Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3341
Peer-review started: April 18, 2019
First decision: August 1, 2019
Revised: September 4, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 26, 2019
Processing time: 191 Days and 19.1 Hours
Hydrofluoric acid (HF) is one of the most common causes of chemical burns. HF burns can cause wounds that deepen and progress aggressively. As a result, HF burns are often severe even if they involve a small area of the skin. Published cases of HF burns have mostly reported small HF burn areas. Few cases of HF inhalation injury have been reported to date.
A 24-year-old man suffered from extensive hydrofluoric acid burns covering 60% of the total body surface area (TBSA), including deep second degree burns on 47% and third degree burns on 13% of the TBSA, after he fell into a pickling pool containing 15% HF. Comprehensive treatments were carried out after the patient was admitted. Ventricular fibrillation occurred 9 times within the first 2 h, and the lowest serum Ca2+ concentration was 0.192 mmol/L. A dose of calcium gluconate (37 g) was intravenously supplied during the first 24 h, and the total amount of calcium gluconate supplementation was 343 g. Extracorporeal membrane oxygenation (ECMO) was applied for 8 d to handle the acute respiratory distress syndrome (ARDS) induced by the HF inhalation injury. The patient was discharged after 99 d of comprehensive treatment, including skin grafting.
Extensive HF burns combined with an inhalation injury led to a potentially fatal electrolyte imbalance and ARDS. Adequate and timely calcium supplementation and ECMO application were the keys to successful treatment of the patient.
Core tip: Hydrofluoric acid (HF) is one of the most common causes of chemical burns. We present a case of extensive HF burns combined with inhalation injury and review the related literature. The patient had extensive HF burns covering 60% of the total body surface area. A dose of calcium gluconate (37 g) was intravenously supplied in the first 24 hours, and the total amount of calcium gluconate supplementation was 343 g. Extracorporeal membrane oxygenation was applied to handle the acute respiratory distress syndrome induced by the HF inhalation injury. We believe that our report makes a significant contribution to the literature.
- Citation: Fang H, Wang GY, Wang X, He F, Su JD. Potentially fatal electrolyte imbalance caused by severe hydrofluoric acid burns combined with inhalation injury: A case report. World J Clin Cases 2019; 7(20): 3341-3346
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3341.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3341
Hydrofluoric acid (HF) is a weak inorganic acid that is widely used in many industrial processes and domestic settings and is a cause of chemical burns. HF burns can cause the wounds to deepen and progress aggressively. As a result, HF burns are often severe even if they involve a small area of the skin. Published cases of HF burns have mostly reported small HF burn areas. To the best of our knowledge, no cases of HF inhalation injury have been reported to date. This paper presents a case of extensive HF burns combined with an inhalation injury.
A 24-year-old man weighing 68 kg fell into a pickling pool containing 15% HF while working in a chrome-plating workshop. The man was pulled out after 20 s of struggling, during which time he might have aspirated HF while coughing and choking. After removing his contaminated clothes and rinsing him with copious amounts of water for approximately 3 min, he was brought to the emergency department 1 h post exposure. On admission, the patient complained of dyspnea and shortness of breath.
His past health status was good, without prior illnesses.
The patient and his family had a free previous medical history.
His blood pressure was 89/65 mmHg, heart rate was 124 beats/min, and no urine output was noted. The burn sites were distributed over approximately 60% of the total body surface area (TBSA), of which approximately 13% were third-degree burns located on the face, right upper limb, and both lower limbs with a brown, tough, leather-like appearance, and the other 47% were deep second-degree burns located on the head, face, neck, trunk, perineum, buttocks, and limbs with a beige and soft appearance (Figure 1).
The electrocardiogram (ECG) results showed sinus tachycardia. The blood gas analysis results were pH 7.262, SpO2 99%, PaO2 323.7 mmHg, PaCO2 18.7 mmHg, Ca2+ 0.192 mmol/L, Mg2+ 0.40 mmol/L, Lac- 7.8 mmol/L, and HCO3- 8.3 mmol/L.
The diagnosis was extensive HF burns covering 60% of the TBSA (deep second degree, 47%; third degree, 13%) with severe HF inhalation injury, hypocalcemia, hypomagnesemia, burn shock (hypovolemic shock), metabolic acidosis, and respiratory alkalosis.
After a number of first-aid measures (volume resuscitation, rapid intravenous administration of 10% calcium gluconate (3 g/h), emergency tracheotomy, washing of the eyes with normal saline solution, etc.) were performed, the patient was admitted to the burn intensive care unit (BICU). Comprehensive treatment measures were carried out, including comprehensive ECG monitoring, fluid resuscitation, dynamic monitoring of serum calcium and magnesium ion levels, intravenous administration of 10% calcium gluconate and magnesium sulfate, wound rinsing, application of topical calcium gluconate, fiber-optic bronchoscopy, and rinsing of the airway with a 1.25% sodium bicarbonate solution.
Two hours after admission, the patient’s condition gradually improved (blood pressure 110/85 mmHg, heart rate 100 beats/min, and urine volume 0.7 mL/kg/h), and the distal parts of the limbs became warm. However, the patient’s blood pressure suddenly dropped, and the pulse disappeared. ECG monitoring indicated ventricular fibrillation. Chest compression and electrical defibrillation (DC two-way waveform, 200 J) were immediately carried out, and the sinus rhythm was recovered. Meanwhile, the blood biochemistry results showed Ca2+ 0.325 mmol/L, Mg2+ 0.55 mmol/L, and Lac- 8.1 mmol/L. Over the next 2 h, ventricular tachycardia reoccurred 8 more times, and chest compression and electrical defibrillation were carried out accordingly. The sinus rhythm was recovered successfully each time. No more ventricular fibrillation occurred after that time, and the infusion rate of calcium gluconate was gradually slowed. At 24 h after admission, the Ca2+ level increased to 0.857 mmol/L with an intravenous calcium gluconate infusion amount of 37 g. During the following 25 d of confinement, a total of 343 g of intravenous calcium supplementation was administered before the calcium concentration stabilized to normal levels.
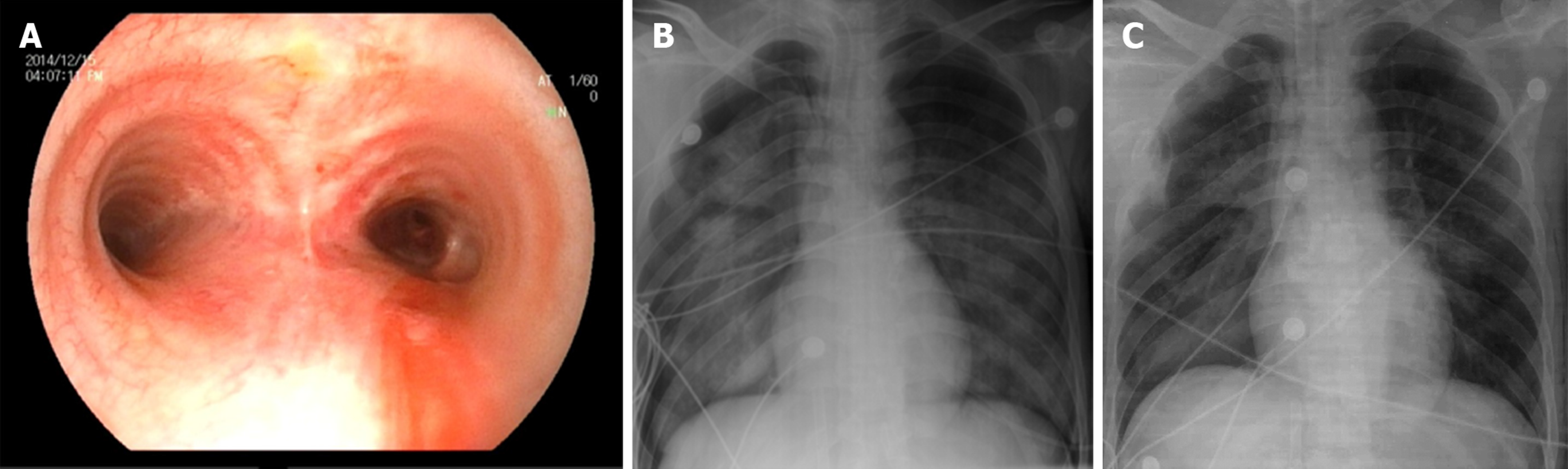
When the patient was admitted to the BICU, bronchoscopy was carried out immediately, which showed obvious injury, paleness, and bleeding of the local tracheal mucosa and varying degrees of necrotic spots and bleeding on the main bronchus and the secondary and tertiary bronchi (Figure 2A). The pH of the airway secretions was 5.2, and a 1.25% sodium bicarbonate airway rinse was administered until the pH increased to 7.2. Then, mechanical ventilation was carried out, and proper sedation and analgesia were achieved using propofol and fentanyl. However, the patient’s oxygenation condition continued to deteriorate, and the SpO2 gradually decreased from 99% to 90%. Five hours after admission, the blood gas analysis showed SpO2 89%, PaO2 51 mmHg, PaCO2 42 mmHg, and Lac- 5.8 mmol/L. The FiO2 gradually increased from 50% to 100% and the PEEP was adjusted to 15 cm H2O, but the blood gas analysis showed SpO2 92% and PaO2 56 mmHg. Furthermore, a bedside chest X-ray showed a decrease in brightness in both lungs and wide exudative changes (Figure 2B).
Considering that the patient had a primary HF inhalation injury and secondary acute respiratory distress syndrome (ARDS), the oxygenation conditions could not be improved in a short time, and extracorporeal membrane oxygenation (ECMO) for cardiopulmonary support was established at 7 h postadmission. After 6 d of ECMO treatment, the patient’s lung condition gradually improved. The arterial blood gas analysis showed PaO2 156 mmHg and PaCO2 35 mmHg. We gradually reduced the ECMO gas flow and oxygen concentration until the ECMO was turned off with no significant changes in oxygenation, and the chest X-ray showed that the lung penetration had improved (Figure 2C). Eight days after admission, the ECMO was removed, and ventilator-assisted respiration was used. Nineteen days after admission, the mechanical ventilation was removed. Thirty-one days after admission, the tracheal tube was removed. The wound from the tracheotomy healed well without airway stenosis or other complications.
The antibiotic drug cefamandole was routinely administered intravenously to prevent infection after admission for several days. The fluid was released from the blisters on the wounds to prevent the persistent toxic effects of the fluoride ion. The wounds were topically infiltrated using 10% calcium gluconate solution (in soaked gauzes) and covered with biological dressing. The wound dressings were changed daily. Nutritional support and analgesia were handled according to the status of the patient.
The deep second-degree wounds on the trunk, buttocks, limbs, neck, and face healed after 3-4 wk of conservative treatment. The third-degree wounds on both legs, feet, and right forearm were repaired after two auto skin grafts. The patient was discharged on the 99th d post burns, and he regained his normal limb and trunk activities.
The severity of HF burns is associated with the HF concentration, involved surface area, and duration of exposure[1]. HF burns are classified as severe if the burn’s TBSA is > 1% at a concentration > 50% or is > 5% at any concentration. HF burn areas are usually small. One study reported that 90% of cases had a burn size < 1% of the TBSA[2]. In this case, the patient had HF burns covering 60% of the TBSA. Such extensive HF burns have not been reported in the published literature.
HF causes tissue damage by two distinct mechanisms[1]. First, the hydrogen ions have corrosive effects similar to other acids. However, HF is a weak acid, and the corrosive effect is not the main pathologic mechanism of HF burns. Second, dissociated F- can bind with Ca2+ and Mg2+, which can induce cell decalcification and cell death. The involvement of F- on nerve endings results in severe pain that cannot be relieved. Furthermore, F- can penetrate deeply into the tissues, leading to deep tissue necrosis. As a result, necrosis of HF burn wounds deepens progressively, and wound healing is difficult. The most dangerous damage induced by HF burns is severe hypocalcemia due to F- uptake, which can lead to fatal arrhythmias[3,4].
Treatment of HF burns is aimed at reducing deep tissue damage and systemic symptoms of poisoning caused by the removal or dilution of HF and neutralization of F-. Treatment methods include decontamination, topical application of calcium, local injection of calcium gluconate, and regional intravenous and intra-arterial infusion[1]. In this case, we mainly used intravenous administration of calcium and local topical application to the burned area. Currently, the calcium dose used to rescue HF burns depends on the clinical symptoms and blood electrolyte test results; however, the amount of calcium supplement is difficult to estimate clinically. In this case, we performed immediate calcium gluconate replenishment in the emergency room in a timely manner. After application of calcium, the patient’s serum calcium level gradually increased from 0.192 mmol/L to 0.325 mmol/L within 2 h, indicating that the treatment was effective. However, despite the high amount of calcium gluconate (37 g) supplied and its rapid intravenous administration (3 g/h) during the first 24 h, the patient still underwent many episodes of ventricular fibrillation due to severe electrolyte disturbance, indicating that a more active and rapid administration of calcium gluconate might have been more advisable. In this case, magnesium treatment was carried out together with calcium treatment, which induced a rapid increase in the blood magnesium concentration and played a vital role in the resuscitation from potentially fatal arrhythmia.
The absorption of F- after severe HF burns is a lasting process that can cause sustained decalcification damage. In this case, calcium treatment was administered for 25 d, and 343 g of intravenous calcium gluconate was administered before the blood calcium level stabilized to normal, suggesting that calcium treatment in severe HF burn cases should be sustained and sequential.
Inhalation injury renders more serious pathophysiological changes in burn patients and increases the treatment difficulty. Inhalation injury is one of the most common causes of death in patients with burns[5]. For a simple thermal inhalation injury, the heat gradually decreases with the depth of the airway and generally does not directly damage the terminal airways and alveolar tissues. Conversely, in a chemical inhalation injury, the chemical substances can be diffused in the inhaled gas and can reach the ends of the airways. As a result, chemical inhalation is more severe than mere thermal damage. We previously reported an event wherein 41 patients had liquid ammonia burns combined with inhalation injury[6]. In the present case, measures were taken to stop the damage caused by the chemicals, such as emergency tracheotomy, bronchoscopy airway lavage, continuous airway rinsing, and so forth, which perhaps partially accounted for the patient’s good prognosis.
For patients with a severe inhalation injury, especially patients with ARDS, the prognosis is often poor[7]. As an in vitro cardiopulmonary support technology, ECMO has been increasingly used for the treatment of severe ARDS in recent years[8]. ARDS occurs frequently in severe burn patients, especially those combined with an inhalation injury. However, only a few reports are available on the use of ECMO in burn patients. One study reported that ECMO did not significantly improve the survival of patients with burns and ARDS[9]. Soussi et al[10] reported ECMO use in burn patients with refractory ARDS and found that the 90-d survival rate was only 28%. However, those studies were limited by their small numbers of cases and thus could not fully explain the role of ECMO in burns combined with ARDS. In this report, ECMO was applied at 8 h post injury even though only mild hypoxic signs were present. This strategy was important for the treatment of ARDS not only to avoid sustained hypoxemic damage of organs but also to let the lungs rest and recover. The ECMO duration was 8 d (190 h), which is consistent with the recommended treatment duration of < 200 h that was reported to have a high survival rate[9]. The successful application of ECMO in this burn case provided an experience with the use of ECMO in patients with extensive burns, especially those with ARDS.
In summary, the patient’s successful treatment benefited from the following factors: (1) Early appropriate and timely emergency treatment, including emergency airway opening and rinsing, timely fluid resuscitation, and other comprehensive treatments; (2) Correct prediction of a potentially fatal electrolyte imbalance, adequate calcium and magnesium supplementation, and timely detection and correction of ventricular fibrillation; and (3) Proper handling of ARDS and timely and effective ECMO treatment of the HF inhalation injury, which was essential for the recovery of respiratory function.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Boots RJ, Kym D S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Hatzifotis M, Williams A, Muller M, Pegg S. Hydrofluoric acid burns. Burns. 2004;30:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Wu ML, Yang CC, Ger J, Tsai WJ, Deng JF. Acute hydrofluoric acid exposure reported to Taiwan Poison Control Center, 1991-2010. Hum Exp Toxicol. 2014;33:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Wu ML, Deng JF, Fan JS. Survival after hypocalcemia, hypomagnesemia, hypokalemia and cardiac arrest following mild hydrofluoric acid burn. Clin Toxicol (Phila). 2010;48:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Graudins A, Burns MJ, Aaron CK. Regional intravenous infusion of calcium gluconate for hydrofluoric acid burns of the upper extremity. Ann Emerg Med. 1997;30:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Lee KC, Joory K, Moiemen NS. History of burns: The past, present and the future. Burns Trauma. 2014;2:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Zhang F, Zheng XF, Ma B, Fan XM, Wang GY, Xia ZF. Mass chemical casualties: treatment of 41 patients with burns by anhydrous ammonia. Burns. 2015;41:1360-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Colohan SM. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Schmidt M, Hodgson C, Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care. 2015;19:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Asmussen S, Maybauer DM, Fraser JF, Jennings K, George S, Keiralla A, Maybauer MO. Extracorporeal membrane oxygenation in burn and smoke inhalation injury. Burns. 2013;39:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Soussi S, Gallais P, Kachatryan L, Benyamina M, Ferry A, Cupaciu A, Chaussard M, Maurel V, Chaouat M, Mimoun M, Mebazza A, Legrand M; PRONOBURN Group. Extracorporeal membrane oxygenation in burn patients with refractory acute respiratory distress syndrome leads to 28 % 90-day survival. Intensive Care Med. 2016;42:1826-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |