Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3217
Peer-review started: April 12, 2019
First decision: August 1, 2019
Revised: August 23, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 26, 2019
Processing time: 198 Days and 12.5 Hours
Colorectal cancer is a common malignant tumor of the digestive tract. The relationship between sentinel polyps (rectal polyps with proximal colon cancer) and proximal colon cancer has received extensive attention in recent years. However, there is still no clear conclusion regarding the relationship.
To investigate the clinical characteristics of sentinel polyps and their correlation with proximal colon cancer.
A retrospective analysis of 2587 patients with rectal polyps from January 2006 to December 2017 was performed. According to whether or not proximal colon cancer was diagnosed, the patients were divided into either a sentinel polyp group (192 patients) or a pure rectal polyp group (2395 patients). The endoscopic features, clinicopathological features, therapeutic effects, and short-term prognosis were analyzed and compared between the two groups.
The mean age of patients in the sentinel polyp group was generally higher than that of the pure rectal polyp group, and the positivity rates of anemia, stool occult blood, and tumor markers of the sentinel polyp group were also significantly higher than those in the rectal polyp group (χ2 = 90.56, P < 0.01; χ2 = 70.30, P < 0.01; χ2 = 92.80, P < 0.01). The majority of the patients in the sentinel polyp group had multiple polyps, large polyps, adenomatous polyps, or sessile polyps (χ2 = 195.96, P < 0.01; χ2 = 460.46, P < 0.01; χ2 = 94.69, P < 0.01; χ2 = 48.01, P < 0.01). Most of the proximal colon cancers were Duke’s A and B stages in the sentinel polyp group. In the pure rectal polyp group, 2203 patients underwent endoscopic treatment, and all of the patients were cured and discharged. In the sentinel polyp group, 65 patients underwent radical operation, and 61 patients received endoscopic submucosal dissection or endoscopic mucosal resection. Additionally, 21 patients were lost to follow-up after 6-12 mo, and the loss rate was 10.94%. A total of 63.16% of patients experienced remission without tumor recurrence or metastasis, 33.33% of patients experienced tumors regression or improved symptoms, and the other 3.51% of the patients died.
If there are multiple, sessile, and adenomatous rectal polyps with a maximum diameter > 1 cm, the possibility of the carcinogenesis of the polyps or of the proximal colon should be monitored closely. These patients should be followed in the short-term and should undergo a whole-colon examination.
Core Tip: This retrospective study included 2587 patients with rectal polyps. According to whether or not proximal colon cancer was diagnosed, the patients were divided into either a sentinel polyp group or a pure rectal polyp group. The endoscopic features, clinicopathological features, therapeutic effects, and short-term prognosis were analyzed. We found that if there were multiple, sessile, and adenomatous rectal polyps with a maximum diameter > 1 cm, the risk of the carcinogenesis of the polyps or of proximal colon cancer is high, and a full colonoscopy and follow-up should be performed.
- Citation: Wang M, Lu JJ, Kong WJ, Kang XJ, Gao F. Clinical characteristics of sentinel polyps and their correlation with proximal colon cancer: A retrospective observational study. World J Clin Cases 2019; 7(20): 3217-3225
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3217.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3217
Colorectal cancer is a common malignant tumor of the digestive tract. With the improvement of living standards, diet and lifestyle have gradually changed, and the incidence and mortality of colorectal cancer have been increasing annually[1,2]. On the other hand, colorectal cancer is also showing a trend of affecting younger individuals. The incidence of colorectal cancer in adolescents and young adults is rising[3]. Early diagnosis and treatment can obviously improve the prognosis and quality of life of patients with colon cancer. In 1991, Foutch et al[4] proposed the concept of sentinel polyps; that is, for patients with rectal polyps and proximal colon carcinoma, sentinel polyps may be a marker of proximal colon cancer (ascending colon and transverse colon cancer). Rectal polyps are the most common type of intestinal polyps, and their relationship with proximal colon cancer has received extensive attention in recent years[5,6]. This study analyzed the characteristics of rectal polyps and explored the correlation between sentinel polyps and proximal colon cancer in northwestern China.
This study was a retrospective study that was approved by the Institutional Review Board (IRB) of People’s Hospital of Xinjiang Uygur Autonomous Region. The informed consent requirements were waived by the IRB due to the retrospective nature of this study, and this work adhered to the applicable STROBE guidelines.
All patients who were first diagnosed with rectal polyps and received treatment at the Medical Center of the People’s Hospital of Xinjiang Uygur Autonomous Region between January 2006 and December 2017 were included as study subjects. The inclusion criteria included the following: (A) Patients who were diagnosed with rectal polyps for the first time; and (B) Patients whose diagnoses were confirmed by colonoscopy and pathological findings. The exclusion criteria included the following: (A) Age ˂ 18 years; (B) No total colonoscopy or no biopsy; (C) Inflammatory bowel disease; (D) History of resection of intestinal polyps; (E) History of resection of intestinal malignant tumors; (F) Rectal tumor; (G) Metastatic colon carcinoma; (H) Polyposis; and (I) Nonpolyposis hereditary colon cancer syndrome. A total of 2587 patients met the inclusion criteria and agreed to participate in the study. According to whether they had proximal colon carcinoma, the patients were divided into either a sentinel polyp group or a pure rectal polyp group.
The clinical data, laboratory findings, colonoscopic characteristics, pathological histology, tumor metastasis, treatment, and short-term prognosis of the patients were retrospectively collected from the electronic medical records.
Statistical analyses were performed using SPSS software (version 17.0). All data are represented as the mean ± SD, or as percentages and ranges. The measurement data and numeration data were evaluated by the t-test and chi-square test, respectively. Fisher’s exact tests with 95%CIs were used when the number of samples was less than 5. P values less than 0.05 were considered statistically significant.
The protocol for this study is available on the Chinese Clinical Trial Registry (ChiCTR1900021639). The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
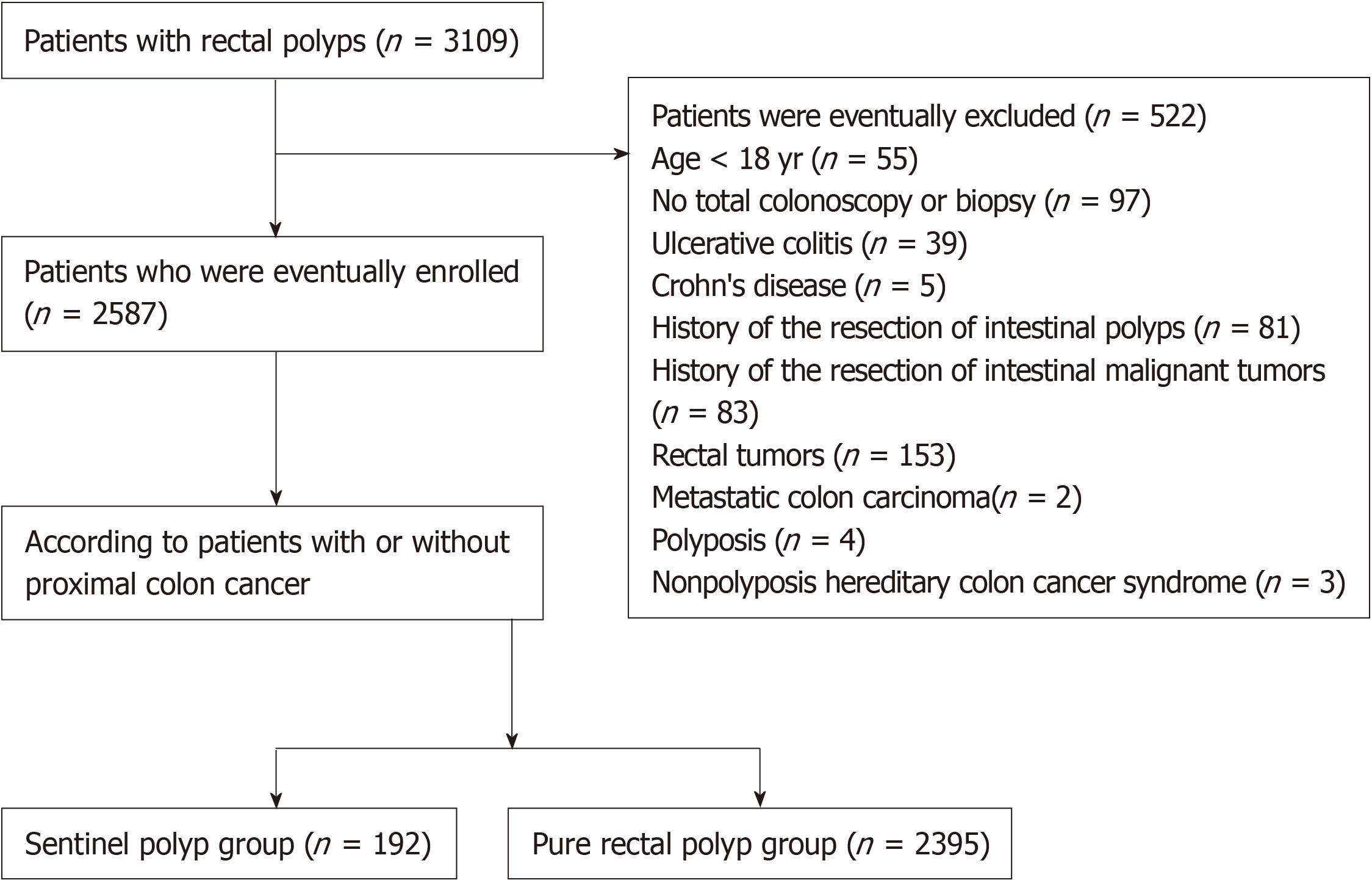
A total of 3109 patients were included in this study, of which 2587 were enrolled in the final analysis. Figure 1 shows the flow chart of the study, which included the subject withdrawal at various stages and the final diagnoses. A total of 1418 males and 1169 females were included. The age ranged from 18 to 88 years old (average, 54.33 ± 12.26 years old), and the total hospitalization days ranged from 2 to 77 d. There were 192 patients in the sentinel polyp group, which contained 118 males and 74 females. The average age was 64.30 ± 11.15 years. There were 44 Uygur patients, 130 Han patients, and 18 patients of other ethnic groups. There were 2395 patients in the pure rectal polyp group, including 1300 males and 1095 females, with an average age of 56.43 ± 11.68 years. There were 631 Uygur individuals, 1525 Han individuals, and 239 individuals of other ethnic groups. The clinical features of the two groups are shown in Table 1. No significant difference was found in gender between the two groups (χ2 = 3.698, P > 0.05), but the proportion of male patients was higher than that of female patients (61.46% vs 38.54%; 54.28% vs 45.72%) in the two groups. The mean age of the sentinel polyp group was significantly higher (χ2 = 79.097, P < 0.01) than that of the pure rectal polyp group, and most of the patients in the sentinel polyp group were over 60 years old. Pure rectal polyps often occurred in patients between 40-60 years old. A pairwise comparison showed that the incidence of sentinel polyps increased significantly with age. There was no significant difference in the ethnicity between the two groups (χ2 = 1.319, P > 0.05).
| Characteristic | Sentinel polyps | Pure rectal polyps | χ2 | P value |
| Total number | 192 | 2395 | ||
| Gender | ||||
| Male | 118 (61.46) | 1300 (54.28) | 3.7 | 0.05 |
| Female | 74 (38.54) | 1095 (45.72) | ||
| Age | ||||
| < 40 yr old | 11 (5.73) | 344 (14.36) | 79.1 | < 0.01 |
| 40-60 yr old | 64 (33.33) | 1334 (55.70) | ||
| > 60 yr old | 117 (60.94) | 717 (29.94) | ||
| Ethnic | ||||
| Uygur | 44 (22.92) | 631 (26.35) | 1.32 | 0.52 |
| Han | 130 (67.71) | 1525 (63.67) | ||
| Others | 18 (9.37) | 239 (9.98) | ||
| Anemia | 58 (30.21) | 206 (8.60) | 90.56 | < 0.01 |
| Positivity for fecal occult blood | 36 (18.75) | 106 (4.43) | 70.3 | < 0.01 |
| Tumor marker-positive | 86 (44.79) | 398 (16.62) | 92.8 | < 0.01 |
| Number of polyps | ||||
| 1 | 56 (29.17) | 1767 (73.78) | 195.96 | < 0.01 |
| 2 | 64 (33.33) | 397 (16.58) | ||
| ≥ 3 | 72 (37.50) | 231 (9.64) | ||
| Size of polyp | ||||
| < 0.5 cm | 65 (33.85) | 1888 (78.83) | 460.46 | < 0.01 |
| 0.5-1 cm | 47 (24.48) | 423 (17.66) | ||
| ≥ 1 cm | 80 (41.67) | 84 (3.51) | ||
| Presence or absence of a pedicle | ||||
| Pedunculated | 62 (32.29) | 328 (13.70) | 48.01 | < 0.01 |
| Sessile | 130 (67.71) | 2067 (86.30) | ||
| Malignant tumors in first-degree relatives | 57 (29.69) | 151 (6.30) | 94.726 | < 0.01 |
Most of the patients showed predominantly nonspecific abdominal symptoms, including abdominal pain (290 patients, 11.21%), black or bloody stools (478 patients, 18.48%), bloating (343 patients, 13.26%), changes in bowel habits (718 patients, 27.75%), and acute intestinal obstruction (3 patients, 0.12%). The average time from the symptom onset to diagnosis was 10 months (range, 1-240 mo). Two hundred and ninety-four (11.36%) patients had lesions found during routine physical examination and no discomfort, including 43 cases of proximal colon cancer, accounting for 22.40% of patients in the sentinel polyp group.
A total of 1891 (73.10%) patients suffered from hypertension, fatty liver, hyperlipidemia, or diabetes; 325 (12.56%) had coronary heart disease, 702 (27.14%) had a history of digestive system disease, and 497 (19.21%) had a history of cholecystectomy.
Two hundred and eight (8.04%) patients had a first-degree relative with malignant tumors, including 57 (29.69%) patients in the sentinel polyp group and 151 (6.30%) in the pure rectal polyp group. No significant difference between the two groups was found in regard to the family history (χ2 = 94.726, P < 0.01). Four (0.15%) patients had a family history of familial adenomatous polyposis (FAP) and three patients had a family history of colon cancer, accounting for 1.56% of the sentinel polyp group.
The liver and kidney function and serum ions had no obvious abnormalities in all patients. There were 264 patients with mild to moderate anemia (hemoglobin range, 63-121 g/L), including 58 (30.21%) patients in the sentinel polyp group and 206 (8.60%) in the pure rectal polyp group. A significant difference was found between the two groups in regard to anemia (χ2 = 90.561, P < 0.01). One hundred and forty-two patients tested positive for fecal occult blood. Thirty-six (18.75%) of these patients were in the sentinel polyp group, and 106 (4.43%) were in the pure rectal polyp group. There was a significant difference between the two groups in regard to fecal occult blood positivity (χ2 = 70.303, P < 0.01). A total of 484 patients were tumor marker-positive (at least one positive indicator among CEA, CA199, CA125, and CA724), including 86 (44.79%) patients in the sentinel polyp group and 398 (16.62%) in the pure rectal polyp group. The positivity rate of tumor markers in the sentinel polyp group was significantly higher than that in the pure rectal polyp group (χ2 = 92.77, P < 0.01) (Table 1).
There were no special colonoscopic findings in the pure rectal polyp group. In the sentinel polyp group, the main manifestation of proximal colon cancer was the encircling of the intestinal lumen in the tumor. However, there were no special manifestations of the intestinal tract between the distal rectal polyps and proximal colon cancer. In the sentinel polyp group, having multiple polyps along the whole colon was rare (2 patients, 1.9%), suggesting that the lesions were not continuous.
As shown in Tables 1 and 2, the detection rates of multiple polyps (n ≥ 3), large polyps (maximum diameter of more than 1 cm), adenomatous polyps (including tubular adenoma, villous tubular adenoma, and villous adenoma), and pedunculated polyps in the sentinel polyps group were significantly higher than those in the pure rectal polyp group (χ2 = 195.964, P < 0.01; χ2 = 460.464, P < 0.01; χ2 = 94.693, P < 0.01; χ2 = 48.014, P < 0.01).
| Group | Adenomatous polyps | Non-adenomatous polyps | ||||||
| Tubular adenoma | Villous tubular adenoma | Villous adenoma | Total | Inflamma-tory | Hyper-plastic | Juvenile | Total | |
| Sentinel polyps | 107 (55.73) | 25 (13.02) | 15 (7.81) | 147 (76.56)a | 36 (18.75) | 8 (4.17) | 1 (0.52) | 45 (23.44) |
| Pure rectal polyps | 898 (37.50) | 46 (1.92) | 24 (1.00) | 968 (40.42) | 1070 (44.68) | 331 (13.82) | 26 (1.08) | 1427 (59.58) |
The pathological types of the 192 patients with colon cancer in the sentinel polyps group were as follows: Well-differentiated adenocarcinoma in 52 (27.08%) patients; moderately differentiated adenocarcinoma in 34 (17.71%); poorly differentiated adenocarcinoma in 13 (6.77%); mucinous adenocarcinoma in 9 (4.69%); signet-ring cell carcinoma in 7 (3.65%); and early carcinoma in 77 (40.10%). In terms of distant metastasis, the number of patients with Duke’s stages A, B, C, and D stage disease was 94 (48.96%), 35 (18.23%), 39 (20.31%), and 24 (12.50%), respectively.
H. pylori was detected by the rapid urease test or 14C-urea breath test. Of the 1972 patients, 1360 were positive for H. pylori. The positivity rate was 80.65%, including 80 patients in the sentinel polyp group and 1280 patients in the pure rectal polyp group. There was no significant difference between the two groups in regard to H. pylori infection (P > 0.05).
In the pure rectal polyp group, 2203 patients underwent endoscopic treatment, and all of these patients were cured and discharged. In the sentinel polyp group, 65 (33.85%) patients received radical surgery, and endoscopic submucosal dissection or endoscopic mucosal resection was performed in 61 (31.77%) patients. Three (1.56%) patients with Duke’s stage D disease underwent endoscopic self-expanding metallic stent implantation. Eight (4.17%) patients underwent palliative surgery (bypass operation or colostomy). Twenty-one (10.94%) patients received chemotherapy, 15 (7.81%) received radiation therapy, and 19 (9.90%) chose to undergo conservative treatment. All patients enrolled were followed for 6-12 mo, and 21 (10.94%) patients were lost to follow-up. Finally, 108 (56.25%) patients were in complete remission without tumor recurrence or metastasis, and 57 (29.69%) had tumor regression or improved symptoms. Additionally, six (3.13%) patients died.
It is clear that there is a lack of effective methods to prevent colon cancer. Early diagnosis and treatment are the most effective ways to improve the prognosis and the survival rate of colon cancer. The morbidity rate and the death rate of colorectal cancer have increased in recent years, and colorectal cancer has become a major cause of death. The expenditure and financial burden involved with the diagnosis and treatment remain at a high level[7,8].
Colorectal cancer is already in a middle or late stage before it is diagnosed in most patients. Early diagnosis and timely treatment are essential to improve the cure rate and survival rate of patients[9,10]. Rectal polyps are one of the most common types of intestinal diseases. The relationship between rectal polyps and colon cancer has received much attention from researchers[5,11]. Sentinel polyps are not uncommon in clinical practice. However, for some patients, poor bowel preparations, being uncooperative, or other reasons make it difficult to perform an examination of the whole colon. Or rectal polyps may have been found during other examinations and not during colonoscopy. All of these situations can lead to the misdiagnosis and missed diagnosis of colon cancer. Therefore, the aim of this study was to explore the characteristics of rectal polyps and their relationship with proximal colon cancer to provide a reference for the clinical diagnosis and early diagnosis of colon cancer.
Our data showed that the onset age of the sentinel polyp group was significantly higher than that of the pure rectal polyp group. Pure rectal polyps often occur at 40 to 60 years old, but sentinel polyps were more common in patients over 60 years old. Great attention should be paid to elderly patients with rectal polyps[12]. However, some researchers have found that the incidence of colorectal cancer in adolescents and young people in the United States is rising, as 5.7% of newly diagnosed colorectal cancer patients are under 45 years old, and 20.5% of patients are younger than 55 years old[12]. Colorectal cancer can largely be prevented by the detection and removal of adenomatous polyps. The survival rate is significantly better when colorectal cancer is diagnosed while the disease is still localized.
Most of the patients showed predominantly nonspecific abdominal symptoms, including abdominal pain, black or bloody stools, bloating, and changes in bowel habits. Additionally, 43 (22.40%) patients with sentinel polyps had no symptoms of digestive tract discomfort and were diagnosed during a routine colonoscopy. This finding indicates that clinical symptoms have limited significance for sentinel polyps. Colonoscopy should be recommended even if the patient has no obvious abdominal symptoms. If there is no relative or absolute contraindication, long-term atypical lower abdominal symptoms should be diagnosed by colonoscopy, thus providing a greater potential for prevention through polypectomy[13]. The proportion of patients who had relatives that were diagnosed with malignant tumors in the sentinel polyp group was higher than that in the pure rectal polyp group. This finding suggested that genetic factors were closely related to the occurrence of colon cancer, which is consistent with the results of Lee et al[14]. The proportion of patients with a family history of FAP in the sentinel polyp group was higher than in the pure rectal polyps group, which is consistent with the findings from another study[15].
There were 1891 (73.10%) patients suffering from hypertension, hyper-lipoproteinemia, fatty liver, or diabetes. These factors were closely related to the prevalence of rectal and colon cancer. Similar results were found in a previous study[16]. It is suggested that we should perform colonoscopy for patients with these metabolic diseases, especially for patients with rectal polyps. Compared with the pure rectal polyp group, the rates of mild to moderate anemia and fecal occult blood were higher in the sentinel polyp group. These symptoms are considered to be related to tumor hemorrhage, poor nutrition, tumor consumption and so on. The positive rate of tumor markers in the sentinel polyp group was higher than that in the pure rectal polyp group. This result implied that the combined detection of multiple tumor markers is valuable in differentiating colon cancer from non-cancerous tissue. This could effectively improve the early diagnosis rate of colon cancer[17].
This study found that patients with multiple total colonic polyps in the colon were relatively rare. There was no special manifestation of the intestinal tube between the rectal polyps and proximal colon cancer, suggesting that the lesion was discontinuous.
Therefore, if a rectal polyp is found during colonoscopy, regardless of whether the intestinal tube is normal, it is necessary to continue to enter the ileum at the end of the ileocecal valve to obtain a full colonic examination. Pullens et al[18] performed computed tomography (CT) colonography on 136 consecutive cases of incomplete colonoscopy. CT colonography revealed 19 polyps in 15 (11.0%) patients and nonsynchronous colorectal cancer in 4 (2.9%) patients after an incomplete colonoscopy. Accordingly, if a total colonic examination cannot be conducted, we could observe the rest intestine by barium enema, CT colonography and so on.
In our study, when comparing the sentinel polyp group with the pure rectal polyp group, the number of polyps, size, presence or absence of a pedicle, and pathological types were significantly different between the two groups. Additionally, when there is a polyp with the maximum diameter > 1 cm, sessile polyps, or multiple rectal polyps or if a pathological examination detected adenomatous polyps, the possibility of proximal colon cancer should be examined. For patients with rectal polyps with the above mentioned characteristics, even if it is difficult to perform an examination or if the patient has difficulty tolerating the examination and give up, the whole colon examination should be attempted again shortly, to avoid missed diagnoses and to improve the early diagnosis and treatment of colon cancer. Even if rectal polyps have been removed, patients should be followed closely, and colonoscopy should be regularly repeated.
In this study, patients with colon cancer were diagnosed based on a pathological examination, most of whom had early-stage cancer, including adenocarcinoma of various differentiation grades, mucinous carcinoma, and signet-ring cell carcinoma. Most patients (approximately 129, 67.19%) had cancer at Duke's stages A and B. This suggests that paying more attention to rectal polyps will be helpful to the early diagnosis and treatment of colon cancer. Most of the patients in the sentinel polyps group underwent either endoscopic treatment or radical surgery, which was related to the colon cancer being in the early stages. During the follow-up of 6 to 12 mo, six patients died, indicating that the survival rate of colon cancer is still not optimal. At present, the main causes of death are tumor recurrence and metastasis in colon cancer patients. Therefore, patients who underwent endoscopic treatment or radical surgery still need to regularly receive follow-up with tumor marker testing and colonoscopy[19].
In summary, sentinel polyps are closely related to proximal colon cancer, but the sequence, the homology of the disease, and the progression of distal rectal polyps after proximal colon cancer resection are still unclear, and multicenter prospective studies are needed. The relevant characteristics of sentinel polyps provide a direction for future research and have clear guiding significance.
Colorectal cancer is a common malignant tumor of the digestive tract. In recent years, the incidence of colorectal cancer has increased with the improvement of people's living standards. The relationship between sentinel polyps (rectal polyps with proximal colon cancer) and proximal colon cancer has also received extensive attention.
There is still no clear conclusion about the relationship between sentinel polyps and proximal colon cancer.
Our main purpose was to investigate the correlation between sentinel polyps and proximal colon cancer.
A retrospective analysis of 2587 patients with rectal polyps from January 2006 to December 2017 was performed. According to whether they had proximal colon carcinoma, the patients were divided into either a sentinel polyp group or a pure rectal polyp group. The endoscopic characteristics, clinical and pathological features, treatment, and prognosis of the two groups were compared.
The sentinel polyps group had a higher average age, and the positivity rates of anemia, stool occult blood, and tumor markers of the sentinel polyp group were also significantly higher than those in the rectal polyp group (P < 0.01). The majority of the patients in the sentinel polyp group had multiple polyps, large polyps, adenomatous polyps, or sessile polyps (P < 0.01). In the pure rectal polyp group, 2203 patients underwent endoscopic treatment, and all of them were cured and discharged. In the sentinel polyp group, 65 patients underwent radical operation, and 61 patients received endoscopic submucosal dissection or endoscopic mucosal resection. A total of 63.16% of patients experienced remission without tumor recurrence or metastasis, 33.33% experienced tumor regression or improved symptoms, and the other 3.51% died.
If there are multiple, sessile, and adenomatous rectal polyps with a maximum diameter > 1 cm, the possibility of polyp or proximal colon cancer should be closely monitored and a whole-colon examination should be performed.
Although the relationship between sentinel polyps and proximal colon cancer has received widespread attention, there are still no clear conclusions. This study shows that sentinel polyps are closely related to proximal colon cancer, and the characteristics of sentinel polyps provide a direction for future research on colon cancer and have clear guiding significance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamburoglu B S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3275] [Article Influence: 409.4] [Reference Citation Analysis (3)] |
| 2. | van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, Iversen LH, van Krieken JH, Marijnen CA, Henning G, Gore-Booth J, Meldolesi E, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Påhlman L, Quirke P, Rödel C, Roth A, Rutten H, Schmoll HJ, Smith JJ, Tanis PJ, Taylor C, Wibe A, Wiggers T, Gambacorta MA, Aristei C, Valentini V. EURECCA colorectal: multidisciplinary management: European consensus conference colon rectum. Eur J Cancer. 2014;50:1.e1-1.e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 306] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Weinberg BA, Marshall JL, Salem ME. The Growing Challenge of Young Adults With Colorectal Cancer. Oncology (Williston Park). 2017;31:381-389. [PubMed] |
| 4. | Foutch PG, DiSario JA, Pardy K, Mai HD, Manne RK. The sentinel hyperplastic polyp: a marker for synchronous neoplasia in the proximal colon. Am J Gastroenterol. 1991;86:1482-1485. [PubMed] |
| 5. | Steele SR, Johnson EK, Champagne B, Davis B, Lee S, Rivadeneira D, Ross H, Hayden DA, Maykel JA. Endoscopy and polyps-diagnostic and therapeutic advances in management. World J Gastroenterol. 2013;19:4277-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Wang HX, Huang YQ, Wang L. Correlation between sentinel polyps and proximal colon carcinoma and analysis of its clinical features. Zhongguo Yishi Jinxiu Zazhi. 2016;39:697-700. [DOI] [Full Text] |
| 7. | Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2051] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 8. | Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, Mao AY, Ren JS, Sun XJ, Zhu XY, Wang L, Song BB, Du LB, Zhu L, Gong JY, Zhou Q, Liu YQ, Cao R, Mai L, Lan L, Sun XH, Ren Y, Zhou JY, Wang YZ, Qi X, Lou PA, Shi D, Li N, Zhang K, He J, Dai M. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin J Cancer. 2017;36:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1302] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 10. | Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 11. | Sievers CK, Grady WM, Halberg RB, Pickhardt PJ. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev Gastroenterol Hepatol. 2017;11:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Chen XY, Zhang H, Zhu Q, Zhen PQ, Chen L. Endoscopic characteristics of colon polyps in elderly patients. Zhonghua Laonian Yixue Zazhi. 2011;30:482-484. [DOI] [Full Text] |
| 13. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, Pickhardt PJ, Rex DK, Smith RA, Thorson A, Winawer SJ; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1455] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 14. | Lee SD, Kim BC, Han KS, Hong CW, Sohn DK, Park JW, Park SC, Kim SY, Baek JY, Chang HJ, Kim DY, Oh JH. Influence of family history on survival in patients with colon and rectal cancer. J Dig Dis. 2014;15:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Recio-Boiles A, Waheed A, Cagir B. Cancer, Colon. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2019; . [PubMed] |
| 16. | Choi YJ, Lee DH, Han KD, Shin CM, Kim N. Abdominal obesity, glucose intolerance and decreased high-density lipoprotein cholesterol as components of the metabolic syndrome are associated with the development of colorectal cancer. Eur J Epidemiol. 2018;33:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Liang XL, Gao HY, Ye LS, Wang YG. Models of logistic regression analysis, support vector machine, and back-propagation neural network based on serum tumor markers in colorectal cancer diagnosis. Genet Mol Res. 2016;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Pullens HJ, van Leeuwen MS, Laheij RJ, Vleggaar FP, Siersema PD. CT-colonography after incomplete colonoscopy: what is the diagnostic yield? Dis Colon Rectum. 2013;56:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | McKeown E, Nelson DW, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, Avital I, Brücher BL, Steele SR. Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer. 2014;5:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |