Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3237
Peer-review started: June 17, 2019
First decision: August 3, 2019
Revised: September 3, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 26, 2019
Processing time: 131 Days and 13.2 Hours
Gastroscopy and colonoscopy are important and common endoscopic methods for the diagnosis and treatment of gastrointestinal and colorectal diseases. However, endoscopy is usually associated with adverse reactions such as nervousness, nausea, vomiting, choking cough, and pain. Severe discomfort, such as vomiting, coughing, or body movement, may lead to aggravation of a pre-existing condition or even interruption of examination or treatment, especially in some critically ill patients with physiological dysfunction (e.g., cardiovascular or respiratory disease). The optimal methods for inducing analgesia and sedation in endoscopy are areas of ongoing debate; nevertheless, determining an appropriate regimen of sedation and analgesia is important.
To evaluate the effects of propofol combined with dezocine, sufentanil, or fentanyl in painless gastroscopy and colonoscopy.
Four hundred patients were randomly assigned to one of four groups for anesthesia: intravenous dezocine, sufentanil, fentanyl, or saline. Propofol was administered intravenously for induction and maintenance of anesthesia.
The dosage of propofol in the dezocine group was significantly lower than those in other groups (P < 0.01). Bispectral index and Steward score (0-6 points, an unresponsive, immobile patient whose airway requires maintenance to a fully recovered patient) after eye opening in the dezocine group were significantly higher than those in other groups (P < 0.01). Awakening time and postoperative pain score (0-10 points, no pain to unbearable pain) in the dezocine group were significantly lower than those in other groups (P < 0.01). Mean arterial pressure and pulse oxygen saturation in the dezocine group were significantly more stable at various time points (before dosing, disappearance of eyelash reflex, and wakeup) than those in other groups (P < 0.01). The rates of hypopnea, jaw thrust, body movements, and usage of vasoactive drugs in the dezocine group were significantly lower than those in other groups (P < 0.01). Additionally, the rates of reflex coughing, nausea, and vomiting were not statistically different between the four groups (P > 0.05).
The combination of propofol and dezocine can decrease propofol dosage, reduce the risk for the development of inhibitory effects on the respiratory and cardiovascular systems, increase analgesic effect, decrease body movement, shorten awakening time, and improve awakening quality.
Core tip: This study aimed to identify a comparatively satisfactory anesthetization regimen for painless gastroscopy and colonoscopy. The combination of propofol and dezocine can decrease propofol dosage, reduce the risk for the development of inhibitory effects on the cardiovascular and respiratory systems, increase analgesic effect, decrease body movement, shorten awakening time, and improve awakening quality. Anesthesia with propofol combined with dezocine is an adequate regimen of anesthesia and analgesia for gastroscopy and colonoscopy, which can increase the patient cooperation, quality and safety of the examination and treatment, and patient and physician satisfaction with anesthesia.
- Citation: Li XT, Ma CQ, Qi SH, Zhang LM. Combination of propofol and dezocine to improve safety and efficacy of anesthesia for gastroscopy and colonoscopy in adults: A randomized, double-blind, controlled trial. World J Clin Cases 2019; 7(20): 3237-3246
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3237.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3237
Gastroscopy and colonoscopy are important and common endoscopic methods for the diagnosis and treatment of gastrointestinal and colorectal diseases. Gastroscopy is used to visualize the upper part of the gastrointestinal tract (i.e., up to the duodenum)[1,2], while colonoscopy is used to examine the large intestine and the distal part of the small intestine[3-6]. However, endoscopy is usually associated with adverse reactions such as nervousness, nausea, vomiting, choking cough, and pain[7,8]. Severe discomfort, such as vomiting, coughing, or body movement, may lead to aggravation of a pre-existing condition or even interruption of examination or treatment, especially in some critically ill patients with physiological dysfunction (e.g., cardiovascular or respiratory disease)[9]. The optimal methods for inducing analgesia and sedation in gastroscopy and colonoscopy are areas of ongoing debate; nevertheless, determining an appropriate regimen of sedation and analgesia is important. It has been reported that the administration of intravenous anesthetics can effectively eliminate patient anxiety, inhibit upper airway reflex, and improve patient comfort during endoscopy, which has led to an increase in patient willingness to undergo follow-up endoscopic examination or treatment[10]. Previous studies have confirmed the efficacy of general anesthesia in endoscopy. The use of propofol in combination with opioids has been reported to improve sedation and analgesia with regard to recovery time, sedative effect, pain, and discomfort[11,12]. The aim of the present trial was to evaluate the effects of propofol combined with dezocine, sufentanil, or fentanyl in gastroscopy and colonoscopy. Based on novel molecular targets of dezocine[13] and our preliminary observations in clinical practice, we hypothesized that a better performance may be achieved with a combination of propofol and dezocine. We observed the incidence of reflex coughing, nausea, and vomiting, cardiovascular and respiratory depression, body movement under sedation, and recovery quality when using propofol alone or combined with dezocine, sufentanil, or fentanyl.
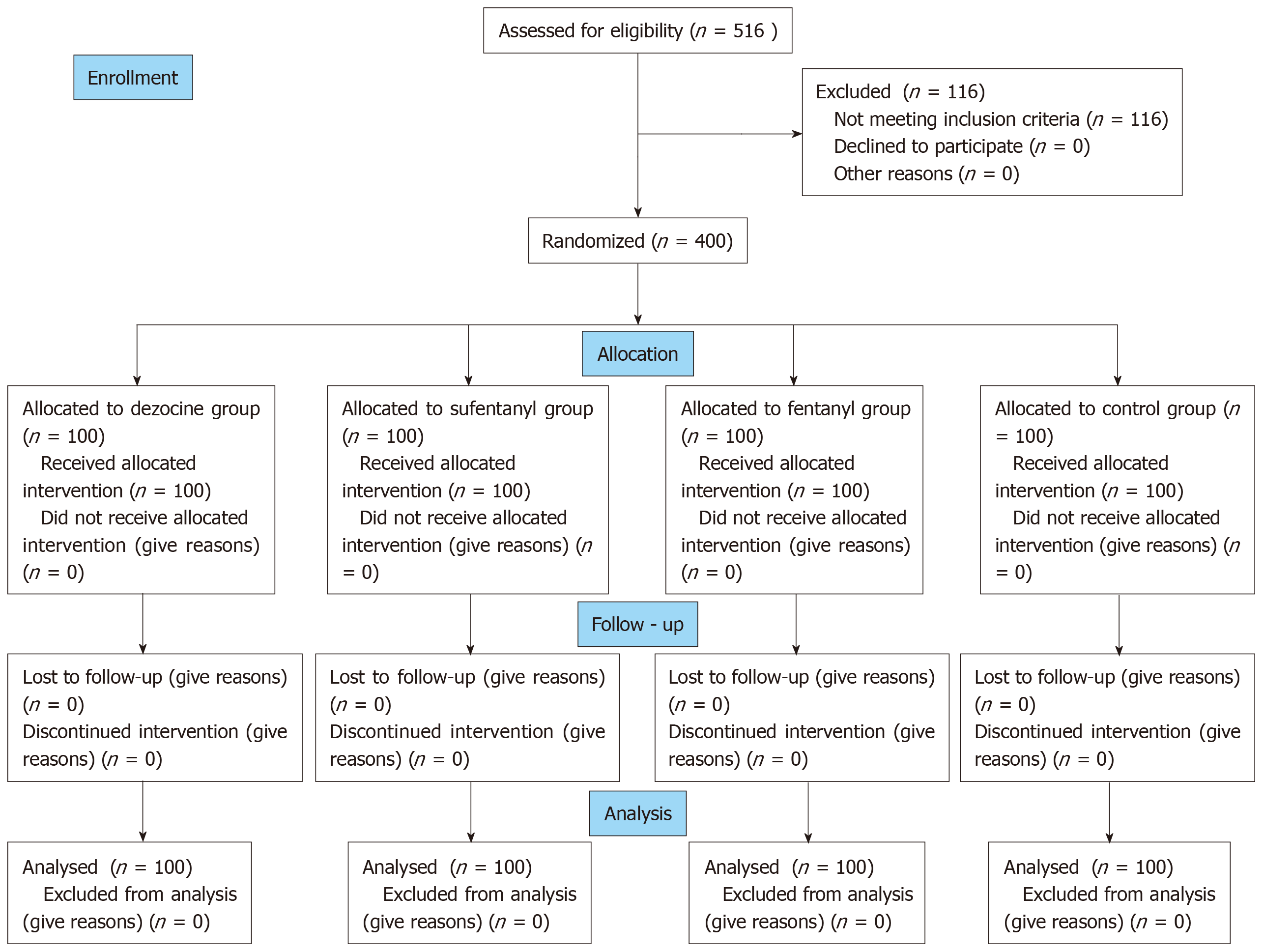
This prospective, randomized, double-blind, controlled trial was conducted with the approval of the Medical Ethics Committee of the Fourth Affiliated Hospital of Harbin Medical University (Harbin, Heilongjian, China), and was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx; Registration number ChiCTR1800017630; August 7, 2018). Stratified randomization was used to assign candidate subjects to one of four groups (Figure 1) according to sex and body mass index (BMI) (two groups). Computer-generated random group numbers were printed and placed into separate sealed envelopes. When recruiting a subject who met the inclusion criteria, the assistant anesthetists assigned the patient to a group according to the number in the envelope. Both anesthesiologists and patients were blinded to the regimen. The drugs were prepared by the assistant anesthetists, labelled with numbers, and then injected by the anesthesiologist. The anesthesiologist was responsible for recording intraoperative and postoperative indexes. In cases of emergency, the anesthesiologist was to be notified of the study group to which the patient belonged by the assistant anesthetist.
Patients with American Society of Anesthesiologists class I or II, 18-85 years of age, and undergoing both gastroscopy and colonoscopy were recruited. Written informed consent for anesthesia was obtained from each participant before anesthesia. Individuals > 85 or < 18 years of age, and those who were pregnant, had a BMI > 30 kg/m2, experienced active gastrointestinal bleeding, had a history of bronchial asthma or chronic obstructive pulmonary disease, upper airway infection in the previous 2 weeks, or had impaired kidney or liver function, drug abuse, sleep apnea, a known hypersensitivity to propofol or opioid, or an expected operation duration > 60 min were excluded from the study. Furthermore, participants were excluded after recruitment if the anesthesia protocol or endoscopic procedure was changed - even temporarily - for any reason. The structure of the study is illustrated in Figure 1.
The sample size was estimated according to the χ2 test of the incidence of the primary study end points and the incidence of hypotension and hypoxemia (30%) during endoscopy. The number of observations allowed detection of a small to moderate effective size (approximately 20%) with a 5% chance of a type I error and 90% power. The test of power remained at 80% or higher if up to 20% of subjects drop out from the study.
A total of 516 patients were recruited for this study (Figure 1). After exclusion, 400 patients were randomly divided into one of four groups for sedation, with 100 subjects in each group: Dezocine (intravenous 1.0-2.5 mg/kg propofol + 0.05 mg/kg dezocine); sufentanil (1.0-2.5 mg/kg propofol + 0.10 μg/kg sufentanil); fentanyl (1.0-2.5 mg/kg propofol + 1.0 μg/kg fentanyl); and control (1.0-3.0 mg/kg propofol + 2-3 mL saline). All drugs, except for propofol, were diluted with saline. Dezocine was diluted to 1 mg/mL, sufentanil to 2.5 μg/mL, and fentanyl to 20 μg/mL.
Peripheral venous access was secured using a 22-gauge intravenous needle in the dorsum of the right hand before the patients entered the operating room. A left-side position was taken, oxygen via a nasal cannula (3 L/min) was administered, and normal saline was infused at a rate of 10 mL/kg/h in the operating room. The bispectral index (BIS), electrocardiogram, non-invasive blood pressure, and pulse oxygen saturation (SpO2) were continuously monitored.
After entering the operating room, the patients were administered one of the three opioids or saline. Ten minutes later, propofol was administered intravenously for induction and maintenance of anesthesia. When the eyelash-conditioned reflex disappeared, the entire body relaxed, and BIS reached 40-60, gastroscopy was performed. A BIS of 40-60 is usually considered to indicate sufficient depth of general anaesthesia. All patients underwent colonoscopy after gastroscopy. During the course of the procedure, no opioids were administered intraoperatively, and anesthesia depth (BIS 40-60) was mainly controlled using propofol. During the course of the procedure, vasoactive drugs were used to maintain the variation range of mean arterial pressure (MAP) and heart rate (HR) less than 20% of pre-induction values.
Intraoperative indexes included the induction time it took for the patient to lose consciousness (from injection of propofol until the eyelash conditioned reflex disappeared), the induction dosage of propofol, additional dosage of propofol, the total dosage of propofol, the incidence of reflex coughing, body movement, respiratory depression, the use of jaw thrust, the need for vasoactive drugs, duration of the procedure, and Steward score [0-6 points: An unresponsive immobile patient whose airway requires maintenance (score = 0) to a fully recovered patient (score = 6)][14] when the patient wake up (open eyes as the anesthesiologist called the patient’s name or lightly tapped the patient on the shoulder). The postoperative indexes included awakening time from pulling out the colonoscope to waking up of the patient, the patient’s postoperative pain score [0-10 points, no pain (score = 0) to unbearable pain (score = 10)] at 30 min in the observation room, and the incidence of nausea and vomiting within a 24-h period. BIS, MAP, HR, SpO2, and respiratory rate (RR) were recorded at time points T1 (before dosing), T2 (disappearance of eyelash reflex), and T3 (patient waking up).
Data were analyzed using SPSS version 22.0 (IBM Corporation, Armonk, NY, United States) for Windows (Microsoft Corporation, Redmond, WA, United States), and are expressed as the mean ± SD, or number and percentage. Group comparisons regarding age, weight, height, operation duration, propofol dosage, and awakening time were performed using one-way analysis of variance (ANOVA). Values of MAP, HR, and SpO2 were compared using repeated-measures ANOVA. Categorical data were compared using the chi-squared test or Fisher’s exact test. Correlation analysis was conducted on propofol dosage and some additional clinical benefits. P < 0.05 was considered to be statistically significant.
A total of 516 patients scheduled to undergo selective painless gastroscopy and colonoscopy were initially assessed for eligibility between August 13, 2018 and March 30, 2019. Of these, 116 patients were excluded for the following reasons: >85 years of age (n = 26); <18 years of age (n = 8); body weight > 30% of ideal body weight (n = 12); tachycardia (n = 19); bradycardia (n = 25); and upper airway infection in the previous 2 wk (n = 26). No severe adverse event leading to study withdrawal was observed. Ultimately, a total of 400 patients (n = 100 in each group) were enrolled in this study (Figure 1).
Demographic information, including sex, age, weight, height, and operation duration, were similar among the groups (P > 0.05) (Table 1). The size, shape, location, and number of polyps were similar among the groups (P > 0.05). The endoscopists were equally skilled and were similar among the groups (P > 0.05).
| Item | Dezocine group | Sufentanyl group | Fentanyl group | Control group | P value |
| Sex, male/female | 48/52 | 53/47 | 51/49 | 49/51 | 0.899 |
| Age, yr | 51 ± 20 | 52 ± 20 | 53 ± 19 | 48 ± 20 | 0.269 |
| Height, cm | 172 ± 7 | 173 ± 7 | 172 ± 8 | 173 ± 8 | 0.876 |
| Weight, kg | 76 ± 15 | 75 ± 15 | 72 ± 16 | 72 ± 15 | 0.157 |
| ASA I/II | 46/54 | 48/52 | 45/55 | 49/51 | 0.940 |
| Induction time, s | 46 ± 8 | 45 ± 9 | 45 ± 9 | 45 ± 9 | 0.807 |
| Operation duration, min | 46 ± 9 | 45 ± 9 | 45 ± 9 | 44 ± 9 | 0.614 |
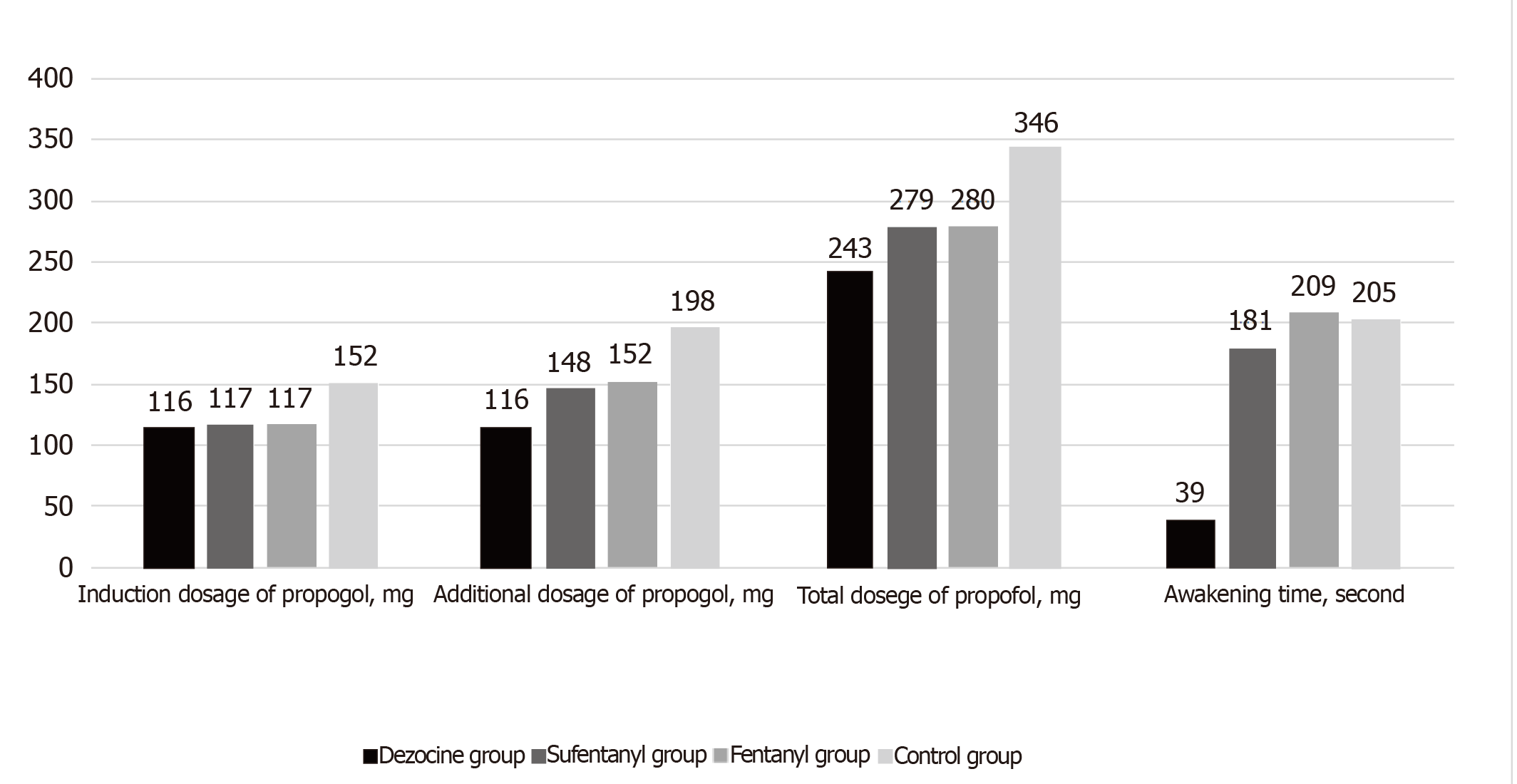
As shown in Table 2 and Figures 2 and 3, the induction dosages, additional dosages, and total dosages of propofol in the dezocine, sufentanil, and fentanyl groups were significantly lower than those in the control group (P < 0.01). The additional dosage and total dosage of propofol in the dezocine group were significantly lower than those in the sufentanil and fentanyl groups (P < 0.01). The awakening time in the dezocine and sufentanil groups was significantly shorter compared with the control group (P < 0.01), and the awakening time in the dezocine group was significantly shorter than that in the sufentanil group (P < 0.01). Steward scores after eye opening in the dezocine and sufentanil groups were significantly higher compared with the control group (P < 0.01), and Steward score after eye opening in the dezocine group was significantly higher than that in the sufentanil group (P < 0.01). Postoperative pain scores in the dezocine group were significantly lower than those in other groups (P < 0.01). Postoperative pain scores in the sufentanil and fentanyl groups were significantly lower than those in the control group (P < 0.01). However, there were no statistical differences in awakening time or Steward score after eye opening between the fentanyl and control groups (P > 0.05). Moreover, correlation analysis showed that awakening time (r = 0.392, P < 0.001) and Steward score (r = -0.306, P < 0.001) were correlated to the total dosage of propofol.
| Item | Dezocine group | Sufentanyl group | Fentanyl group | Control group | P value |
| Induction dosage of propofol, mg | 116 ± 21a | 117 ± 21a | 117 ± 23a | 152 ± 28 | <0.001 |
| Additional dosage of propofol, mg | 116 ± 20a | 148 ± 29ac | 152 ± 27ac | 198 ± 28 | <0.001 |
| Total dosage of propofol, mg | 243 ± 42a | 279 ± 50ac | 280 ± 45ac | 346 ± 37 | <0.001 |
| Awakening time, second | 39 ± 12a | 181 ± 35abc | 209 ± 55c | 205 ± 50 | <0.001 |
| Steward score | 4.74 ± 1.088a | 4.07 ± 0.856abc | 2.97 ± 0.797c | 3.15 ± 0.833 | <0.001 |
| Postoperative pain score | 1.62 ± 1.117a | 2.25 ± 1.672ac | 2.68 ± 2.024ac | 4.61 ± 1.136 | <0.001 |
Compared with the dezocine group, BIS was comparatively lower at T3 in the sufentanil, fentanyl, and control groups (P < 0.01) (Table 3; Figure 4A). Compared with T1, MAP and HR decreased after anesthesia induction in the fentanyl and control groups at T2 (P < 0.01), and gradually increased as the operation progressed, but was still lower than the baseline value in the fentanyl and control groups at T3 (P < 0.01). Compared with the dezocine and sufentanil groups, MAP was comparatively lower in the fentanyl and control groups at T2 (P < 0.01) (Table 3; Figure 4B). Compared with T1, SpO2 and RR decreased after anesthesia induction in the sufentanil, fentanyl, and control groups at T2 (P < 0.01), and gradually increased as the operation progressed; however, there were no significant statistical differences among the four groups at T3 (P > 0.05). Compared with the dezocine group, SpO2 and RR were comparatively lower at T2 in the sufentanil, fentanyl, and control groups (P < 0.01) (Table 3; Figure 4C and D).
| Item | Time point | Dezocine group | Sufentanyl group | Fentanyl group | Control group | P value |
| BIS | T1 | 92.77 ± 4.824 | 91.89 ± 4.694 | 92.94 ± 4.610 | 92.11 ± 4.197 | 0.302 |
| T2 | 52.01 ± 4.768a | 52.47 ± 4.768a | 53.02 ± 4.216a | 52.44 ± 4.685a | 0.492 | |
| T3 | 85.14 ± 9.072 | 77.85 ± 7.512c | 75.83 ± 5.927c | 73.21 ± 5.761c | 0.000 | |
| MAP (mmHg) | T1 | 88.47 ± 10.538 | 86.08 ± 10.003 | 87.50 ± 9.630 | 86.18 ± 11.397 | 0.308 |
| T2 | 86.43 ± 10.415 | 84.72 ± 8.078 | 74.76 ± 9.007ab | 75.33 ± 8.766ab | 0.000 | |
| T3 | 87.36 ± 9.733 | 84.62 ± 8.262 | 82.93 ± 9.907ac | 79.79 ± 8.308ac | 0.000 | |
| HR (beats/min) | T1 | 74.90 ± 13.654 | 72.56 ± 15.408 | 74.49 ± 14.797 | 77.41 ± 14.749 | 0.138 |
| T2 | 65.94 ± 9.389a | 63.36 ± 9.130a | 61.37 ± 10.333ac | 62.25 ± 10.930ac | 0.009 | |
| T3 | 71.18 ± 11.111 | 68.50 ± 12.156 | 69.58 ± 11.560 | 72.06 ± 11.581 | 0.131 | |
| SpO2 (%) | T1 | 97.01 ± 1.480 | 97.29 ± 1.431 | 97.14 ± 1.484 | 96.84 ± 1.441 | 0.162 |
| T2 | 97.15 ± 1.579 | 94.02 ± 1.348ac | 92.64 ± 1.812ac | 92.61 ± 1.576ac | 0.000 | |
| T3 | 97.04 ± 1.421 | 97.12 ± 1.387 | 97.03 ± 1.460 | 97.07 ± 1.409 | 0.970 | |
| RR (times/min) | T1 | 18.51 ± 1.661 | 18.57 ± 1.671 | 18.37 ± 1.668 | 18.42 ± 1.571 | 0.827 |
| T2 | 17.35 ± 1.678 | 14.48 ± 1.605ac | 13.54 ± 1.690ac | 13.22 ± 1.873ac | 0.000 | |
| T3 | 17.01 ± 1.425 | 16.79 ± 1.274 | 16.72 ± 1.464 | 17.05 ± 1.452 | 0.264 |
As shown in Table 4, usage of vasoactive drugs, rates of hypopnea, jaw thrust, and body movement in the dezocine group were significantly lower than those of other groups (P < 0.01). Moreover, correlation analysis showed that usage of vasoactive drugs was correlated to total dosage of propofol (r = 0.204, P < 0.001). However, no statistically significant differences in reflex coughing, nausea, or vomiting were observed among the four groups (P > 0.05). Furthermore, no patient in any group experienced therapy interruption, aspiration, or intra-operative awareness.
| Item | Dezocine group | Sufentanyl group | Fentanyl group | Control group | P value |
| Usage of vasoactive drugs | 7 | 8 | 36 | 39 | 0.000 |
| Hypopnea | 2 | 7 | 14 | 18 | 0.001 |
| Jaw thrust | 2 | 7 | 14 | 18 | 0.001 |
| Body movement | 5 | 15 | 16 | 25 | 0.001 |
| Reflex coughing | 3 | 8 | 9 | 11 | 0.182 |
| Nausea and vomiting | 6 | 4 | 5 | 4 | 0.895 |
This study aimed to identify a comparatively satisfactory anesthetization regimen for painless gastroscopy and colonoscopy. The combination of propofol and dezocine can decrease propofol dosage, reduce the risk for the development of inhibitory effects on the cardiovascular and respiratory systems, increase analgesic effect, decrease body movement, shorten awakening time, and improve awakening quality.
The duration of gastroscopy and colonoscopy is typically approximately 45 min, and addition of propofol is needed. However, high additional dosage of propofol can significantly prolong recovery time, increase the risk for post-procedure respiratory depression and hypoxemia, and the workload for recovery management. Dezocine can also decrease the additional dosage of propofol in patients undergoing gastroscopy and colonoscopy, and reduce the side effects of propofol.
In recent years, painless electronic endoscopy has been widely used in clinical practice, reducing discomfort and suffering in patients undergoing gastroscopy or colonoscopy[1,3]. Presently, the generally used anesthetic drug is propofol, which is an intravenously administered sedative with a favourable sedative effect, rapid onset, and short duration of action, which results in a decreased level of consciousness and lack of memory of events[4,11,12,15]. Propofol also strongly inhibits the contraction of gastrointestinal smooth muscle, antagonizes the vomiting reflex, and reduces cough and physical movement[3,16-18]. However, because it lacks an obvious analgesic effect, the dosages of propofol are relatively high in painless gastroscopy and colonoscopy. High dosages of propofol usually cause inhibitory effects on the cardiovascular and respiratory systems. Similar to other opioids, dezocine has been reported to decrease anesthetic requirements by up to 50%[19].
Dezocine belongs to a class of opioid receptor agonist and antagonist drugs that exhibit strong affinity for both the mu and kappa receptors, but relatively weak interactions with the delta receptor[13,20,21], and has been used in procedures requiring propofol sedation[22] and postoperative pain management[20]. Because dezocine is a partial mu receptor agonist and a kappa receptor antagonist[13,23], common side effects observed in opioids with full agonism, such as sufentanil and fentanyl, are significantly reduced. Dezocine, however, exhibits a “ceiling effect” for respiratory depression (a notorious and fatal side effect caused by commonly used clinical opiates). In an in vitro study, dezocine inhibited norepinephrine and serotonin reuptake in a concentration-dependent manner through two novel molecular targets (the norepinephrine transporter and serotonin transporter)[13]. The interaction of dezocine with three major opioid receptors and two novel molecular targets helped to elucidate the mechanisms underlying the pharmacological effects of dezocine.
The major limitation of this study was its single-center design, which may limit the generalizability of the results. Therefore, multicenter, prospective, randomized studies will be needed to further assess the clinical effect of propofol combined with dezocine in painless gastroscopy and colonoscopy.
In conclusion, this study demonstrated that anesthesia with propofol combined with dezocine is an adequate regimen of anesthesia and analgesia for gastroscopy and colonoscopy, which can increase the patient cooperation, quality and safety of the examination and treatment, and patient and physician satisfaction with anesthesia.
Endoscopy is usually associated with severe adverse reactions.
The optimal methods for inducing analgesia and sedation in endoscopy are areas of ongoing debate.
To evaluate the effects of propofol combined with dezocine, sufentanil, or fentanyl in painless gastroscopy and colonoscopy.
Patients were randomly assigned to one of four groups for anesthesia: intravenous dezocine, sufentanil, fentanyl, or saline.
Propofol dosage, bispectral index, Steward score, awakening time, postoperative pain score, mean arterial pressure, pulse oxygen saturation, rates of hypopnea, jaw thrust, body movements, and usage of vasoactive drugs in the dezocine group were significantly better than those of the other three groups.
The combination of propofol and dezocine can decrease propofol dosage, reduce the risk for the development of inhibitory effects on the respiratory and cardiovascular systems, increase analgesic effect, decrease body movement, shorten awakening time, and improve awakening quality.
Anesthesia with propofol combined with dezocine is an adequate regimen of anesthesia and analgesia for gastroscopy and colonoscopy, which can increase the patient cooperation, quality and safety of the examination and treatment, and patient and physician satisfaction with anesthesia.
The authors are grateful to their colleagues in the Department of Gastroenterology for their help.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poskus T, Yeoh SW S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Kim JH, Eun HW, Hong SS, Auh YH. Early gastric cancer: virtual gastroscopy. Abdom Imaging. 2006;31:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Liu J, Wang B, Hu W, Sun P, Li J, Duan H, Si J. Global and Local Panoramic Views for Gastroscopy: An Assisted Method of Gastroscopic Lesion Surveillance. IEEE Trans Biomed Eng. 2015;62:2296-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (3)] |
| 4. | Gasparović S, Rustemović N, Opacić M, Premuzić M, Korusić A, Bozikov J, Bates T. Clinical analysis of propofol deep sedation for 1,104 patients undergoing gastrointestinal endoscopic procedures: a three year prospective study. World J Gastroenterol. 2006;12:327-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | IJspeert JEG, Bevan R, Senore C, Kaminski MF, Kuipers EJ, Mroz A, Bessa X, Cassoni P, Hassan C, Repici A, Balaguer F, Rees CJ, Dekker E. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017;66:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Repici A, Pagano N, Hassan C, Carlino A, Rando G, Strangio G, Romeo F, Zullo A, Ferrara E, Vitetta E, Ferreira Dde P, Danese S, Arosio M, Malesci A. Balanced propofol sedation administered by nonanesthesiologists: The first Italian experience. World J Gastroenterol. 2011;17:3818-3823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Xiao Q, Yang Y, Zhou Y, Guo Y, Ao X, Han R, Hu J, Chen D, Lan C. Comparison of Nasopharyngeal Airway Device and Nasal Oxygen Tube in Obese Patients Undergoing Intravenous Anesthesia for Gastroscopy: A Prospective and Randomized Study. Gastroenterol Res Pract. 2016;2016:2641257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Meining A, Semmler V, Kassem AM, Sander R, Frankenberger U, Burzin M, Reichenberger J, Bajbouj M, Prinz C, Schmid RM. The effect of sedation on the quality of upper gastrointestinal endoscopy: an investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy. 2007;39:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Shen XC, Ao X, Cao Y, Lan L, Liu XM, Sun WJ, Li P, Lan CH. Etomidate-remifentanil is more suitable for monitored anesthesia care during gastroscopy in older patients than propofol-remifentanil. Med Sci Monit. 2015;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Adams MA, Prenovost KM, Dominitz JA, Kerr EA, Krein SL, Saini SD, Rubenstein JH. National Trends in Use of Monitored Anesthesia Care for Outpatient Gastrointestinal Endoscopy in the Veterans Health Administration. JAMA Intern Med. 2017;177:436-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Ho WM, Yen CM, Lan CH, Lin CY, Yong SB, Hwang KL, Chou MC. Comparison between the recovery time of alfentanil and fentanyl in balanced propofol sedation for gastrointestinal and colonoscopy: a prospective, randomized study. BMC Gastroenterol. 2012;12:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | LaPierre CD, Johnson KB, Randall BR, Egan TD. A simulation study of common propofol and propofol-opioid dosing regimens for upper endoscopy: implications on the time course of recovery. Anesthesiology. 2012;117:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Kiriyama S, Naitoh H, Kuwano H. Propofol sedation during endoscopic treatment for early gastric cancer compared to midazolam. World J Gastroenterol. 2014;20:11985-11990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Lee TL, Ang SB, Dambisya YM, Adaikan GP, Lau LC. The effect of propofol on human gastric and colonic muscle contractions. Anesth Analg. 1999;89:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Fanti L, Testoni PA. Sedation and analgesia in gastrointestinal endoscopy: what's new? World J Gastroenterol. 2010;16:2451-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Rowlingson JC, Moscicki JC, DiFazio CA. Anesthetic potency of dezocine and its interaction with morphine in rats. Anesth Analg. 1983;62:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Fragen RJ, Caldwell N. Comparison of dezocine (WY 16, 225) and meperidine as postoperative analgesics. Anesth Analg. 1978;57:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | O'Brien JJ, Benfield P. Dezocine. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1989;38:226-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ramirez-Ruiz M, Smith I, White PF. Use of analgesics during propofol sedation: a comparison of ketorolac, dezocine, and fentanyl. J Clin Anesth. 1995;7:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 3.3] [Reference Citation Analysis (0)] |