Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3259
Peer-review started: April 8, 2019
First decision: September 9, 2019
Revised: September 24, 2019
Accepted: October 5, 2019
Article in press: October 5, 2019
Published online: October 26, 2019
Processing time: 203 Days and 19.1 Hours
Pituitary apoplexy represents one of the most serious, life threatening endocrine emergencies that requires immediate management. Gonadotropin-releasing hormone agonist (GnRHa) can induce pituitary apoplexy in those patients who have insidious pituitary adenoma coincidentally.
A 46-year-old woman, with a history of hypertension and menorrhagia was transferred to our hospital from a secondary care hospital after complaints of headache and vomiting, with loss of consciousness 5 min after an injection of GnRHa. The drug was prescribed by her gynecologist due to the presence of uterine myomas. The clinical neurological examination revealed right cranial nerve III palsy, ptosis and movement limitation of the right eye. Our first clinical consideration was a pituitary apoplexy. Blood hormonal analysis revealed mild hyperprolactinemia and high follicle stimulating hormone level; PTH and calcium was high with glomerular filtration rate mildly to moderately decrease. A computed tomography scan, revealed an enlarged pituitary gland (3.5 cm) impinging upon the optic chiasm with bone involvement of the sella. Following contrast media administration, the lesion showed homogeneous enhancement with high-density focus that suggests hemorrhagic infarction of the tumor. Transsphenoidal endoscopic surgery was perfomed and adenomatous tissue was removed. Immunohistochemistry was positive for luteinizing hormone (LH) and follicular-stimulating hormone (FSH). A solid hypoechoic nodule (14 mm x 13 mm x 16 mm) was found in the caudal portion of the right thyroid lobe after a parathyroid ultrasound. A genetic test of Multiple Endocrine Neoplasia type 1 (MEN1) was negative. A right lower parathyroidectomy was performed and the pathologic study showed the presence of an encapsulated parathyroid carcinoma of 1.5 cm. A MEN type 4 genetic test was performed result was negative.
This case demonstrates an uncommon complication of GnRH agonist therapy in the setting of a pituitary macroadenoma and the casual finding of parathyroid carcinoma. It also highlights the importance of suspecting the presence of a multiple endocrine neoplasia syndrome and to carry out relevant genetic studies.
Core tip: Pituitary apoplexy represents one of the most serious, life threatening endocrine emergencies. Gonadotropin-releasing hormone agonist can develop pituitary apoplexy in those who have insidious pituitary adenoma coincidentally. What will be discussed in this article is the case of the first female patient who has develop pituitary apoplexy after receiving triptorelin, which is prescribed to regulate the menstrual cycle due menorrhagia, and uterine fibroids through pituitary down regulation. This case demonstrates an uncommon complication of gonadotropin-releasing hormone agonist therapy in the setting of a pituitary macroadenoma and the casual finding of parathyroid carcinoma. It also highlights the importance to suspect the presence of a multiple endocrine neoplasia syndrome and to carry out relevant genetic studies.
- Citation: Triviño V, Fidalgo O, Juane A, Pombo J, Cordido F. Gonadotrophin-releasing hormone agonist-induced pituitary adenoma apoplexy and casual finding of a parathyroid carcinoma: A case report and review of literature. World J Clin Cases 2019; 7(20): 3259-3265
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3259.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3259
Gonadotropin-releasing hormone agonists (GnRHa), like triptorelin, are used to treat menorrhagia and uterine fibroids through pituitary down regulation. Pituitary Apoplexy (PA) is characterized by bleeding or impaired blood supply of the pituitary gland that usually occurs in the presence of a pituitary tumor[1]. GnRHa will rarely induce PA in those who coincidentally have insidious pituitary adenoma[2,3], although most cases have not been diagnosed previously. PA represents one of the most serious, life- threatening endocrine emergencies that requires immediate management. It is a clinical syndrome associated with sudden headache, vomiting, visual impairment and meningismus caused by the rapid enlargement of a pituitary adenoma usually due to infarction of the tumor[4,5].
Recent data suggests that pituitary adenomas occur in almost 20% of the general population[3]. The majority of these are non-functioning gonadotropinomas. Functioning Gonadotropins Adenomas (FGAs) are very rare and for premenopausal females tend to be universally associated with an elevated follicular-stimulating hormone (FSH), with varying levels of the luteinizing hormone (LH)[6]. The most common clinical manifestations presented include menstrual irregularity, infertility, galactorrhea, and mass effects[7]. A few patients were reported to have hormonal hypersecretion syndromes such as ovarian hyperstimulation, testicular enlargement, and precocious puberty[8].
The diagnosis can be very difficult in postmenopausal women because they exhibit elevated serum FSH in this period, making indistinguishable the FSH tumoral secretion, but in reproductive aged women can be difficult too because of de fluctuating levels of FSH during menstrual cycle[9].
Here we will discuss the case of the first female patient who has developed PA after receiving triptorelin for the treatment of menorrhagia and uterine fibroids through pituitary down regulation. We also discuss the casual finding of parathyroid carcinoma in the same patient.
A 46-year-old woman was transferred to our hospital from a secondary care hospital after complaints of headache and vomiting, with loss of consciousness 5 min after an injection of GnRH agonist.
Patient’s symptoms started 5 min after the injection of GnRH agonist prescribed by her gynecologist due to the presence of menorrhagia.
Hypertension under regular control. Left renal lithiasis treated with lithotripsy three times. Menarquia at the age of 10. Uterine myomas and long-standing menorrhagia, with secondary iron deficiency anemia. There was no history of migraine or any type of headache.
The patient’s temperature was 35.6 °C, heart rate was 56 bpm, respiratory rate was 16 breaths per minute, blood pressure was 200/85 mmHg and oxygen saturation in the room air was 98%. The clinical neurological examination revealed third right cranial nerve palsy, ptosis and movement limitation of the right eye, without any other pathological signs. Our first clinical consideration was a pituitary apoplexy.
Blood hormonal analysis revealed a mild hyperprolactinemia (prolactin 68 ng/mL normal range 2.8-29.2 ng/mL). High FSH (76 mUI/mL). Insulin growth factor-1 and TSH level was normal; PTH was high 384 pg/mL. (normal range 14-72 pg/mL) serum calcium was high 12.9 mg/dL (normal range 8.5-10.1 mg/dL) with glomerular filtration rate mildly to moderately decreased.
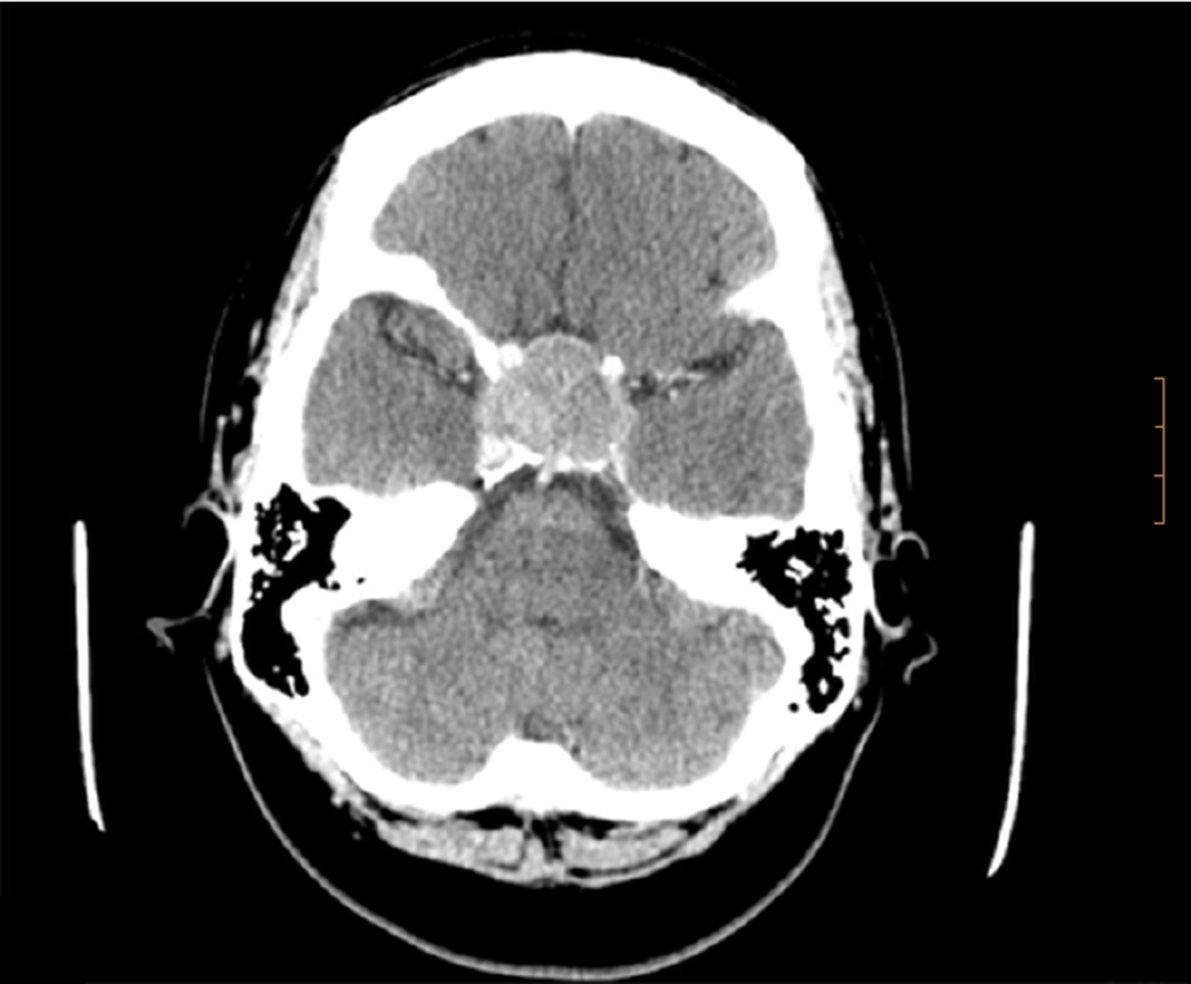
An initial imaging evaluation with brain computed tomography scan, revealed an enlarged pituitary gland (3.5 cm) impinging upon the optic chiasm with bone involvement of the sella. The tumor invaded the cavernous sinuses. Following the contrast media administration, the lesion showed homogeneous enhancement with high density focus, suggesting hemorrhagic infarction of the tumor (Figure 1).
Due to the presence of pituitary gonadotroph macroadenoma and primary hyperparathyroidism, Multiple Endocrine Neoplasia type 1 (MEN1) was suspected.
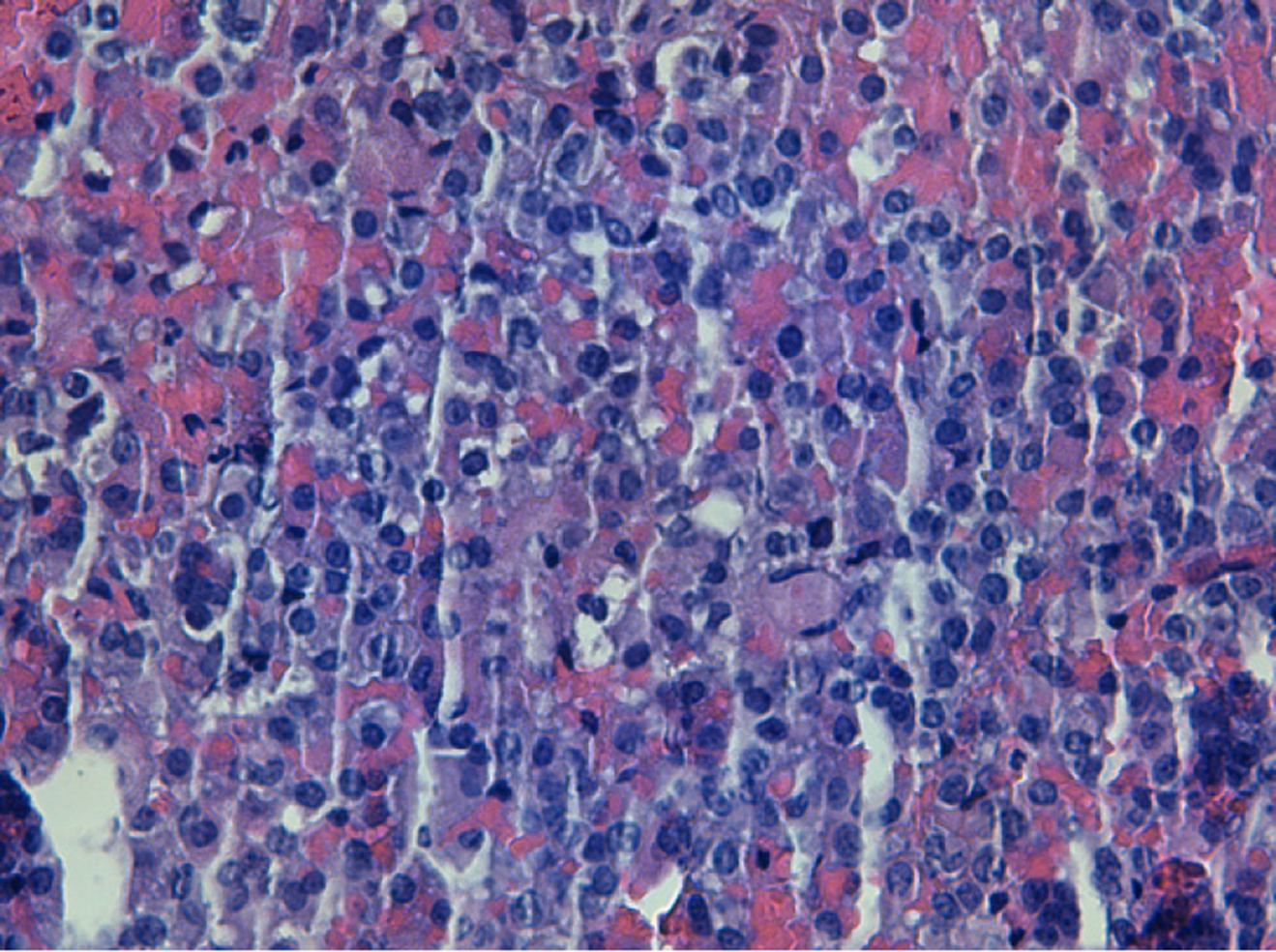
Transsphenoidal endoscopic surgery was performed and adenomatous tissue with hemorrhagic foci was removed. Immunohistochemistry was positive for LH and FSH, the tissue did not express p53 and the cell proliferation index (MIB-1) was less than 3% (Figure 2).
Postoperative evolution was good, with progressive recovery of right cranial nerve III palsy, ptosis and movement of the right eye. Subsequent testing found hypothyroidism and hypoadrenalism, for which the patient was treated with replacement drugs. Prolactin and FSH level decreased to normal range and LH was suppressed. An elevated value of PTH was found, 469 pg/mL with serum calcium 13.9 mg/dL.
A parathyroid ultrasound was performed. In the caudal portion of the right thyroid lobe a solid hypoechoic nodule (14 mm x 13 mm x 16 mm), with small hyperechoic images inside it suggesting microcalcifications was found.
A parathyroid sestamibi scan was negative.
After clinical suspicion, a genetic test of MEN1 was requested since it can change the type of parathyroid surgery to be performed. Genetic counseling was provided to the patient, upon which she provided written authorization for MEN1 testing. Genomic DNA obtained was screened for 2-10 coding exons of the MEN1 gene by bidirectional sequence analysis and the result was negative.
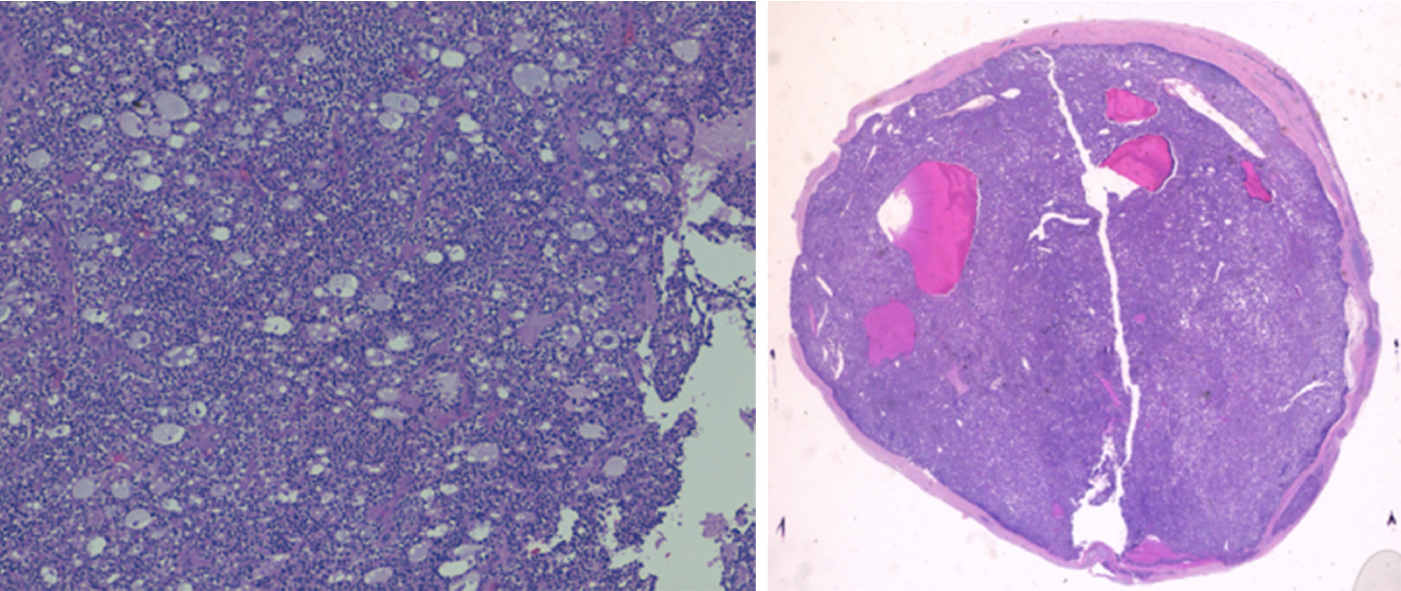
A right lower parathyroidectomy was performed and the pathologic study showed the presence of an encapsulated parathyroid carcinoma of 1.5 cm, with capsular and vascular invasion (Figure 3). A MEN type 4 (MEN4) genetic test was performed that was negative.
Pituitary apoplexy may result from spontaneous hemorrhage into a pituitary adenoma; after head trauma with skull base fracture; in association with hypertension, diabetes mellitus, sickle cell anemia, or acute hypovolemic shock. Until now precipitating factors include major surgery, pregnancy, gamma knife irradiation, anticoagulant therapy, coagulopathy secondary to liver failure and administration of thyrotropin releasing hormone, bromocriptine, cabergoline or gonadotrophin releasing hormone agonist[1].
FGAs are adenomas expressing and secreting biologically active gonadotropins and causing distinct clinical manifestations, in premenopausal females mainly menstrual irregularities (oligo/amenorrhea, spotting-menorrhagia) and the ovarian hyperstimulation syndrome[7]. The vast majority of the immunohistochemically confirmed gonadotroph adenomas are hormonally silent (in surgical series accounted for 64% of all clinically nonfunctioning pituitary adenomas), and the FGAs are very rare with a prevalence unknown. These tumors most frequently produce FSH and less frequently produce both FSH and LH[6]. The published literature includes only small case-series or individual case reports[10].
In the present case, triptorelin was administered to control menorrhagia due to the presence of uterine fibroids. To our knowledge, this is the first case of pituitary apoplexy after the use of triptorelin for pituitary down-regulation in conjunction with uterine fibroids. The exact mechanism by which GnRH agonists precipitate pituitary apoplexy in patients with pituitary adenomas is unknown[11]. However, multiple factors that increase the risk or are directly associated with the cause of the bleeding have been implicated, in any case the temporal relationship with the administration of the drug strongly suggests a causal relationship. Guerra et al[12]. suggest a biphasic phenomenon, because in the series of cases presented the symptoms of the patients began in the first 24 h after the administration of the drug and in others up to 10 d later. Therefore, an acute and subacute mechanism is suggested[12].
The acute pathophysiologic events due to cell degranulation and cell shrinking, in association with the abnormal capillaries, would explain the apoplexy that occur hours after giving GnRHa[13]. The subacute mechanism would explain the events occurring more than a week after the medication was given, when GnRH-induced cell growth and protein synthesis can have an effect on intrasellar pressure, causing generalized ischemia and consequent bleeding[12,14].
The principle initial acute symptom is a headache that can be moderate to severe, possibly attributed to irritation or stretching of dura mater in the sella supplied by branches of the trigeminal nerve. Other clinical features commonly seen are nausea and vomiting and can be extremely severe. Visual symptoms occur after headache, including ptosis, ophthalmoplegia, decreased visual acuity, loss of visual field, and anisocoria, consistent with the imaging finding of extrasellar extension[1,5]. Prompt and proper intervention is crucial for patients with pituitary apoplexy, especially in presence of consciousness change or cranial nerve palsy. Randeva showed that visual acuity could be remarkably improved by transsphenoidal surgery. In patients operated on within 8 d, recovery was complete, but the rate dropped to only 46% after this cut-out point. Fortunately, our case patient completely recovered, although necessarily receiving transsphenoidal surgery[1].
During the follow up of the pituitary adenoma, hypercalcemia with high PTH and a parathyroid adenoma was detected. This data suggest MEN1, because she had two of the three main endocrine features of MEN 1[15,16,17].
In an analysis of 324 patients with MEN1, pituitary adenomas were found in 136 patients, and of these 85 were lactotroph, 12 somatotroph, 6 corticotroph, 13 cosecreting (5 PRL + GH; 2 PRL + FSH; 4 PRL +ACTH, 1 PRL + alfa-subunit, and 1 GH + alfa-subunit) and 20 nonsecreting. when 15 of the 20 nonsecreting adenomas were examined by immunocytochemistry, two stained for LH and FSH, suggesting that they were gonadotroph adenomas[18]. Adenomas categorized as nonfunctioning have also been reported in other series of patients with MEN1[15], and it is well recognized that most clinically nonfunctioning adenomas are composed of cells with variable gonadotropic differentiation.
Primary hyperparathyroidism with parathyroid carcinoma was found in the present case. One of the most common endocrine diseases is primary hyperparathyroidism. It usually results from a single parathyroid adenoma, and less frequently tumors can develop in multiple parathyroid glands. Parathyroid carcinoma (PC) is a rare cause of hyperparathyroidism[19,20,21]. In a retrospective study of two European cohorts of patients with primary hyperparathyroidism, the frequency of PC ranged from 0.3 to 2.1 percent. Around half of PC cases are diagnosed at an average age of 50 years with no sex or race predilection. Most of the patients present clinical and biochemical manifestations of severe hyperparathyroidism, including severe hypercalcemia, elevated parathyroid hormone level, as well as renal complications like the ones we are counting in this case[22]. A palpable neck mass is present in 30%–76% of patients. However, the diagnosis of PC is rarely made preoperatively[23]. There is no evidence that PC arises from pre-existing benign parathyroid lesions. PC can occur sporadically and, in 15% of cases, in association with familial isolated primary hyperparathyroidism and hyperparathyroidism-jaw tumor syndrome[24,25]. This is not the case for other syndromes like multiple endocrine neoplasia (MEN1, MEN2A) where the occurrence of PC is very rare; only one case was reported in a series of 348 cases of MEN1 (0.28 percent) from the Mayo Clinic from 1977 to 2013[26,27].
The genetic study of MEN1 was performed due to the presence of pituitary gonadotroph macroadenoma in addition to hyperparathyroidism, although in patients with MEN1, there are tumors in three or all four parathyroid glands[16]. Beside the affectation with MEN1 it is recommended to change the type of operation to perform. Minimally invasive parathyroidectomy is not recommended, because it does not support the routine identification of all four parathyroid glands. Subtotal parathyroidectomy with transcervical near-total thymectomy is the most common initial neck operation performed[16,28]. The genetic test for MEN1 was negative in our patient. Approximately 5%-10% of patients with MEN1 do not have mutations of the MEN1 gene, and these patients may have mutations involving other genes. One of these genes is the CDNK1B and was identified to be involved by investigations of a recessive MEN-like syndrome in a naturally occuring rat model, referred to as MEN4[29,30], where the most common phenotypic features are parathyroid and pituitary adenomas[31,32]. In our patient the presence of this mutation was negative too.
Pituitary adenoma apoplexy could be due to the administration of gonadotrophin-releasing hormone agonist. The presence of both pituitary adenoma and parathyroid carcinoma is infrequent and can be related to multiple endocrine neoplasia syndrome, or mutations that have not been discovered or are sporadic.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mastoraki A S-Editor: Zhang L L-Editor: A E-Editor: Liu JH
| 1. | Huang TY, Lin JP, Lieu AS, Chen YT, Chen HS, Jang MY, Shen JT, Wu WJ, Huang SP, Juan YS. Pituitary apoplexy induced by Gonadotropin-releasing hormone agonists for treating prostate cancer-report of first Asian case. World J Surg Oncol. 2013;11:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Hands KE, Alvarez A, Bruder JM. Gonadotropin-releasing hormone agonist-induced pituitary apoplexy in treatment of prostate cancer: case report and review of literature. Endocr Pract. 2007;13:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Keane F, Egan AM, Navin P, Brett F, Dennedy MC. Gonadotropin-releasing hormone agonist-induced pituitary apoplexy. Endocrinol Diabetes Metab Case Rep. 2016;2016:160021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sasagawa Y, Tachibana O, Nakagawa A, Koya D, Iizuka H. Pituitary apoplexy following gonadotropin-releasing hormone agonist administration with gonadotropin-secreting pituitary adenoma. J Clin Neurosci. 2015;22:601-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Chanson P, Schaison G. Pituitary apoplexy caused by GnRH-agonist treatment revealing gonadotroph adenoma. J Clin Endocrinol Metab. 1995;80:2267-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mete O, Asa SL. Clinicopathological correlations in pituitary adenomas. Brain Pathol. 2012;22:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Ntali G, Capatina C, Grossman A, Karavitaki N. Clinical review: Functioning gonadotroph adenomas. J Clin Endocrinol Metab. 2014;99:4423-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Chaidarun SS, Klibanski A. Gonadotropinomas. Semin Reprod Med. 2002;20:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hirano M, Wada-Hiraike O, Miyamamoto Y, Yamada S, Fujii T, Osuga Y. A case of functioning gonadotroph adenoma in a reproductive aged woman. Endocr J. 2019;66:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Shimada H, Sasaki H, Kasai K, Egawa S. [Gonadotroph Pituitary Adenoma Causing Severe Headache Following Repeated Use of GnRH Agonist for Prostate Cancer]. Hinyokika Kiyo. 2019;65:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Christin-Maitre S, Rongières-Bertrand C, Kottler ML, Lahlou N, Frydman R, Touraine P, Bouchard P. A spontaneous and severe hyperstimulation of the ovaries revealing a gonadotroph adenoma. J Clin Endocrinol Metab. 1998;83:3450-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Guerra Y, Lacuesta E, Marquez F, Raksin PB, Utset M, Fogelfeld L. Apoplexy in non functioning pituitary adenoma after one dose of leuprolide as treatment for prostate cancer. Pituitary. 2010;13:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Engel G, Huston M, Oshima S, Beck C, Harsh G, Rosenthal MH, Camargo CA. Pituitary apoplexy after leuprolide injection for ovum donation. J Adolesc Health. 2003;32:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Guerrero-Pérez F, Marengo AP, Planas-Vilaseca A, Flores-Escobar V, Villabona-Artero C. Pituitary apoplexy induced by triptorelin in patient with prostate cancer. Endocrinol Nutr. 2015;62:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Benito M, Asa SL, Livolsi VA, West VA, Snyder PJ. Gonadotroph tumor associated with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2005;90:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 17. | Alberto F. Genetics of parathyroids disorders: Overview. Best Pract Res Clin Endocrinol Metab. 2018;32:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Yoshimoto K, Endo H, Tsuyuguchi M, Tanaka C, Kimura T, Iwahana H, Kato G, Sano T, Itakura M. Familial isolated primary hyperparathyroidism with parathyroid carcinomas: clinical and molecular features. Clin Endocrinol (Oxf). 1998;48:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Invêncio da Costa LF, Alzate Amaya F, Herranz González Botas J. Parathyroid carcinoma, an unusual cause of primary hyperparathyroidism. Acta Otorrinolaringol Esp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Cristina EV, Alberto F. Management of familial hyperparathyroidism syndromes: MEN1, MEN2, MEN4, HPT-Jaw tumour, Familial isolated hyperparathyroidism, FHH, and neonatal severe hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32:861-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Ozolins A, Narbuts Z, Vanags A, Simtniece Z, Visnevska Z, Akca A, Wirowski D, Gardovskis J, Strumfa I, Goretzki PE. Evaluation of malignant parathyroid tumours in two European cohorts of patients with sporadic primary hyperparathyroidism. Langenbecks Arch Surg. 2016;401:943-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Singh Ospina N, Sebo TJ, Thompson GB, Clarke BL, Young WF. Prevalence of parathyroid carcinoma in 348 patients with multiple endocrine neoplasia type 1 - case report and review of the literature. Clin Endocrinol (Oxf). 2016;84:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kolsi N, Jellali S, Koubaa J. [Parathyroid carcinoma: about a case and review of the literature]. Pan Afr Med J. 2017;27:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol. 2014;386:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | Omi Y, Horiuchi K, Haniu K, Tokura M, Nagai E, Isozaki O, Nagashima Y, Okamoto T. Parathyroid carcinoma occurred in two glands in multiple endocrine neoplasia 1: a report on a rare case. Endocr J. 2018;65:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Veldhuis JD, Norton JA, Wells SA, Vinik AI, Perry RR. Surgical versus medical management of multiple endocrine neoplasia (MEN) type I. J Clin Endocrinol Metab. 1997;82:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Angelousi A, Dimitriadis GK, Zografos G, Nölting S, Kaltsas G, Grossman A. Molecular targeted therapies in adrenal, pituitary and parathyroid malignancies. Endocr Relat Cancer. 2017;24:R239-R259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | McDonnell JE, Gild ML, Clifton-Bligh RJ, Robinson BG. Multiple endocrine neoplasia: an update. Intern Med J. 2019;49:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Lee M, Pellegata NS. Multiple endocrine neoplasia type 4. Front Horm Res. 2013;41:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Frederiksen A, Rossing M, Hermann P, Ejersted C, Thakker RV, Frost M. Clinical Features of Multiple Endocrine Neoplasia Type 4: Novel Pathogenic Variant and Review of Published Cases. J Clin Endocrinol Metab. 2019;104:3637-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |