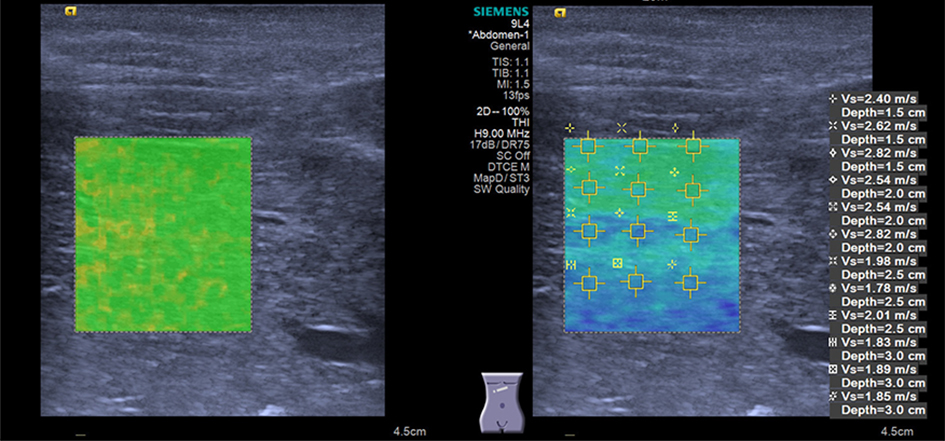
In all subjects, shear wave elastography (SWE) was performed by a senior pediatric radiologist with more than 1 year of experience in this technology using an Acuson Oxana2 system with a 9L4 (4.0-9.0 MHz) liner transducer (Siemens Medical Solutions, Mountain View, CA, United States). All children were placed in a spine position with free breathing. SWE was performed using the VTIQ method to measure SWV in several ROIs within the liver parenchyma while avoiding the identifiable biliary and vascular structures. SWE was conducted in both lobes (segment IV and V, respectively); an intercostal approach was employed to make the measurements for the right liver lobe, while in the left lobe, measurements were performed by placing the transducer under the xiphoid of the sternum. Three valid measurements of SWV were performed and the average value was calculated at each depth of 1.5, 2.0, 2.5 cm, and 3.0 cm beneath the skin surface. The results are expressed in meter per second (m/s). The quality of the elastogram was evaluated upon the quality mode of VTIQ; the elastogram box with a uniform green color fill-in was referred to as “superior” (Figure 1), while the elastogram box with > 80% green color fill-in was referred to as “good” quality (Figure 2).
Continuous variables are presented as the mean ± SD, range, or 95% confidence interval (CI), while categorical data are summarized as counts and percentages (%). Differences between two groups were determined by the Student’s t-test or Mann-Whitney U test when data were not normally distributed. For comparison among more than two groups, Kruskal-Wallis test or analysis of variance (ANOVA) was used as appropriate; when the differences were significant, the post hoc test was performed using the Bonferroni’s adjustment or Bonferroni method accordingly. The relationship between SWV and continuous variables was investigated using Pearson liner correlation analysis. The chi-square test or Fisher’s exact test was used for comparing the categorical data. All statistical analyses were performed using SPSS software for Mac, Version 23.0. A two-tailed P-value less than 0.05 was considered to indicate a significant difference.
Eighteen children were excluded, fifteen because of bad quality of elastogram or lack of cooperation, and three because of hepatic calcifications observed on gray-scale ultrasound. After exclusion, a collective of 202 subjects (116 girls and 86 boys) were enrolled in this study. The study group was divided into four age groups: Group 1 (≤2 years old, n = 28), group 2 (>2 but ≤6 years old, n = 68), group 3 (>6 but ≤10 years old, n = 58), and group 4 (>10 years old, n = 48). The participants were also divided into four BMI groups: Group 1 (BMI ≤ 15, n = 23), group 2 (15 < BMI ≤ 16, n = 65), group 3 (16 < BMI ≤ 17, n = 54), and group 4 (BMI > 17, n = 60). Table 1 shows the gender, age, and the BMI information of the subjects. No significant differences were identified in age or BMI between the gender groups.
| Female (%) | Male (%) | All | P value | |
| 116 (42.6) | 86 (57.4) | 202 | ||
| Age | ||||
| Mean ± SD (yr) | 7.2 ± 3.4 | 6.3 ± 4.0 | 6.8 ± 3.7 | 0.081 |
| Range | 0.1-15 | 0.1-15 | 0.1-15 | |
| BMI | ||||
| Mean ± SD (kg/m2) | 16.7 ± 2.6 | 16.4 ± 1.6 | 16.6 ± 2.1 | 0.335 |
| Range | 13.8-25.5 | 13.2-24.2 | 13.2-25.5 |
There was no significant difference in SWV values measured in the same lobe and at the same depth between the gender groups (Table 2).
| Liver lobe/depth | Male | Female | P value | ||
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | ||
| Left/1.5 cm | 2.56 ± 0.41 | 2.46-2.66 | 2.58 ± 0.47 | 2.47-2.68 | 0.788 |
| Left/2.0 cm | 2.15 ± 0.44 | 2.05-2.24 | 2.06 ± 0.37 | 2.00-2.13 | 0.144 |
| Left/2.5 cm | 1.77 ± 0.27 | 1.71-1.83 | 1.77 ± 0.30 | 1.71-1.82 | 0.901 |
| Left/3.0 cm | 1.64 ± 0.19 | 1.60-1.69 | 1.66 ± 0.25 | 1.61-1.72 | 0.565 |
| Right/1.5 cm | 2.51 ± 0.33 | 2.42-2.60 | 2.55 ± 0.44 | 2.45-2.65 | 0.591 |
| Right/2.0 cm | 2.10 ± 0.33 | 2.03-2.18 | 2.10 ± 0.37 | 2.04-2.17 | 0.982 |
| Right/2.5 cm | 1.75 ± 0.28 | 1.69-1.81 | 1.74 ± 0.29 | 1.69-1.79 | 0.752 |
| Right/3.0 cm | 1.66 ± 0.22 | 1.60-1.72 | 1.61 ± 0.25 | 1.55-1.67 | 0.284 |
Significant correlations were identified between age and SWV values in the left lobe at all measurement depths, and in the right lobe at depths of 1.5 cm, 2.0 cm, and 3.0 cm (P < 0.05). The correlation between age and SWV value in the right lobe at a depth of 2.5 cm was marginally significant (P = 0.09). After grouping the age into four classes, only in cases of measurement at a depth of 1.5 cm and 2.0 cm in the left lobe were there significant differences among the age groups (P < 0.05, Table 3). There was significant difference in SWV values at a depth of 1.5 cm between groups 1 and 3. And SWV values at a depth of 2.0 cm in groups 3 and 4 were significantly higher than those in group 1 (Table 3).
| Liver lobe/depth | Age group (yr) | P value | Compar-ison | P value | |||
| Group 1 (n = 28) | Group 2 (n = 68) | Group 3 (n = 58) | Group 4 (n = 48) | ||||
| Left/1.5 cm | 0.038 | ||||||
| Mean ± SD | 2.41 ± 0.40 | 2.53 ± 0.40 | 2.70 ± 0.46 | 2.63 ± 0.53 | G1-G3 | 0.038 | |
| 95%CI | 2.25-2.56 | 2.43-2.63 | 2.56-2.85 | 2.34-2.92 | |||
| Left/2.0 cm | 0.002 | ||||||
| Mean ± SD | 1.92 ± 0.25 | 2.07 ± 0.39 | 2.08 ± 0.29 | 2.27 ± 0.55 | G1-G4 | 0.002 | |
| 95%CI | 1.82-2.02 | 1.97-2.16 | 2.01-2.16 | 2.11-2.43 | G2-G4 | 0.042 | |
| Left/2.5 cm | 0.116 | ||||||
| Mean ± SD | 1.69 ± 0.23 | 1.75 ± 0.28 | 1.76 ± 0.26 | 1.84 ± 0.33 | |||
| 95%CI | 1.60-1.78 | 1.69-1.82 | 1.69-1.83 | 1.75-1.94 | |||
| Left/3.0 cm | 0.091 | ||||||
| Mean ± SD | 1.56 ± 0.15 | 1.65 ± 0.21 | 1.68 ± 0.25 | 1.69 ± 0.24 | |||
| 95%CI | 1.49-1.62 | 1.58-1.72 | 1.60-1.75 | 1.62-1.77 | |||
| Right/1.5 cm | 0.160 | ||||||
| Mean ± SD | 2.37 ± 0.29 | 2.53 ± 0.41 | 2.61 ± 0.44 | 2.57 ± 0.38 | |||
| 95%CI | 2.25-2.50 | 2.41-2.65 | 2.46-2.75 | 2.41-2.72 | |||
| Right/2.0 cm | 0.131 | ||||||
| Mean ± SD | 1.98 ± 0.22 | 2.09 ± 0.36 | 2.12 ± 0.30 | 2.18 ± 0.43 | |||
| 95%CI | 1.90-2.07 | 2.00-2.18 | 2.04-2.20 | 2.05-2.30 | |||
| Right/2.5 cm | 0.419 | ||||||
| Mean ± SD | 1.69 ± 0.23 | 1.75 ± 0.29 | 1.73 ± 0.23 | 1.79 ± 0.35 | |||
| 95%CI | 1.60-1.77 | 1.68-1.82 | 1.67-1.79 | 1.69-1.90 | |||
| Right/3.0 cm | 0.061 | ||||||
| Mean ± SD | 1.53 ± 0.24 | 1.65 ± 0.32 | 1.59 ± 0.18 | 1.70 ± 0.22 | |||
| 95%CI | 1.42-1.65 | 1.52-1.78 | 1.53-1.66 | 1.63-1.76 | |||
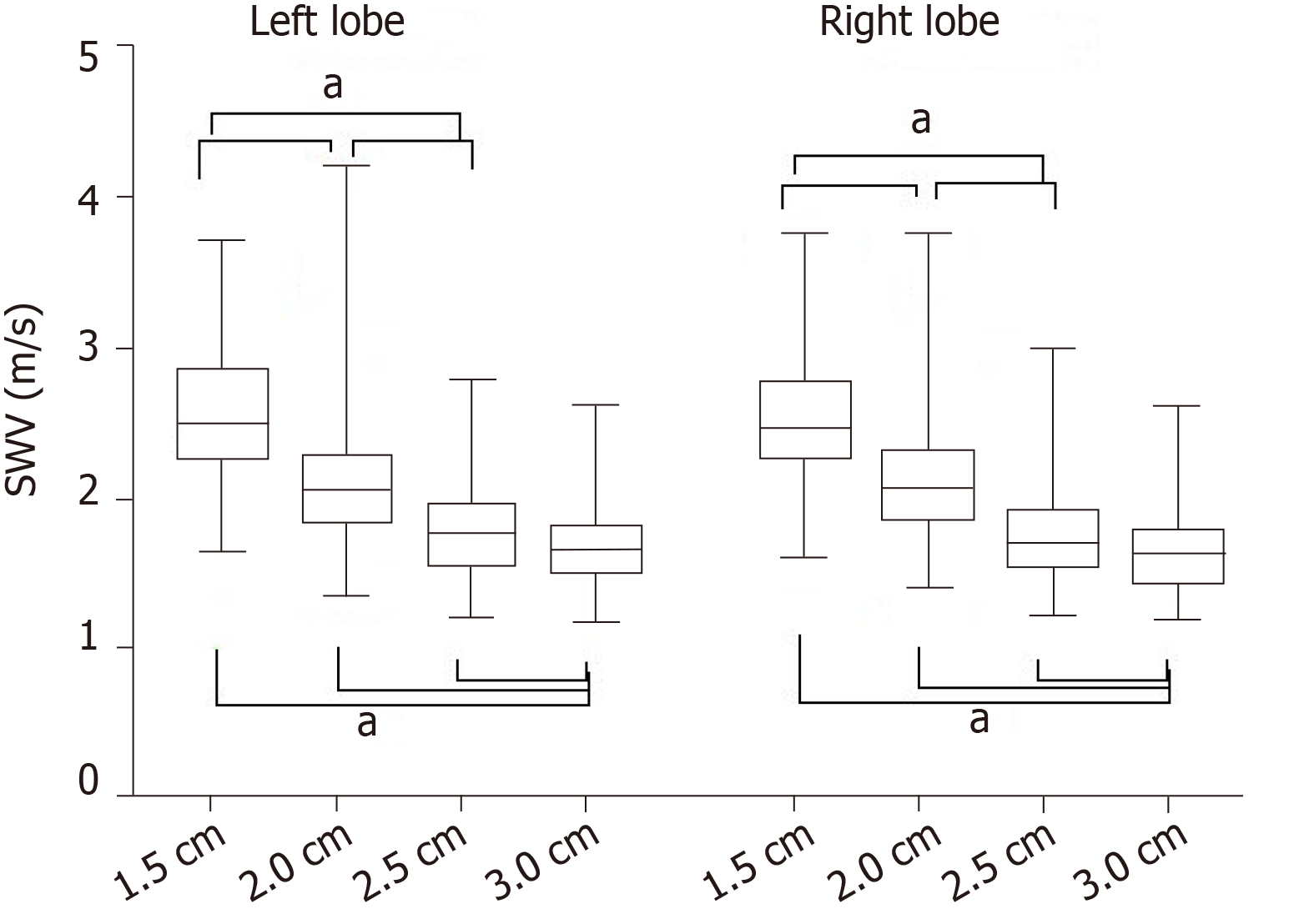
SWV values were significantly negatively correlated with the measurement depth in each lobe (P < 0.001). The deeper the depth, the lower the SWV values. And there were significant differences in the SWV value between any two of the measurement depths (P < 0.05, Figure 3). The SWV values at a depth of 3.0 cm had the lowest variance (P < 0.05). There was no significant difference in SWV values between the left and right lobes at the same depth (Figure 3), and there was no significant difference in SWV value variance between the left and right lobes.
No statistically significant correlations were found between BMI and the SWV in either lobe at each depth in our study group. After categorizing BMI into four groups, no significant differences were detected among the four groups at each measurement depth in either lobe (Table 4).
| Liver lobe/depth | BMI group (kg/m2) | P value | |||
| <15 (n = 23) | 15-16 (n = 65) | 16-17 (n = 54) | >17 (n = 60) | ||
| Left/1.5 cm | 0.599 | ||||
| Mean ± SD | 2.44 ± 0.38 | 2.59 ± 0.42 | 2.59 ± 0.50 | 2.58 ± 0.43 | |
| 95%CI | 2.27-2.62 | 2.48-2.70 | 2.44-2.75 | 2.40-2.77 | |
| Left/2.0 cm | 0.235 | ||||
| Mean ± SD | 1.94 ± 0.29 | 2.01 ± 0.40 | 1.99 ± 0.27 | 2.06 ± 0.50 | |
| 95%CI | 1.82-2.06 | 1.92-2.11 | 1.91-2.06 | 1.93-2.19 | |
| Left/2.5 cm | 0.363 | ||||
| Mean ± SD | 1.72 ± 0.25 | 1.77 ± 0.29 | 1.73 ± 0.26 | 1.81 ± 0.31 | |
| 95%CI | 162-1.82 | 1.70-1.84 | 1.66-1.80 | 1.73-1.90 | |
| Left/3.0 cm | 0.427 | ||||
| Mean ± SD | 1.58 ± 0.19 | 1.69 ± 0.22 | 1.67 ± 0.26 | 1.64 ± 0.22 | |
| 95%CI | 1.48-1.68 | 1.62-1.75 | 1.58-1.75 | 1.58-1.70 | |
| Right/1.5 cm | 0.218 | ||||
| Mean ± SD | 2.48 ± 0.36 | 2.60 ± 0.41 | 2.43 ± 0.40 | 2.57 ± 0.39 | |
| 95%CI | 2.29-2.26 | 2.48-2.72 | 2.30-2.56 | 2.42-2.71 | |
| Right/2.0 cm | 0.312 | ||||
| Mean ± SD | 2.04 ± 0.26 | 2.11 ± 0.39 | 2.03 ± 0.27 | 2.18 ± 0.39 | |
| 95%CI | 1.93-2.16 | 2.02-2.21 | 1.96-2.10 | 2.08-2.28 | |
| Right/2.5 cm | 0.064 | ||||
| Mean ± SD | 1.65 ± 0.22 | 1.79 ± 0.29 | 1.69± 0.21 | 1.79 ± 0.34 | |
| 95%CI | 1.56-1.75 | 1.71-1.86 | 1.63-1.75 | 1.70-1.87 | |
| Right/3.0 cm | 0.347 | ||||
| Mean ± SD | 1.55 ± 0.26 | 1.65 ± 0.27 | 1.60 ± 0.19 | 1.65 ± 0.24 | |
| 95%CI | 1.38-1.71 | 1.58-1.77 | 1.52-1.67 | 1.58-1.72 | |
Lastly, we investigated the potential factors which might influence the quality of elastic images (Table 5). The gender did not have any effect on the image quality (Table 5). A significant association was found between age and the quality of the elastic image; subjects with a relatively younger age tended to have a superior image quality in each liver lobe (P < 0.001). BMI also had an impact on the image quality, and it was significantly associated with the elastic image quality of the left lobe; subjects with a lower BMI tended to have a superior image quality (P < 0.001). However, the significant association could not be identified between BMI and the image quality of the right lobe (P = 0.075).
| Factor | Image quality | P value | Image quality | P value | ||
| Superior | Good | Superior | Good | |||
| Gender | 0.875 | 0.423 | ||||
| Male | 61 (70.9) | 25 (29.1) | 70 (81.4) | 16 (18.6) | ||
| Female | 84 (72.4) | 32 (27.6) | 89 (76.7) | 27 (23.3) | ||
| Age group (yr) | <0.001 | <0.001 | ||||
| <2 | 24 (85.7) | 4 (14.3) | 25 (89.3) | 3 (10.7) | ||
| 3-6 | 58 (85.3) | 10 (14.7) | 57 (83.8) | 11 (16.2) | ||
| 7-10 | 41 (70.7) | 17 (29.3) | 44 (75.9) | 14 (24.1) | ||
| >10 | 22 (45.8) | 26 (54.2) | 27 (56.3) | 21 (43.8) | ||
| BMI group (kg/m2) | <0.001 | 0.075 | ||||
| <15 | 21 (91.3) | 2 (8.7) | 17 (73.9) | 6 (26.1) | ||
| 15-16 | 60 (92.3) | 5 (7.7) | 56 (86.2) | 9 (13.8) | ||
| 16-17 | 34 (63.0) | 20 (37.0) | 45 (83.3) | 9 (16.7) | ||
| >17 | 30 (50.0) | 30 (50.0) | 41 (68.3) | 19 (31.7) | ||
In recent years, a variety of non-invasive ultrasound-based methods have been developed with the hope of eliminating the necessity for traditional liver biopsy in as many individuals as possible[18]. SWE is one of the latest methods, and offers a non-invasive, non-ionizing, low-cost, and well-tolerated approach to quantify liver stiffness and presumably liver fibrosis. Up to date, several studies[6,19,20] have assessed the usefulness of SWE techniques of ARFI for detecting liver fibrosis in children. Hence, a normal range of liver stiffness in children is expected to be established, to discriminate between normal and abnormal livers. To the best of our knowledge, this is the first study to define the normal SWV values in healthy children using the newest VTIQ mode of ARFI technology.
A few published articles about liver stiffness using VTQ technique had some contradictory results. The study of Eiler et al[5] showed that there was a significant effect of gender on the SWV value, and girls had lower SWV values. The explanation might be that estrogen in females might have an anti-fibrosis effect and there could be an intrinsic difference between genders in elasticity. Whereas, other researchers[4,6] had different results, which showed no significant differences in SWV values of the liver in children with different sex. Furthermore, the studies of Rifai et al[21] and Fontanilla et al[4] demonstrated that aging process might contribute to the increase of liver elasticity. Other researchers[5,6], however, reported that age showed no significant effect on SWV value. Similar contradictory results were shown about the effects of the location of ARFI measurements and the impact of BMI on SWV[22-30]. One important reason for all the discrepancies could be that VTQ technique with fixed and big ROI may not be a very repetitive method for children’s liver.
In our study, we used VTIQ to measure the stiffness of normal children liver and the results showed that no significant difference in SWV value was observed between boys and girls at each measurement depth in either lobe. Our results on the effects of age on SWV revealed a tendency toward higher SWV values in the relatively elder children. This did not reach statistical significance when the measurements were made in the right lobe, yet some significant differences were noted between measurements in different age groups in the left lobe (at depths of 1.5 and 2.0 cm, with higher SWV value in group 4). Also, our results indicated a significant correlation between SWV value and measurement depth. There was a significant difference in SWV among the measurement depths, with lower SWV values and lower variance at a deeper measurement depth. And these differences were observed in both hepatic lobes. Therefore, we recommend that the VTIQ measurement be conducted at the deep region of the liver parenchyma where the elasticity box with a homogeneous green color filling can be chosen.
With respect to the location of TVIQ measurements, no significant inter-lobe difference in SWV values at each measurement depth was found in our study. When we performed the elastography examination, the elastogram box in each lobe was only confined within the segments IV and V to minimize the measurement error. Despite that there is no significant inter-lobe difference, the elastography examination is recommended to perform in the right lobe, because the biopsy procedure for evaluating the liver fibrosis was performed on the right lobe, so was the elastic measurement. Moreover, the right lobe is larger, making it easier to place ROI in various regions and avoid vessels.
Data from the present study failed to demonstrate either a significant correlation between SWV and BMI, or the significant differences in SWV across the individual BMI classes. This result indicated that SWV values were reliable for people regardless of their BWI.
Lastly, we analyzed the factors which might influence the quality of the elastogram. Interestingly, the superior image quality in each lobe could frequently be found in relatively younger subjects, and BMI of the subjects might have a greater influence on the image quality in the left lobe. Therefore, as mentioned above, the right lobe of the liver may be a better choice for elastography measurement.
The present study has several limitations. First, since all repeated measurements were made by a single examiner, the inter-observer reproducibility could not be evaluated. Second, the choice of healthy children was based solely on normal laboratory liver tests, medical history, and normal appearance of the abdominal ultrasound examination. No liver biopsy was performed to exclude the liver diseases; however, its application in healthy population is not justified. Third, the sample volume in our study was not large enough. The results of this study need to be testified by further studies with more subjects.
In conclusion, our study suggested that VTIQ is a feasible technique to measure liver stiffness in children, and reported the normal liver stiffness measured by the VTIQ method. The lower SWV values are expected in younger children, and at a deeper measurement depth. Liver elasticity is not affected by gender, BMI, and the location of measurement. When available, the VTIQ measurement in the right lobe at deep regions is preferably suggested.
Non-invasive assessment of liver stiffness in children with chronic liver disease plays a critical role in clinical evaluation of liver fibrosis. Virtual touch imaging quantification (VTIQ) based on acoustic radiation force impulse imaging has been developed as a latest and noninvasive method for assessing liver stiffness in children.
Given the diversity of children in all aspects, a normal range of liver stiffness measurements must be established in children to differentiate the normal liver from abnormalities.
To determine the standard shear wave velocity (SWV) values in healthy children, and to identify possible factors that might influence the VTIQ measurement.
We conducted a prospective study. After exclusion, a total of 202 children between 1 mo and 15 years old were included in this study. None of them had any liver or systematic diseases. All children had a normal ultrasound scan and normal body mass index (BMI) range. The subjects were divided into four age and BMI groups. The effects of gender, age, liver lobe, measurement depth, and BMI on liver elasticity were investigated.
A significant correlation was found between age and SWV values. At measurement depths of 1.5 cm and 2.0 cm in the left lobe, there were significant differences among the four age groups. Negative correlations were found between the measurement depth and SWV values. Gender, liver lobe, and BMI showed no significant effect on the SWV values. Age and BMI may influence the quality of the elastogram.
As a recently developed noninvasive technique, VTIQ is feasible for physicians to measure liver stiffness in children. Lower SWV values are expected in younger children, and at a deeper measurement depth. Liver elasticity is not affected by gender, BMI, and the location of measurement. Therefore, when available, the VTIQ measurement at deep regions in the right lobe of the liver is preferably suggested.
The results of this study provide a range of normal reference values for measuring the liver elasticity in normal children. In the future, more subjects should be incorporated to further confirm the results of this study, and the consistency between observers needs to be evaluated so that VTIQ technology can be better applied to clinical practice. More importantly, with the reliable normal reference value, this noninvasive method can widely be used to detect and evaluate liver fibrosis in children.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim KC, Lin JK S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Tanner MS. Mechanisms of liver injury relevant to pediatric hepatology. Crit Rev Clin Lab Sci. 2002;39:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, Grulich-Henn J, Schenk JP, Teufel U. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 2012;171:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Dillman JR, Heider A, Bilhartz JL, Smith EA, Keshavarzi N, Rubin JM, Lopez MJ. Ultrasound shear wave speed measurements correlate with liver fibrosis in children. Pediatr Radiol. 2015;45:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Fontanilla T, Cañas T, Macia A, Alfageme M, Gutierrez Junquera C, Malalana A, Luz Cilleruelo M, Roman E, Miralles M. Normal values of liver shear wave velocity in healthy children assessed by acoustic radiation force impulse imaging using a convex probe and a linear probe. Ultrasound Med Biol. 2014;40:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Eiler J, Kleinholdermann U, Albers D, Dahms J, Hermann F, Behrens C, Luedemann M, Klingmueller V, Alzen GF. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med. 2012;33:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol. 2013;43:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Galina P, Alexopoulou E, Zellos A, Grigoraki V, Siahanidou T, Kelekis NL, Zarifi M. Performance of two--dimensional ultrasound shear wave elastography: reference values of normal liver stiffness in children. Pediatr Radiol. 2019;49:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Goldschmidt I, Streckenbach C, Dingemann C, Pfister ED, di Nanni A, Zapf A, Baumann U. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. 2013;57:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Matos H, Trindade A, Noruegas MJ. Acoustic radiation force impulse imaging in paediatric patients: normal liver values. J Pediatr Gastroenterol Nutr. 2014;59:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lee MJ, Kim MJ, Han KH, Yoon CS. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013;82:e290-e294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Özkan MB, Bilgici MC, Eren E, Caltepe G, Yilmaz G, Kara C, Gun S. Role of Point Shear Wave Elastography in the Determination of the Severity of Fibrosis in Pediatric Liver Diseases With Pathologic Correlations. J Ultrasound Med. 2017;36:2337-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Boursier J, Cassinotto C, Hunault G, Shili S, Lebigot J, Lapuyade B, Lannes A, Hiriart JB, Cartier V, Le Bail B, Michalak S, Mouries A, Oberti F, Chermak F, Fouchard-Hubert I, Cales P, Aube C, de Ledinghen V. Criteria to Determine Reliability of Noninvasive Assessment of Liver Fibrosis With Virtual Touch Quantification. Clin Gastroenterol Hepatol. 2019;17:164-171.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Yoo H, Lee JM, Yoon JH, Lee DH, Chang W, Han JK. Prospective Comparison of Liver Stiffness Measurements between Two Point Shear Wave Elastography Methods: Virtual Touch Quantification and Elastography Point Quantification. Korean J Radiol. 2016;17:750-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Sherman D, Lung P, Shorvon P. Virtual touch quantification (VTq) elastography for non-invasive assessment of liver disease and its complications: what the clinician needs to know. Frontline Gastroenterol. 2017;8:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Chen YP, Han T, Wu R, Yao MH, Xu G, Zhao LX, Liu H, Pu H, Fang Y. Comparison of Virtual Touch Tissue Quantification and Virtual Touch Tissue Imaging Quantification for diagnosis of solid breast tumors of different sizes. Clin Hemorheol Microcirc. 2016;64:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Yang YP, Xu XH, Bo XW, Liu BJ, Guo LH, Xu JM, Sun LP, Xu HX. Comparison of Virtual Touch Tissue Imaging & Quantification (VTIQ) and Virtual Touch Tissue Quantification (VTQ) for diagnosis of thyroid nodules. Clin Hemorheol Microcirc. 2017;65:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Leschied JR, Dillman JR, Bilhartz J, Heider A, Smith EA, Lopez MJ. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol. 2015;45:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L'abbate L, Nunnari G, Bronte F, Di Marco V, Craxì A, Cammà C. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Monti L, Manco M, Lo Zupone C, Latini A, D'Andrea ML, Alghisi F, Lucidi V, Tomà P, Bonomo L. Acoustic radiation force impulse (ARFI) imaging with Virtual Touch Tissue Quantification in liver disease associated with cystic fibrosis in children. Radiol Med. 2012;117:1408-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Shima H, Igarashi G, Wakisaka M, Hamano S, Nagae H, Koyama M, Kitagawa H. Noninvasive acoustic radiation force impulse (ARFI) elastography for assessing the severity of fibrosis in the post-operative patients with biliary atresia. Pediatr Surg Int. 2012;28:869-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Rifai K, Sebagh M, Karam V, Saliba F, Azoulay D, Adam R, Castaing D, Bismuth H, Reynès M, Samuel D, Féray C. Donor age influences 10-year liver graft histology independently of hepatitis C virus infection. J Hepatol. 2004;41:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Zhao H, Song P, Urban MW, Kinnick RR, Yin M, Greenleaf JF, Chen S. Bias observed in time-of-flight shear wave speed measurements using radiation force of a focused ultrasound beam. Ultrasound Med Biol. 2011;37:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med. 2013;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | D'Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Son CY, Kim SU, Han WK, Choi GH, Park H, Yang SC, Choi JS, Park JY, Kim DY, Ahn SH, Chon CY, Han KH. Normal liver elasticity values using acoustic radiation force impulse imaging: a prospective study in healthy living liver and kidney donors. J Gastroenterol Hepatol. 2012;27:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Galgenmueller S, Jaeger H, Kratzer W, Schmidt SA, Oeztuerk S, Haenle MM, Mason RA, Graeter T. Parameters affecting different acoustic radiation force impulse applications in the diagnosis of fibrotic liver changes. World J Gastroenterol. 2015;21:8425-8432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |