Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3394
Peer-review started: June 6, 2019
First decision: September 9, 2019
Revised: September 20, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 6, 2019
Processing time: 156 Days and 18.5 Hours
Secondary haemophagocytic lymphohistiocytosis (sHLH) is a rare life-threatening condition mainly associated with underlying infections, malignancies, and autoimmune or immune-mediated diseases.
To analyse all sHLH cases that were diagnosed and managed under real-world circumstances in our department focusing on the treatment schedule and the outcome.
Prospectively collected data from all adult patients fulfilling the criteria of sHLH who diagnosed and managed from January 1, 2010 to June 1, 2018, in our department of the tertiary care university hospital of Larissa, Greece, were analysed retrospectively (n = 80; 52% male; median age: 55 years). The electronic records and/or written charts of the patients were reviewed for the demographic characteristics, clinical manifestations, underlying causes of sHLH, laboratory parameters, treatment schedule and 30-d-mortality rate. Most of patients had received after consent intravenous γ-immunoglobulin (IVIG) for 5 d (total dose 2 g/kg) in combination with intravenous steroid pulses followed by gradual tapering of prednisolone.
Seventy-five patients (94%) reported fever > 38.5 °C, 47 (59%) had liver or spleen enlargement and 76 (95%) had ferritin > 500 ng/mL including 20 (25%) having considerably high levels (> 10000 ng/mL). Anaemia and thrombocytopenia occurred in 72% and leucopoenia in 47% of them. Underlying infections were diagnosed in 59 patients (74%) as follows: leishmaniasis alone in 15/80 (18.9%), leishmaniasis concurrently with Coxiella Burnetti or non-Hodgkin lymphoma in 2/80 (2.5%), bacterial infections in 14/80 (17.5%) including one case with concurrent non-Hodgkin lymphoma, viral infections in 13/80 (16.3%), fungal infections in 2/80 (2.5%), infections by mycobacteria in 1/80 (1.3%) and unidentified pathogens in 12/80 (15%). Seventy-two patients (90%) had received combination treatment with IVIG and intravenous steroids. Overall, sHLH resolved in 76% of patients, 15% died within the first month but 82.5% of patients were still alive 6 mo after diagnosis. Univariate analysis showed older age, anaemia, thrombocytopenia, low fibrinogen, disseminated intravascular coagulation (DIC), and delay of diagnosis as factors that negatively affected remission. However, multivariate analysis showed low platelets and DIC as the only independent predictors of adverse outcome.
sHLH still carries a remarkable morbidity and mortality. Underlying infections were the major cause and therefore, they should be thoroughly investigated in patients with sHLH. Early recognition and combination treatment with IVIG and corticosteroids seem an efficient treatment option with successful outcome in this life-threatening condition.
Core tip: This retrospective study analysed all adult patients (n = 80) with secondary haemophagocytic lymphohistiocytosis (sHLH) diagnosed in our tertiary care hospital during 2010-2018 paying considerable attention on the treatment schedule and the outcome of patients. In our large cohort, infections were the major cause of sHLH (74% of patients) and therefore, they should be thoroughly investigated in such patients. sHLH still carries a remarkable morbidity and mortality as 15% of patients died within the first month. Early recognition and treatment with intravenous γ-immunoglobulin in association with intravenous corticosteroids seem an efficient treatment option for successful outcome in this life-threatening condition (resolution in 76%; 6-mo survival: 82.5%).
- Citation: Georgiadou S, Gatselis NK, Stefos A, Zachou K, Makaritsis K, Rigopoulou EI, Dalekos GN. Efficient management of secondary haemophagocytic lymphohistiocytosis with intravenous steroids and γ-immunoglobulin infusions. World J Clin Cases 2019; 7(21): 3394-3406
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3394.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3394
Haemophagocytic lymphohistiocytosis (HLH) also known as haemophagocytotic syndrome, is a rare but life-threatening immune disorder characterized by clinical and laboratory evidence of uncontrolled hyper-inflammation due to massive proliferation of T cells and macrophages[1,2]. The most common manifestations are unremitting fever, hepatosplenomegaly, peripheral blood cytopenias, liver dysfunction, high ferritin levels and haemophagocytosis[3]. Depending on the aetiology, this syndrome can be caused by genetic mutations affecting cytotoxic function (primary or familial HLH) or secondary to infectious, autoimmune, or malignant conditions (acquired or secondary HLH; sHLH). However, in some cases no underlying disease or any triggering factor is identified (idiopathic cases)[4]. Ideally, the diagnosis of HLH is based upon fulfilling the published diagnostic criteria used in the HLH-2004 trial of Histiocyte Society[5]. Early recognition of the syndrome is crucial because without prompt initiation of treatment, HLH is often fatal. Nevertheless, in every-day clinical practice the assessment of haemophagocytosis on bone marrow aspirates is not feasible for all patients while frequently, there is also lack of data on natural killer (NK) cell activity and soluble CD25 levels[6-10]. Therefore, clinicians often confront the dilemma to start treatment earlier in patients who do not fulfil all the above criteria. Treatment of HLH may vary according to the cause including immunosuppressive and immunomodulatory agents, treatment of the underlying disease, chemotherapeutic agents and hematopoietic stem cell transplantation[11-13].
The aim of the present study was to analyse a large cohort of adult patients with sHLH who were diagnosed and managed in our department during 2010-2018 paying particular attention on the treatment schedules and the outcome of the patients.
All adult patients with sHLH who were prospectively diagnosed and managed at the Department of Medicine of the University Hospital of Larissa, Greece, from January 1, 2010 to June 1, 2018, were assessed retrospectively. The electronic records and/or written charts of the patients were reviewed for demographic characteristics, clinical manifestations, underlying causes of sHLH, laboratory parameters, treatment and the 30-d-mortality rate. All cases met at least 5 out of the following 8 2004-HLH international criteria for the diagnosis of HLH[5]: Fever ≥ 38.5 °C, splenomegaly, peripheral blood cytopenias with at least two of the following three [haemoglobin (Hb) < 9g/dL; platelets (PLTs) < 100000/μL; absolute neutrophil count < 1000/μL], high serum triglycerides (fasting triglycerides > 265 mg/dL) and/or low fibrinogen levels (fibrinogen < 150 mg/dL), haemophagocytosis (showed in bone marrow, lymph node, or liver specimens), low or absent NK cell activity, ferritin > 500 ng/mL, and elevated soluble CD25 (soluble IL-2 receptor alpha) above two SD the age-adjusted laboratory-specific norms.
Serum levels of aspartate aminotransferase, alanine aminotransferase (ALT), gamma-glutamyl transferase, alkaline phosphatase, lactate dehydrogenase, C-reactive protein, ferritin, triglycerides, fibrinogen, and the erythrocyte sedimentation rate were determined using standard techniques. Available biopsy specimens from bone marrow, lymph node or liver were peer examined by two experienced pathologists.
Clinical response was assessed at the completion of treatment and was defined as cure (absence of fever and restoration of the abnormal laboratory parameters i.e., peripheral blood cytopenias, high triglycerides, low fibrinogen, ferritin > 500 ng/mL) or failure (persistent or worsening of clinical and laboratory findings).
The ethical committee of the University Hospital of Larissa approved the protocol which conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. Patients agreed to receive the treatment schedule by written consent at the time of interview. In case patients were not able to give consent (e.g., critically ill patients), a first-degree relative authorized to give consent.
Kolmogorov-Smirnov test was used to assess the normality of the distribution of variables. Quantitative values are expressed as mean ± SD or median (with interquartile range-IQR) where applicable. Data were analysed by Mann-Whitney U test, χ2 (two by two with Yates’s correction), independent-samples T test and binary logistic regression analysis. Survival analysis was carried out using Kaplan-Meier plot and the comparisons were done by log-rank test. Two-sided P values less than 0.05 were considered statistically significant. The statistical review of the study was performed by Gatselis NK and Zachou K who have MSc in biostatistics. All data analyses were performed using the statistical software SPSS version 20.0.
Over the period of 8-years of the study, 80 patients (52.5% males; mean age 52.1 ± 19.2 years) with sHLH were identified. The main clinical, physical, and laboratory findings of these patients are shown in Table 1. The median duration of symptoms until admission was 8 d (range 1-120 d) and the median time of diagnosis after admission was also 8 d (range 1-45 d). Overall, 75 patients (94%) reported high fever (≥ 38.5 °C), 24 (30%) respiratory symptoms while 20 (25%) presented skin rash. Physical examination revealed splenomegaly, hepatomegaly and lymphadenopathy in 59%, 59% and 46% of patients, respectively.
| Characteristic | n (%) |
| Clinical symptoms | |
| Fever | 75 (94) |
| Respiratory symptoms | 24 (30) |
| Skin rash | 20 (25) |
| Gastrointestinal symptoms | 8 (10) |
| CNS symptoms1 | 7 (9) |
| Physical findings | |
| Splenomegaly | 47 (59) |
| Hepatomegaly | 47 (59) |
| Lymphadenopathy | 37 (46) |
| Laboratory findings | |
| Elevated ferritin (≥ 500 ng/mL) | 76 (95) |
| (≥ 10000 ng/mL) | 20 (25) |
| Elevated inflammatory markers2 | 69 (86) |
| Complete blood count | |
| Anaemia | 58 (72) |
| Thrombocytopenia | 57 (71) |
| Leucopoenia/neutropenia | 38 (47)/9 (11) |
| Pancytopenia | 24 (30) |
| Elevated LFTs | 70 (87) |
| Elevated LDH | 61 (76) |
| High triglycerides | 55 (70) |
| Low fibrinogen levels | 18 (22) |
| DIC | 9 (11) |
Of interest, 76 patients (95%) had ferritin > 500 ng/mL, while 20 (25%) had significantly high levels (>10000 ng/mL). Moreover, 24 patients (30%) had pancytopenia and the vast majority of them had high levels of triglycerides (69%) and elevated liver enzymes (87.5%). The median levels of ALT, aspartate aminotransferase and lactate dehydrogenase were 77 (range 6-2192) U/L, 75 (range 7-1823) U/L and 390 (range 73-3920) U/L, respectively. Histological examination (specimens from bone marrow, lymph node, or liver) was available in 45 patients (56%). Haemophagocytosis was established in 14/45 samples (31%).
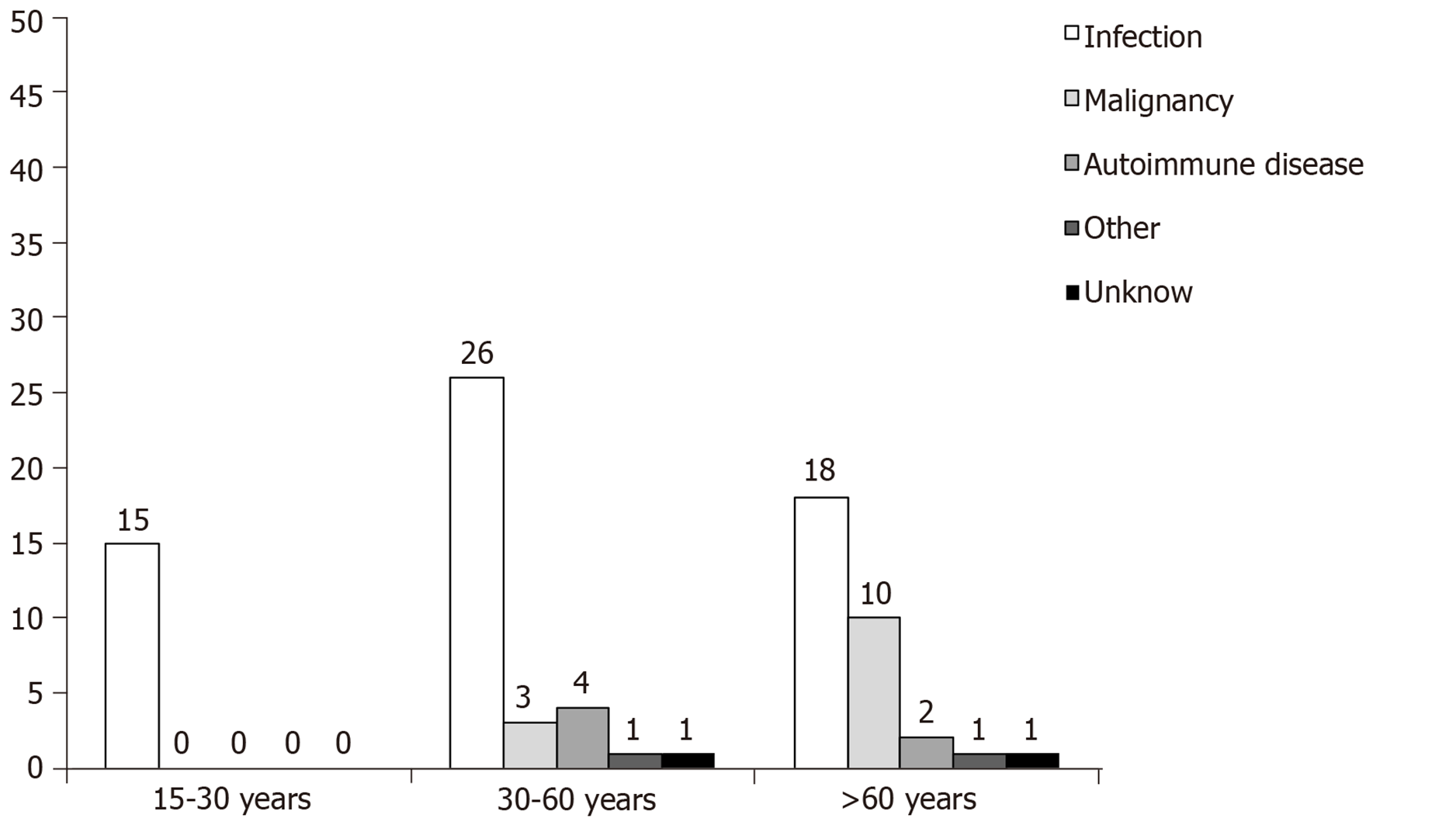
Infection was identified as the underlying cause of sHLH in the majority of cases (59/80; 74%) followed by neoplastic disease in 13/80 patients (16.2%) including two cases with concurrent infectious disease (Table 2). In more detail, leishmaniasis alone identified in 15/80 (18.9%), leishmaniasis concurrently with Coxiella Burnetti or non-Hodgkin lymphoma in 2/80 (2.5%), bacterial infections in 14/80 (17.5%) including one case with concurrent non-Hodgkin lymphoma, viral infections in 13/80 (16.3%), fungal infections in 2/80 (2.5%), infections by mycobacteria in 1/80 (1.3%) and unidentified pathogens in 12/80 (15%) patients (Table 2). In six patients (7.5%) the underlying cause was an autoimmune disease. The precise underline condition in the sHLH patients of the study is shown in Table 2. Infections were exclusively the underlying cause of sHLH in the young age group (15-30 years old) while in the middle and older age groups (30-60 years and > 60 years, respectively) the frequency of infections albeit predominant, was declined (74.3% and 60%, respectively; Figure 1) followed by the presence of neoplastic disease (33.3%) in the older patients. In two patients, the underlying cause was not identified (idiopathic sHLH cases).
| Disease | n |
| Infection | |
| Parasite | |
| Leishmania spp. alone | 15 |
| Leishmania spp. plus Coxiella Burnetti | 1 |
| Bacteria | |
| Coxiella Burnetti alone | 3 |
| Staphylococcus aureus | 2 |
| Acinetobacter baumannii plus Achromobacter xylosoxidans | 1 |
| Acinetobacter baumannii plus Aspergillus spp. | 1 |
| Achromobacter xylosoxidans alone | 1 |
| E. Coli | 2 |
| Pseudomonas aeruginosa | 1 |
| Rickettsia spp. | 1 |
| Brucella spp. | 1 |
| Virus | |
| EBV | 7 |
| Influenza (A/H3N2) | 3 |
| HSV | 1 |
| CMV1 | 1 |
| Measles | 1 |
| Fungi | |
| Aspergillus spp. | 1 |
| Candida spp. | 1 |
| Mycobacteria | |
| Mycobacterium Fortuitum | 1 |
| Unidentified pathogen | 12 |
| Neoplasia | |
| Non-Hodgkin lymphoma | 10 |
| Non-Hodgkin lymphoma accompanied by Leismaniasis or Coxiella Burnetti | 2 |
| Solid tumour | 1 |
| Autoimmune disease | |
| Adult Still’s disease | 5 |
| Psoriatic arthritis | 1 |
| Other | |
| Toxic epidermal necrolysis | 1 |
| Wilson disease | 1 |
| Unknown | 2 |
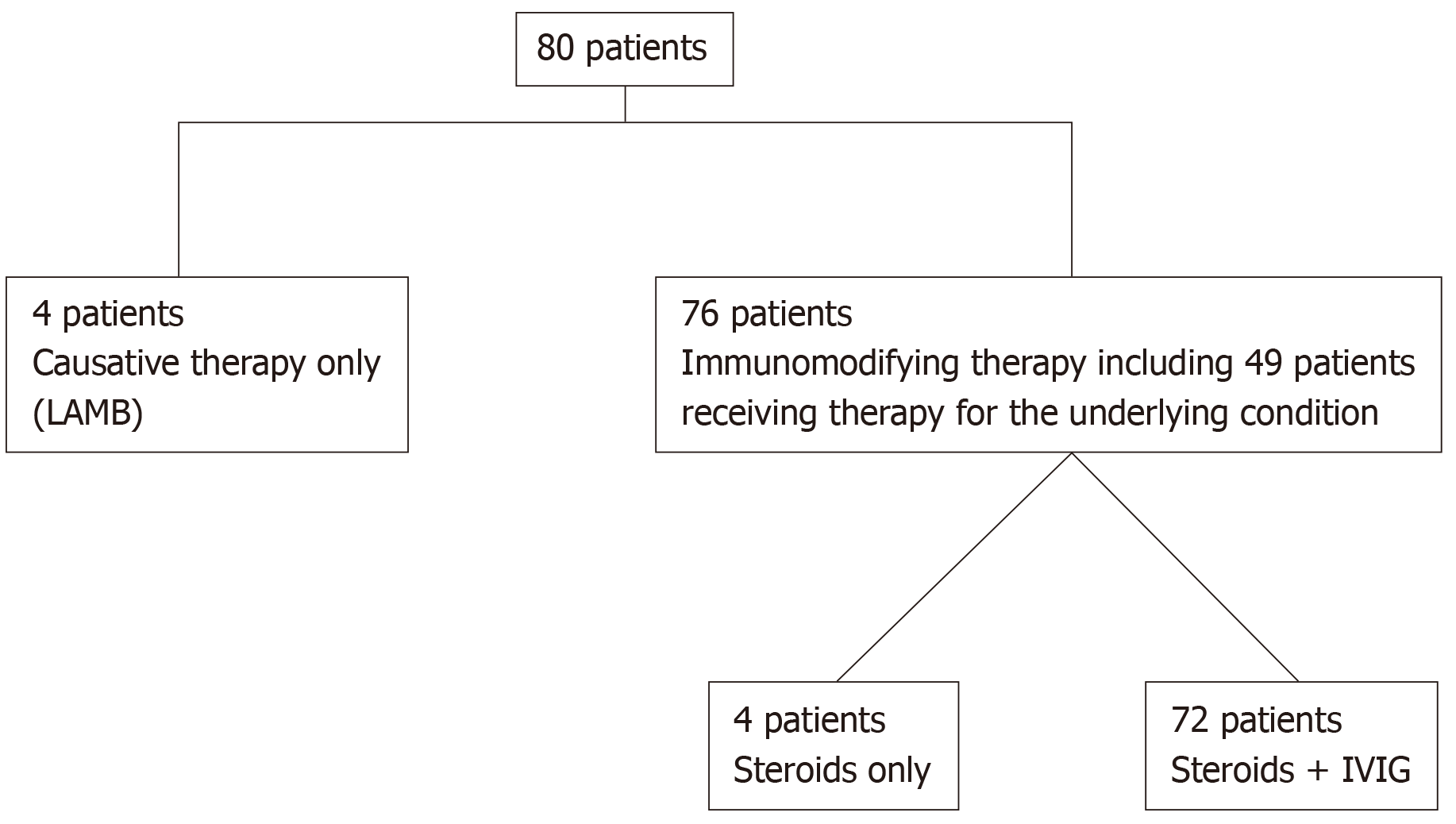
The majority of patients (76/80; 95%) received immune modifying treatment with either only intravenous steroid pulses (1 g of methylprednisolone for 1-3 consecutive days) followed by intravenous 1 mg/kg/d of prednisolone with appropriate rapid tapering of 10 mg every fourth day (n = 4 patients) or the above schedule of corticosteroids in combination with intravenous γ-immunoglobulin (IVIG; 0.4 g/kg/d for 5 d; n = 72 patients). Forty-nine out of these 76 patients (64.5%) received also treatment for the underlying cause either with antibiotic, antiviral, antifungal or antiparasitic therapy for infections, chemotherapy or other immunosuppressive treatment for malignancies or rheumatologic diseases, respectively. The remaining 4 patients (5%) received only causative treatment for visceral leishmaniasis (VL) with liposomal amphotericin B (LAMB; total dose: 21 mg/kg; Figure 2). The mean duration of treatment after diagnosis of sHLH was 35 ± 30 d. Six patients (7%) complicated with nosocomial infection and one developed LAMB-related myocarditis with a very low ejection fraction and acute pulmonary oedema that resolved after drug withdrawal. IVIG administration was well-tolerated by most of the 72 patients. Only three patients developed dyspnoea and hypertension which were managed by temporary discontinuation of the infusion and symptomatic therapy.
The majority of patients (76%) were cured following treatment. No association was found between remission and sex, clinical symptoms, and physical findings (data not shown). However, remission rates were significantly different in the three age groups (100% in the young age group, 85.7% in the middle and 53.3% in the older, P < 0.01). Patients with remission were younger compared to those without remission (47 ± 6 vs 66.6 ± 12.4 years, respectively, P < 0.001). In addition, anaemia, low fibrinogen and disseminated intravascular coagulation (DIC) at baseline were more frequent in patients that did not achieve remission during treatment (anaemia was present in 100% of non-responders vs 63.9% of responders, P < 0.01; low fibrinogen levels in 50% of non-responders vs 14.8% of responders, P < 0.01 and DIC in 44.4% of non-responders vs 1.6% of responders, P < 0.001). Treatment complications were also more frequent in patients without remission (38.9% vs 18%, P < 0.05). Of note, although remission was more frequent in patients with underlying infections (81.4%) and autoimmune diseases (83.3%) compared to patients with underlying neoplastic diseases (60%), this difference was not statistically significant. Furthermore, Hb and PLTs were lower in patients without remission compared to patients with remission [Hb: 7.8 (1.3) vs 9.1 (3.1) g/dL, P < 0.001; PLTs 35000 (57500) vs 94000 (87000)/μL, P < 0.001)]. On the contrary, ALT, alkaline phosphatase and time-to-diagnosis were higher in patients without remission [ALT 192 (48) vs 103 (151) U/L, P < 0.05; alkaline phosphatase 163 (162) vs 95 (118) U/L, P < 0.05; time-to-diagnosis 10 (20) vs 4 (6) d, P < 0.05)]. When all the above parameters that were associated with remission in the univariate analysis entered in a multivariate logistic regression model, remission was independently associated only with higher PLTs (OR = 1.045, 95%CI: 1.008-1.085, P = 0.01) and the absence of DIC (OR = 0.037, 95%CI: 0.002-0.848, P < 0.05).
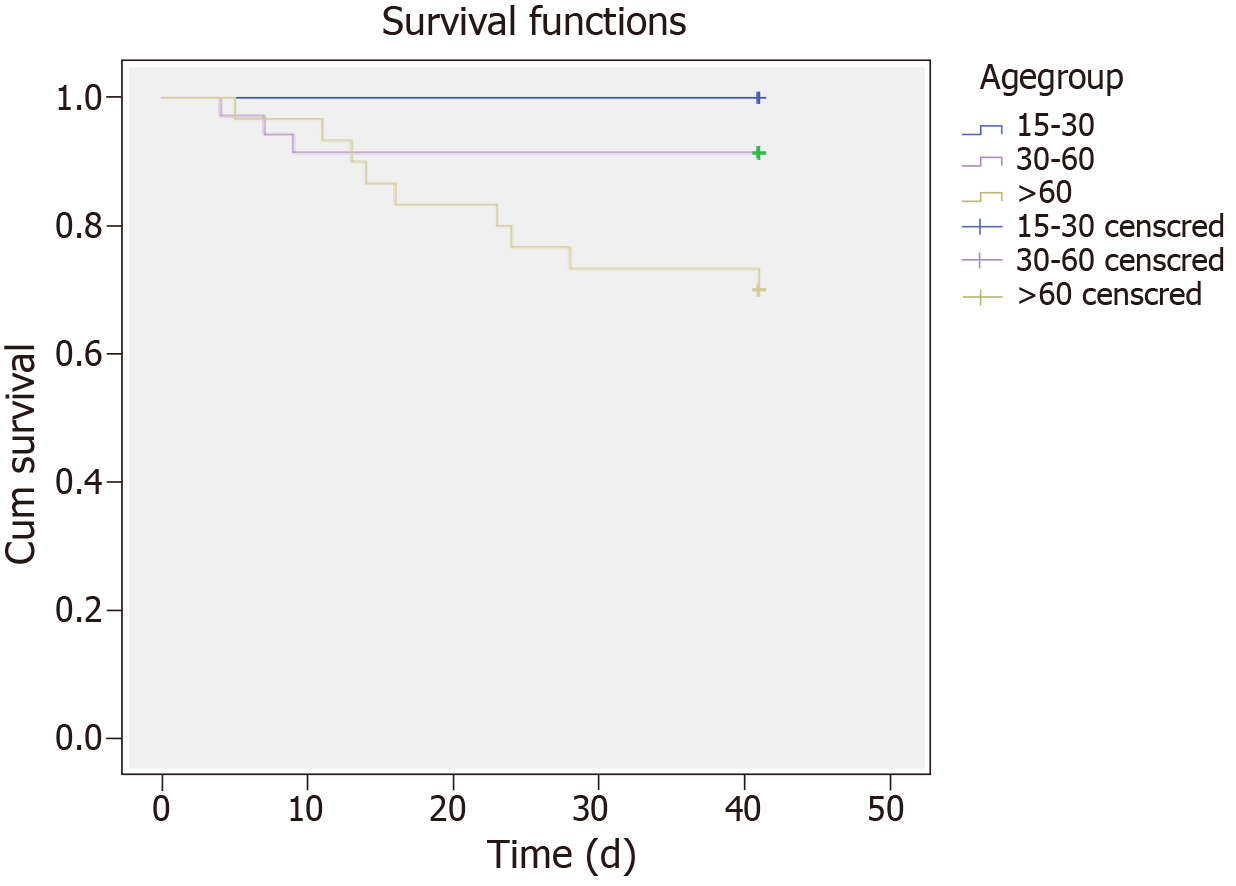
Twelve patients (15%) died within the first month after diagnosis. The underlying cause of sHLH was infection in nine patients (75%), malignancy in one patient (8.3%) and unknown in the remaining two patients (16.7%). Survival was significantly worse for patients in the older age group (log-rank test χ2 = 8.510, P = 0.01; Figure 3). However, 66 patients (82.5%) were still alive 6 mo after diagnosis of sHLH.
In this single-centre, real-world study we describe our experience regarding the diagnosis and management of a large cohort of sHLH cases admitted to an Internal Medicine department. The main findings arose from our large study were: (A) sHLH patients characterised by variable clinical and laboratory features including high fever, hepatosplenomegaly, cytopenias, high triglycerides, low fibrinogen levels, elevated LFTs along with a progressive disease course; however, no single clinical or laboratory finding was specific for the diagnosis of sHLH; (B) In accordance with previous studies[14-16], infections were the most prevalent underlying cause (74%) in all age groups, although declined with increasing age[17]; (C) Although older age, anaemia, thrombocytopenia, low fibrinogen levels, DIC, and delay of diagnosis were factors that negatively affected response to treatment in the univariate analysis, only the development of DIC and low PLTs were independently associated with adverse outcome; and (D) Most importantly by using a less toxic therapeutic schedule than etoposide containing regimen, we achieved a quite high remission rate (76%) and 6-mo survival (82.5%) albeit a 30-d mortality rate of 15%.
Typically, HLH diagnosis is based upon the published diagnostic criteria used in the HLH-2004 trial[5]. Interestingly, these criteria were developed for the diagnosis of paediatric HLH, but have also been applied - although not validated - in adult patients with sHLH. During the study period there were no internationally accepted and validated criteria for the diagnosis of sHLH in the adult population and therefore, a timely diagnosis which is crucial for remission and survival was challenging because of the rarity of this syndrome, its heterogeneous presentation, and the time and/or availability which is required to perform the diagnostic testing[18-21]. In any case, a high index of suspicion is necessary for the early diagnosis of this catastrophic syndrome and treatment could potentially be started while awaiting either the results of complex immunologic testing or until the fulfilment of at least 5 out of 8 HLH-2004 diagnostic criteria[22]. Of note, soluble CD25 levels and NK cell activity cannot be determined in every-day clinical practice in our hospital which is also common for many other institutes[6-10]. Thus, our clinical decision for the diagnosis of sHLH had to be based on the rest 6/8 HLH-2004 criteria.
Considering infections, it has been shown that many infectious agents can act as triggering factors for the development of sHLH, including viruses, bacteria, fungi and parasites[22]. The most common underlying infection in our cohort was VL which is endemic in our country[23-25]. Therefore, it should be always investigated in patients with sHLH from endemic areas such as the Mediterranean basin[25,26]. A high clinical suspicion, as well as the use of modern, molecular diagnostic techniques such as polymerase chain reaction, may lead to the early diagnosis of VL-associated sHLH, minimizing unnecessary hospitalization, potentially harmful investigations, overtreatment and unnecessary immunosuppression for sHLH[23,25]. Of interest, another zoonotic disease -Q fever, which is in general a very rare cause of sHLH[27] - was also diagnosed in a few cases in our cohort. This disease is also endemic in our country. Moreover, among underlying viral infections, Epstein-Barr virus was the most prevalent which is not surprising as it is considered one of the leading triggers associated with sHLH[22,28]. Autoimmune diseases were the second more frequent underlying cause of sHLH in the middle age group, while neoplastic diseases were in the older age group[17]. The most common autoimmune disease was adult Still’s disease, a well-known cause of sHLH[29]. Considering the neoplastic diseases, the most frequent underlying cause was non-Hodgkin lymphomas (high grade B-cell or T-cell); sHLH has already been reported in association with lymphoproliferative disorders, including T, NK, and anaplastic large cell lymphomas and leukaemia with a very poor prognosis[16,30]. Interestingly, the diagnosis of sHLH may occur either concurrently with malignancy, before or during chemotherapy, but very rarely in case of remission of the neoplastic disease[8]. Depending on the clinical department of admission, several other investigators have reported neoplastic diseases, and not infections, as the most frequent underlying cause of sHLH[7,10,20].
Several clinical and laboratory parameters have been associated with worse prognosis. Our results are in agreement with previous studies who also underscore the association of both decreased PLTs count[14,20,31] and the presence of DIC[32] with poor outcome. In addition, although remission was more frequent in patients with underlying infections (81.4%) and autoimmune diseases (83.3%) compared to patients with underlying neoplastic diseases (60%), this difference did not achieve statistical significance. Underlying malignancies have already been implicated for worse survival[9,10,16,20,31,33]. In our cohort only 13 patients (16.2%) had an underlying neoplastic disease which could also contribute partly to the low total 30-d mortality rate of our patients (15%). One month-survival was also worse for patients in the older age group (> 60 years) although the age was not an independent predictor of treatment response in the multivariate analysis. Increased age, especially above 50 years, seems to be a significant factor associated with mortality as these patients are more susceptible to multiple severe organ dysfunction[16]. Contrary to our findings, many other negative prognostic factors have already been reported in previous studies including male sex, central nervous system involvement, remarkably high ferritin levels (> 50000 ng/mL), persisting fever for more than 3 d despite treatment, low albumin levels, and infection with more than one microbiological agent[7,15,16,32,34,35].
Treatment of HLH is challenging and has been developed primarily for the paediatric patients where familial HLH is the leading cause of HLH. The Histiocyte Society Study Group for HLH developed the HLH-94 and HLH-2004 treatment protocols, which are also frequently used –although not validated for adults- arbitrarily by physicians treating sHLH in adults irrespective of the underlying cause[5,36]. These protocols include etoposide, corticosteroids, and cyclosporine that aim to eliminate activated cytotoxic lymphocytes and macrophages, as well as to restrain the cytokine cascade that promotes HLH[5,36]. However, as there are no randomized trials for the optimal management of sHLH in adults, treatment in every-day clinical practice varies widely among medical institutes. Notably, long-term results of the HLH-94 treatment protocol showed a quite poor 5-year survival rate of 54%with 89% of deaths recorded during the first year[37]. In general, the 30-d mortality rate of HLH is quite high, estimated in about 20%-44% in several case-series using various immunomodulatory therapeutic schemes[12] while the use of etoposide or cyclosporine containing regimens apart from the risk of several severe side effects[38-40], do not seem to be associated with a better outcome[7,34].
In our retrospective study which based on prospectively collected data, treatment was started as soon as possible (the sooner the better) with intravenous steroid pulses followed by gradual tapering of prednisolone in combination with intravenous IVIG as we have already published in a small series of infectious-related sHLH[41]. In parallel, appropriate treatment for the underlying cause was administered. Remission rate was quite high (76%) with a 30-d mortality rate of 15%. Similar remission rates were reported recently in two small retrospective studies from United States and Turkey in patients with sHLH by using a personalized diagnostic and less immunosuppressive and cytotoxic treatment approach which included treatment with anakinra, cyclosporine, IVIG and steroids[33,42]. In this context, it is worthy to state that the use of anakinra as a treatment option has already been described with favourable results in small studies[43] while on the other hand, IVIG has been proved effective in at least a subgroup of adults with sHLH when started at the beginning of the macrophage activation process, during the ferritin run-up to peak values[44]. However, the role of IVIG for the treatment of sHLH is not fully understood. IVIG has several well-known potential anti-inflammatory and immunomodulatory actions through broad and possibly synergistic mechanisms including alteration of activation, differentiation and functions of T-cells and B-cells and suppression of cytokine production[45-48]. This broad spectrum of mechanisms may explain the rationale and effectiveness of IVIG in sHLH. Unfortunately, in four patients IVIG was in short supply and thus they received treatment with intravenous steroid pulses followed by gradual tapering of prednisolone only. Two of them did not achieve remission and eventually died.
Although therapy directed against the underlying pathogen is usually not sufficient to control sHLH, recognition and management of the underlying disease seems of major importance. Thus, a careful microbiological, immunological and haematological workup is required to identify existing triggers of sHLH and especially infections due to Epstein-Barr virus, Cytomegalovirus or VL. Controlling of infectious diseases is an important component of sHLH management particularly in cases of VL, as specific treatment with LAMB alone, may be successful in cases of VL-associated sHLH[11,24]. Under this context, four of our patients with VL who received early treatment only with LAMB (without corticosteroids and/or IVIG infusions) achieved complete remission of the sHLH.
Our study has some limitations: (A) It is a retrospective single-centre real-world study; and (B) Long-term follow-up data is not available for all patients although 66 patients (82.5%) were still alive 6 mo after diagnosis of sHLH. However, our study showed that if management with intravenous pulsed steroids and IVIG starts promptly enough, can prevent deterioration of the syndrome.
During the period of the study (January 2010 - June 2018) the guidelines in use for the treatment of HLH, whether primary or secondary, included an initial 8 wk of chemo-immune therapy[5]. However, several investigators –including us[24,41] - questioned whether it is rationale all these patients with sHLH, particularly those with infections- or sepsis-associated haemophagocytosis, to receive intense immunosuppressive treatment[33,42,44,49-51]. Indeed, there are several reports for patients with sHLH who treated successfully after the administration of less immunosuppressive and less cytotoxic treatment modalities outside the HLH-2004 protocol including primarily anti-inflammatory agents and IVIG[24,33,41-44,49-51]. In this regard, although the grade of evidence in support of the latter intervention in a recent review was moderate[52] (level of evidence 3: obtained from well-designed non-experimental descriptive studies, such as comparative studies, correlation studies and case studies), the use of IVIG seems to be safe and effective as first-line treatment in a number of studies avoiding in parallel unnecessary toxicity[24,33,41,42,44,50,53-57]. Taking into account our preliminary results[24,41] as well as the abovementioned uncertainties we chose to treat our adult patients outside the HLH-2004 protocol with less cytotoxic agents as the vast majority of them (74%) suffered from underlying serious infections. Of note, the recent updated recommendations of the HLH Steering Committee of the Histiocyte Society for the management of HLH was published after the end of our study (October 2018[58] and April 2019[59]) so, it was impossible to take into account these new recommendations.
In conclusion, sHLH still carries a remarkable morbidity and mortality while adult-specific diagnostic criteria, are missing. In the absence of randomised controlled trials, the management of sHLH has in principle two targets. The first is to interrupt the severe hyper-inflammation and the second to identify the underlying trigger. In this context, we found infections as the major cause of sHLH and therefore, they should be thoroughly investigated in these patients. In addition, our real-world study showed that IVIG in association with intravenous corticosteroids seems efficient and safe first-line treatment option for successful outcome of this life-threatening condition, avoiding in parallel overtreatment and unnecessary toxicity. However, future prospective multicentre randomised control studies will be needed in order to definitely address the potential therapeutic benefit of IVIG in sHLH and in particular in infections- or autoimmunity-related sHLH.
Acquired or secondary haemophagocytic lymphohistiocytosis (sHLH) in adults is a heterogeneous disease triggered by infectious, autoimmune, or neoplastic disorders. sHLH is still associated with high morbidity and mortality. At the time of our study (January 2010-June 2018) the guidelines in use for the treatment of HLH, whether primary or secondary, included intensive immunosuppressive treatment.
As there are no randomized trials for the optimal management of sHLH in adults, treatment in every-day clinical practice varies widely among medical institutes. Nevertheless, several investigators are reluctant to treat patients with intense chemo-immune agents, particularly in cases with infection-associated sHLH.
To analyze all adult sHLH cases that were diagnosed and managed under real-world circumstances between 2010 and 2018 in our tertiary care hospital focusing on the treatment schedule and the outcome.
All adult patients with well-established sHLH who were diagnosed and managed at the Department of Medicine of the Larissa University Hospital, Greece from January 1, 2010 to June 1, 2018, were assessed retrospectively. The electronic records and/or written charts of the patients were reviewed for demographic characteristics, clinical manifestations, underlying causes of sHLH, laboratory parameters, treatment schedule and the 30-d-mortality rate.
Over this 8-year study period, 80 patients (52.5% males; mean age 52.1 ± 19.2 years) with sHLH were identified. In the majority of cases (74%), the underlying cause of sHLH was infection followed by neoplastic disease (16.2%) and autoimmune disease (7.5%). Seventy-two patients (90%) received combination treatment of intravenous γ-immunoglobulin (IVIG) and intravenous steroids, 4 patients received corticosteroids only (due to IVIG short supply) and 4 patients received treatment only for their underlying disease (visceral leishmaniasis) with liposomal amphotericin B. The majority of patients (76%) were cured following treatment. Twelve patients (15%) died within the first month after diagnosis but the 6-mo survival was 82.5%. Although older age, anaemia, thrombocytopenia, low fibrinogen, disseminated intravascular coagulation (DIC), and delay of diagnosis were factors that negatively affected response to treatment in the univariate analysis, only the development of DIC and low platelets were independently associated with adverse outcome.
Infections identified as the major cause of sHLH in our study and therefore, they should be thoroughly investigated in these patients. In addition, IVIG in combination with intravenous corticosteroids seems efficient and safe first-line treatment option for successful outcome of this life-threatening condition, avoiding in parallel overtreatment and unnecessary toxicity by using less immunosuppressive and less cytotoxic treatment modalities.
Using a less cytotoxic and less immunosuppressive therapeutic schedule, we achieved a quite high remission rate (76%) and 6-mo survival (82.5%) albeit a 30-day mortality rate of 15%. Future prospective multicenter randomized control studies are needed in order to definitely address the potential therapeutic benefit of IVIG in patients with sHLH and particularly in those with infections- or autoimmunity-related sHLH.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maher J S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16:S82-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 954] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 4. | Bode SF, Lehmberg K, Maul-Pavicic A, Vraetz T, Janka G, Stadt UZ, Ehl S. Recent advances in the diagnosis and treatment of hemophagocytic lymphohistiocytosis. Arthritis Res Ther. 2012;14:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3579] [Article Influence: 198.8] [Reference Citation Analysis (1)] |
| 6. | Ferreira DG, do Val Rezende P, Murao M, Viana MB, de Oliveira BM. Hemophagocytic lymphohistiocytosis: a case series of a Brazilian institution. Rev Bras Hematol Hemoter. 2014;36:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Li J, Wang Q, Zheng W, Ma J, Zhang W, Wang W, Tian X. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, Allen CE, Pierce SA, Cortes JE, Ravandi F, Konopleva MY, Garcia-Manero G, Benton CB, Chihara D, Rytting ME, Wang S, Abdelall W, Konoplev SN, Daver NG. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: Relation to hemophagocytosis, characteristics, and outcomes. Cancer. 2016;122:2857-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Rivière S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O, Fardet L. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Schram AM, Comstock P, Campo M, Gorovets D, Mullally A, Bodio K, Arnason J, Berliner N. Haemophagocytic lymphohistiocytosis in adults: a multicentre case series over 7 years. Br J Haematol. 2016;172:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | La Rosée P. Treatment of hemophagocytic lymphohistiocytosis in adults. Hematology Am Soc Hematol Educ Program. 2015;2015:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 13. | Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2013;2013:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Li F, Yang Y, Jin F, Dehoedt C, Rao J, Zhou Y, Li P, Yang G, Wang M, Zhang R, Yang Y. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: a retrospective study of increasing awareness of a disease from a single-center in China. Orphanet J Rare Dis. 2015;10:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Guo Y, Bai Y, Gu L. Clinical features and prognostic factors of adult secondary hemophagocytic syndrome: Analysis of 47 cases. Medicine (Baltimore). 2017;96:e6935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 17. | Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, Yamamoto K, Horiuchi H, Takada K, Ohshima K, Nakamura S, Kinukawa N, Oshimi K, Kawa K. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Davì S, Minoia F, Pistorio A, Horne A, Consolaro A, Rosina S, Bovis F, Cimaz R, Gamir ML, Ilowite NT, Kone-Paut I, Feitosa de Oliveira SK, McCurdy D, Silva CA, Sztajnbok F, Tsitsami E, Unsal E, Weiss JE, Wulffraat N, Abinun M, Aggarwal A, Apaz MT, Astigarraga I, Corona F, Cuttica R, D'Angelo G, Eisenstein EM, Hashad S, Lepore L, Mulaosmanovic V, Nielsen S, Prahalad S, Rigante D, Stanevicha V, Sterba G, Susic G, Takei S, Trauzeddel R, Zletni M, Ruperto N, Martini A, Cron RQ, Ravelli A; Paediatric Rheumatology International Trials Organisation, the Childhood Arthritis and Rheumatology Research Alliance, the Pediatric Rheumatology Collaborative Study Group, and the Histiocyte Society. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:2871-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 913] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 20. | Barba T, Maucort-Boulch D, Iwaz J, Bohé J, Ninet J, Hot A, Lega JC, Guérin C, Argaud L, Broussolle C, Jamilloux Y, Richard JC, Sève P. Hemophagocytic Lymphohistiocytosis in Intensive Care Unit: A 71-Case Strobe-Compliant Retrospective Study. Medicine (Baltimore). 2015;94:e2318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Batu ED, Erden A, Seyhoğlu E, Kilic L, Büyükasık Y, Karadag O, Bilginer Y, Bilgen SA, Akdogan A, Kiraz S, Ertenli AI, Özen S, Kalyoncu U. Assessment of the HScore for reactive haemophagocytic syndrome in patients with rheumatic diseases. Scand J Rheumatol. 2017;46:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 23. | Georgiadou SP, Stefos A, Spanakos G, Skrimpas S, Makaritsis K, Sipsas NV, Dalekos GN. Current clinical, laboratory, and treatment outcome characteristics of visceral leishmaniasis: results from a seven-year retrospective study in Greece. Int J Infect Dis. 2015;34:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Saitis A, Gatselis NK, Dalekos GN. Leishmaniasis-associated haemophagocytic syndrome revisited: Not an uncommon clinical presentation of leishmaniasis. J Med Cases. 2012;3:315-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Georgiadou SP, Makaritsis KP, Dalekos GN. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J Transl Int Med. 2015;3:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Koubâa M, Mâaloul I, Marrakchi Ch, Mdhaffar M, Lahiani D, Hammami B, Makni F, Ayedi A, Jemâa MB. Hemophagocytic syndrome associated with visceral leishmaniasis in an immunocompetent adult-case report and review of the literature. Ann Hematol. 2012;91:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Lecronier M, Prendki V, Gerin M, Schneerson M, Renvoisé A, Larroche C, Ziol M, Fain O, Mekinian A. Q fever and Mediterranean spotted fever associated with hemophagocytic syndrome: case study and literature review. Int J Infect Dis. 2013;17:e629-e633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Lai W, Wang Y, Wang J, Wu L, Jin Z, Wang Z. Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults and adolescents-a life-threatening disease: analysis of 133 cases from a single center. Hematology. 2018;23:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Fukaya S, Yasuda S, Hashimoto T, Oku K, Kataoka H, Horita T, Atsumi T, Koike T. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: analysis of 30 cases. Rheumatology (Oxford). 2008;47:1686-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Strenger V, Merth G, Lackner H, Aberle SW, Kessler HH, Seidel MG, Schwinger W, Sperl D, Sovinz P, Karastaneva A, Benesch M, Urban C. Malignancy and chemotherapy induced haemophagocytic lymphohistiocytosis in children and adolescents-a single centre experience of 20 years. Ann Hematol. 2018;97:989-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Arca M, Fardet L, Galicier L, Rivière S, Marzac C, Aumont C, Lambotte O, Coppo P. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Tseng YT, Sheng WH, Lin BH, Lin CW, Wang JT, Chen YC, Chang SC. Causes, clinical symptoms, and outcomes of infectious diseases associated with hemophagocytic lymphohistiocytosis in Taiwanese adults. J Microbiol Immunol Infect. 2011;44:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Kumar B, Aleem S, Saleh H, Petts J, Ballas ZK. A Personalized Diagnostic and Treatment Approach for Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Adults. J Clin Immunol. 2017;37:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 2014;89:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Brito-Zerón P, Kostov B, Moral-Moral P, Martínez-Zapico A, Díaz-Pedroche C, Fraile G, Pérez-Guerrero P, Fonseca E, Robles A, Vaquero-Herrero MP, Calvo MA, Forner MJ, Morcillo C, Larrañaga J, Rodriguez-Carballeira M, Ruiz-Muñoz M, Hurtado-García R, Prieto-González S, Aguilar AA, Caminal-Montero L, Hernández-Jiménez P, Fernández-Viagas CR, Castro P, Massó VM, Flores-Chavez A, Ramos-Casals M; REGHEM-GEAS-SEMI Study Group. Prognostic Factors of Death in 151 Adults With Hemophagocytic Syndrome: Etiopathogenically Driven Analysis. Mayo Clin Proc Innov Qual Outcomes. 2018;2:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Henter JI, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, Gadner H, Imashuku S, Komp D, Ladisch S, Webb D, Janka G; Histocyte Society. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 617] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 37. | Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G, Henter JI; Histiocyte Society. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 462] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 38. | Thompson PA, Allen CE, Horton T, Jones JY, Vinks AA, McClain KL. Severe neurologic side effects in patients being treated for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2009;52:621-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Seiter K. Toxicity of the topoisomerase II inhibitors. Expert Opin Drug Saf. 2005;4:219-234. [PubMed] |
| 40. | Seiter K, Feldman EJ, Sreekantaiah C, Pozzuoli M, Weisberger J, Liu D, Papageorgio C, Weiss M, Kancherla R, Ahmed T. Secondary acute myelogenous leukemia and myelodysplasia without abnormalities of chromosome 11q23 following treatment of acute leukemia with topoisomerase II-based chemotherapy. Leukemia. 2001;15:963-970. [PubMed] |
| 41. | Argyraki CK, Gabeta S, Zachou K, Boulbou M, Polyzos A, Dalekos GN. Favourable outcome of life-threatening infectious-related haemophagocytic syndrome after combination treatment with corticosteroids and intravenous immunoglobulin infusions. Eur J Intern Med. 2011;22:e155-e157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Haytoglu Z, Yazici N, Erbay A. Secondary Hemophagocytic Lymphohistiocytosis: Do We Really Need Chemotherapeutics for All Patients? J Pediatr Hematol Oncol. 2017;39:e106-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Sönmez HE, Demir S, Bilginer Y, Özen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. 2018;37:3329-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 44. | Emmenegger U, Frey U, Reimers A, Fux C, Semela D, Cottagnoud P, Spaeth PJ, Neftel KA. Hyperferritinemia as indicator for intravenous immunoglobulin treatment in reactive macrophage activation syndromes. Am J Hematol. 2001;68:4-10. [PubMed] |
| 45. | Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 46. | Kaveri SV, Lacroix-Desmazes S, Bayry J. The antiinflammatory IgG. N Engl J Med. 2008;359:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011;127:315-23; quiz 324-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Ballow M. Mechanisms of immune regulation by IVIG. Curr Opin Allergy Clin Immunol. 2014;14:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Demirkol D, Yildizdas D, Bayrakci B, Karapinar B, Kendirli T, Koroglu TF, Dursun O, Erkek N, Gedik H, Citak A, Kesici S, Karabocuoglu M, Carcillo JA; Turkish Secondary HLH/MAS Critical Care Study Group. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care. 2012;16:R52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Rajasekaran S, Kruse K, Kovey K, Davis AT, Hassan NE, Ndika AN, Zuiderveen S, Birmingham J. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children*. Pediatr Crit Care Med. 2014;15:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 52. | Gilardin L, Bayry J, Kaveri SV. Intravenous immunoglobulin as clinical immune-modulating therapy. CMAJ. 2015;187:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Álvarez-Cardona A, Rodríguez-Lozano AL, Blancas-Galicia L, Rivas-Larrauri FE, Yamazaki-Nakashimada MA. Intravenous immunoglobulin treatment for macrophage activation syndrome complicating chronic granulomatous disease. J Clin Immunol. 2012;32:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Gupta AA, Tyrrell P, Valani R, Benseler S, Abdelhaleem M, Weitzman S. Experience with hemophagocytic lymphohistiocytosis/macrophage activation syndrome at a single institution. J Pediatr Hematol Oncol. 2009;31:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Tristano AG, Casanova-Escalona L, Torres A, Rodríguez MA. Macrophage activation syndrome in a patient with systemic onset rheumatoid arthritis: rescue with intravenous immunoglobulin therapy. J Clin Rheumatol. 2003;9:253-258. [PubMed] |
| 56. | Seidel MG, Kastner U, Minkov M, Gadner H. IVIG treatment of adenovirus infection-associated macrophage activation syndrome in a two-year-old boy: case report and review of the literature. Pediatr Hematol Oncol. 2003;20:445-451. [PubMed] |
| 57. | Rajajee S, Ashok I, Manwani N, Rajkumar J, Gowrishankar K, Subbiah E. Profile of hemophagocytic lymphohistiocytosis; efficacy of intravenous immunoglobulin therapy. Indian J Pediatr. 2014;81:1337-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Ehl S, Astigarraga I, von Bahr Greenwood T, Hines M, Horne A, Ishii E, Janka G, Jordan MB, La Rosée P, Lehmberg K, Machowicz R, Nichols KE, Sieni E, Wang Z, Henter JI. Recommendations for the Use of Etoposide-Based Therapy and Bone Marrow Transplantation for the Treatment of HLH: Consensus Statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6:1508-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 59. | La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 636] [Article Influence: 106.0] [Reference Citation Analysis (0)] |