Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3175
Peer-review started: June 23, 2019
First decision: July 31, 2019
Revised: August 23, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 26, 2019
Processing time: 126 Days and 14.9 Hours
Antibiotic resistance has become a global threat for human health, calling for rational use of antibiotics.
To analyze the distribution and drug resistance of the bacteria, providing the prerequisite for use of antibiotics in emergency patients.
A total of 2048 emergency patients from 2013 to 2017 were enrolled. Their clinical examination specimens were collected, followed by isolation of bacteria. The bacterial identification and drug susceptibility testing were carried out.
A total of 3387 pathogens were isolated. The top six pathogens were Acinetobacter baumannii (660 strains), Staphylococcus aureus (436 strains), Klebsiella pneumoniae (347 strains), Pseudomonas aeruginosa (338 strains), Escherichia coli (237 strains), and Candida albicans (207 strains). The isolation rates of these pathogens decreased year by year except Klebsiella pneumoniae, which increased from 7.1% to 12.1%. Acinetobacter baumannii is a widely-resistant strain, with multiple resistances to imipenem, ciprofloxacin, minocycline and tigecycline. The Staphylococcus aureus had high resistance rates to levofloxacin, penicillin G, and tetracycline. But the susceptibility of it to vancomycin and tigecycline were 100%. Klebsiella pneumoniae had high resistance rates to imipenem, cefoperazone/sulbactam, amikacin, and ciprofloxacin, with the lowest resistance rate to tigecycline. The resistance rates of Pseudomonas aeruginosa to cefoperazone/sulbactam and imipenem were higher, with the resistance rate to amikacin below 10%. Besides, Escherichia coli had high resistance rates to ciprofloxacin and cefoperazone/sulbactam and low resistance rates to imipenem, amikacin, and tigecycline.
The pathogenic bacteria isolated from the emergency patients were mainly Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. The detection rates of drug-resistant bacteria were high, with different bacteria having multiple drug resistances to commonly used antimicrobial agents, guiding the rational use of drugs and reducing the production of multidrug-resistant bacteria.
Core tip: The purpose of this study is to analyze the distribution and drug resistance of the bacteria isolated from the emergency department specimens, providing the prerequisite for rational use of antibiotics in emergency patients. The top six pathogens were Acinetobacter baumannii (660 strains), Staphylococcus aureus (436 strains), Klebsiella pneumoniae (347 strains), Pseudomonas aeruginosa (338 strains), Escherichia coli (237 strains), and Candida albicans (207 strains). The detection rates of drug-resistant bacteria were high, with different bacteria having multiple drug resistances to commonly used antimicrobial agents, guiding the rational use of drugs and reducing the production of multidrug-resistant bacteria.
- Citation: Huai W, Ma QB, Zheng JJ, Zhao Y, Zhai QR. Distribution and drug resistance of pathogenic bacteria in emergency patients. World J Clin Cases 2019; 7(20): 3175-3184
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3175
With the wide application of antibacterial drugs, the sensitivity of common bacteria to antibiotics is decreasing. Especially, the resistance to β-lactams, aminoglycosides, fluoroquinolones, and sulfonamides extremely plagues the effective anti-infective treatment and seriously threatens the lives of patients. At the same time, the emergence of new antibacterial drugs makes problem of drug abuse increasingly serious. It has become one of the most important medical problems worldwide. The recent discovery of superbacteria in India and other countries is a serious consequence of the abuse of antimicrobial drugs, but currently there is still no effective treatment that can replace the antibiotics to inhibit and exterminate the pathogens. Therefore, the rational use of antibiotics has become an important topic for clinical use[1]. However, the premise of rational drug use is to understand the distribution of pathogens and the trends and characteristics of drug resistance.
Generally, patients in the emergency department have a relatively rapid disease progression. Without clear etiology and bacteriological basis, early and empirical use of antibiotics is required. In China, the emergency department of the central hospitals is an important department for the treatment and rescue of critically ill patients. It is difficult to avoid the body injury of patients caused by invasive treatment while rescuing patients, so it is also inevitable to be complicated with various hospital infectious diseases[2]. Besides, most of the emergency departments are open management, with more patients’ family members, more hospital staff flow, and more severe ward environmental pollution, further increasing the probability of hospital-acquired infection in critically ill patients in the emergency department[3]. For patients with severe infections, a combination of multiple antibiotics is often required, which may lead to bacterial resistance. Due to the great difference in the resistance of different pathogenic bacteria to antibacterial drugs, it brings great inconvenience to clinical medication, followed by poor therapeutic effect[4,5]. In this study, we analyzed the distribution characteristics and drug resistance to different antibiotics of the bacteria isolated from the emergency department specimens in our hospital from 2013 to 2017. This study is of great significance for the selection of drugs for initial empirical treatment in the emergency department and the careful selection of antibiotics to reduce the generation of drug-resistant strains.
This study was approved by the Ethics Committee of Peking University Third Hospital, China, and all patients provided written informed consent.
A total of 2048 critically ill patients hospitalized in the emergency department of Peking University Third Hospital from January 2013 to December 2017 were enrolled. The clinical examination specimens of these patients were collected, including deep sputum retained after adequate gargling (secretions from deep respiratory tract in patients with tracheotomy or intubation), urine, localized secretions, and blood from the apical segment of the catheter in patients with central venous catheterization1. Finally, the respiratory specimens accounted for 51.6% of all samples, blood specimens accounted for 23.3%, urine specimens accounted for 18.7%, sterile body fluid specimens accounted for 4.0 % and other specimens accounted for 2.4%. The bacteria were then isolated from these specimens. Only the first isolated strains were used for patients with replicate strains isolated. Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, Escherichia coli ATCC25922, and Pseudomonas aeruginosa ATCC27853 were used as control strains.
Bacterial identification and drug susceptibility testing were carried out according to the routine methods of the National Clinical Laboratory Procedures for bacteria culture, isolation, and identification.
The isolation media including China-blue plate and blood agar plate were purchased from Oxoid, United Kingdom. The bacterial identification was then performed using VITEK 2 Compact automatic microorganism analysis system (bioMerieux, France) and BD-Bruker MALDI Biotyper microorganism mass spectrometry rapid identification system (BD, United Kingdom).
Machine-based and paper-based susceptibility tests were used for evaluating drug sensitivity. Among them, the machine-based susceptibility test applied VITEK 2 Compact automatic microbiological analysis system to determine the minimum inhibitory concentration (MIC) of commonly used antibacterial drugs; the paper-based susceptibility test used Mueller Hinton Agar plates (Oxoid, United Kingdom). Cefoperazone-sulbactam, imipenem, tigecycline, amikacin, ciprofloxacin, and minocycline were purchased from Oxoid, United Kingdom. According to the Clinical and Laboratory Standards Institute (CLSI) M100-S27 document, 2017 edition, the sensitivity of both tests was determined as sensitive (S), intermediate resistant (I), and resistant (R). The determination of susceptibility to cefoperazone-sulbactam referred to cefoperazone. The susceptibility to tigecycline of Enterobacteriaceae and Acinetobacter was determined based on the United States Food and Drug Administration standard: S (MIC ≤ 2 μg/mL), I (MIC ≤ 4 μg/mL), and R (MIC ≥ 8 μg/mL).
Data were analyzed using the R 3.5.1 software. The rate was expressed as a percentage. The Cochran-Armitage trend test (CATT) was used to analyze the change trend of drug resistance rates with time. P < 0.05 was considered to be statistically significant.
A total of 3387 pathogens were isolated from various clinical specimens of 2048 critically ill patients, including 1805 strains of Gram-negative bacteria (53.29%), 1141 Gram-positive bacteria (33.69%), and 441 fungi (13.02%). The Gram-negative bacteria were mainly Acinetobacter baumannii (660 strains), Klebsiella pneumoniae (347 strains), Pseudomonas aeruginosa (338 strains), and Escherichia coli (237 strains). The Gram-positive bacteria mainly included Staphylococcus aureus (436 strains), Staphylococcus epidermidis (181 strains), Staphylococcus haemolyticus (161 strains), and Enterococcus faecalis (140 strains). Besides, the fungi were mainly Candida albicans (207 strains), followed by Candida tropicalis (95 strains). As a result, the top six bacteria isolated from emergency patients were Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans.
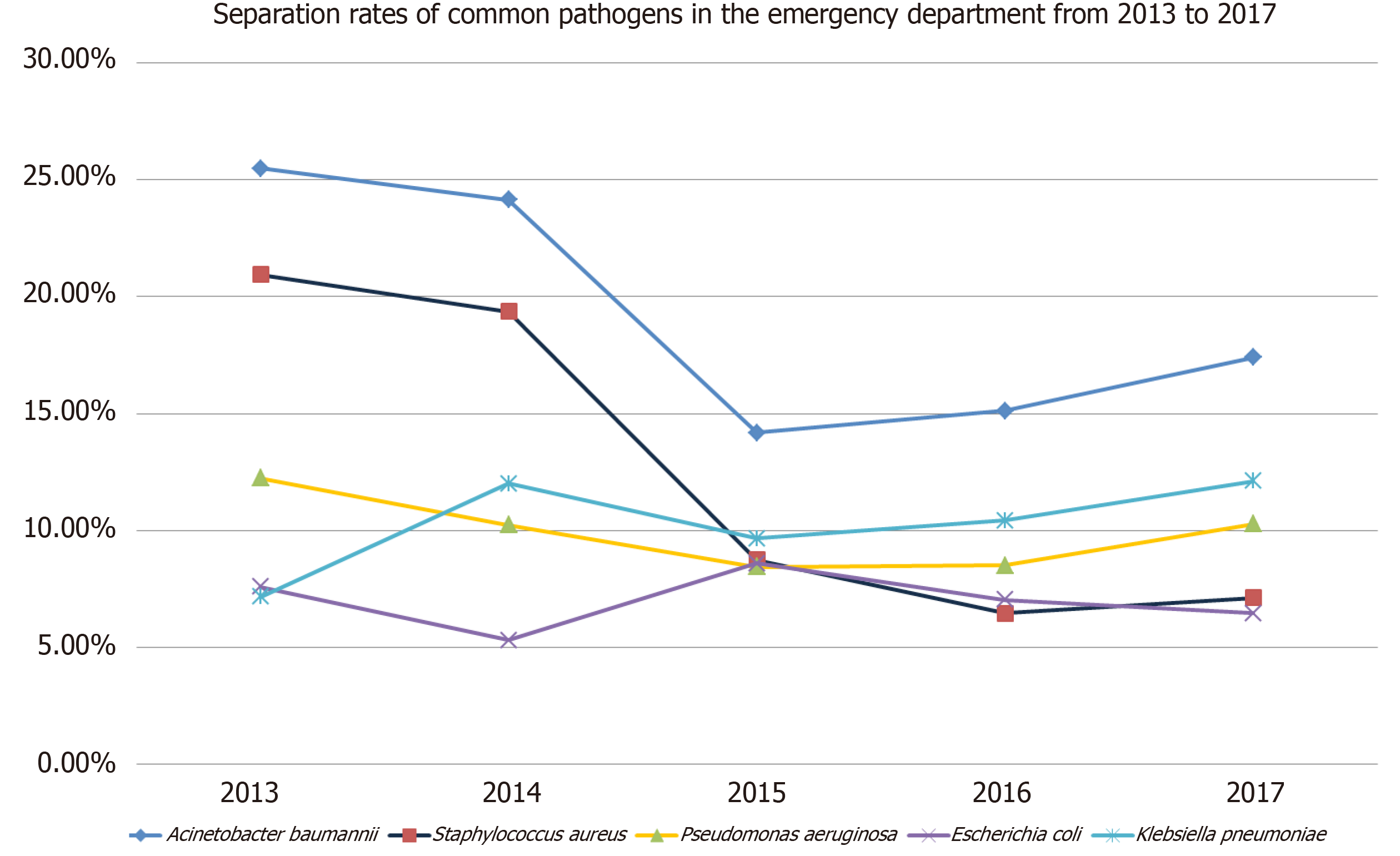
From 2013 to 2017, the isolation rates of common pathogens in the emergency department decreased, including Acinetobacter baumannii (25.5% to 17.4%), Staphylococcus aureus (21. 0% to 7.1%), Pseudomonas aeruginosa (12.3% to 10.3%), and Escherichia coli (7.6% to 6.5%). However, the isolation rate of Klebsiella pneumoniae increased from 7.1% to 12.1% (Figure 1).
Although the isolation rate of Acinetobacter baumannii has been decreasing these year, it was still ranked first for many years and was a widely-resistant strain, whose resistance rates to imipenem and ciprofloxacin were above 90%. The drug with the lowest resistance rate of Acinetobacter baumannii to common clinical antibiotics was tigecycline, followed by minocycline, but the sensitivity to the two drugs has been also decreasing each year (P < 0.01).
The resistance rates of Pseudomonas aeruginosa to cefoperazone/sulbactam and imipenem were higher, with the resistance to cefoperazone/sulbactam increasing year over year (P < 0.01). But its resistance rate to amikacin was below 10%.
Klebsiella pneumoniae had higher resistance rates to imipenem, cefoperazone/sulbactam, amikacin, and ciprofloxacin, with the lowest resistance rate to tigecycline. The resistance rate to minocycline was also high but it decreased year over year (P < 0.01).
For Escherichia coli, the resistance to ciprofloxacin was high, and the resistance rate to cefoperazone/sulbactam showed an upward trend year after year (P < 0.01). Besides, the resistance rates to imipenem, amikacin, and tigecycline were low, with the resistance rate to tigecycline being zero for the last three years (Table 1).
| Gram-negative bacteria | Drugs | 2013 | 2014 | 2015 | 2016 | 2017 | CATT | P value | |||||
| R | I/S | R | I/S | R | I/S | R | I/S | R | I/S | ||||
| Acinetobacter baumannii | Cefoperazone/sulbactam | 84 | 97 | 91 | 86 | 79 | 15 | 80 | 23 | 80 | 25 | 47.72 | < 0.01 |
| Imipenem | 174 | 7 | 170 | 7 | 89 | 5 | 97 | 6 | 102 | 3 | 0.05 | 0.82 | |
| Tigecycline | 7 | 174 | 6 | 171 | 0 | 94 | 10 | 93 | 12 | 93 | 11.13 | < 0.01 | |
| Amikacin | 91 | 90 | 89 | 89 | - | - | - | - | - | - | 0.00 | 1.00 | |
| Ciprofloxacin | 171 | 10 | 167 | 10 | 89 | 5 | 98 | 5 | 103 | 2 | 1.46 | 0.23 | |
| Minocycline | 116 | 65 | 70 | 107 | 17 | 77 | 29 | 74 | 15 | 90 | 79.13 | < 0.01 | |
| Pseudomonas aeruginosa | Cefoperazone/sulbactam | 18 | 69 | 17 | 58 | 7 | 49 | 19 | 39 | 27 | 35 | 10.17 | < 0.01 |
| Imipenem | 29 | 58 | 39 | 36 | 28 | 28 | 28 | 30 | 24 | 38 | 0.31 | 0.58 | |
| Tigecycline | - | - | - | - | - | - | - | - | - | - | - | - | |
| Amikacin | 5 | 82 | 5 | 70 | 2 | 54 | 5 | 53 | 2 | 60 | 0.06 | 0.80 | |
| Ciprofloxacin | 19 | 68 | 20 | 55 | 16 | 40 | 15 | 44 | 10 | 52 | 0.47 | 0.50 | |
| Minocycline | - | - | - | - | - | - | - | - | - | - | - | - | |
| Klebsiella pneumoniae | Cefoperazone/sulbactam | 12 | 39 | 58 | 30 | 23 | 41 | 33 | 38 | 40 | 33 | 1.94 | 0.16 |
| Imipenem | 5 | 46 | 54 | 34 | 25 | 39 | 32 | 39 | 34 | 39 | 3.91 | 0.05 | |
| Tigecycline | - | - | 6 | 82 | 5 | 59 | 12 | 59 | 7 | 66 | 1.48 | 0.22 | |
| Amikacin | 1 | 50 | 36 | 52 | 19 | 45 | 28 | 43 | 25 | 48 | 6.86 | < 0.01 | |
| Ciprofloxacin | 20 | 31 | 58 | 30 | 35 | 29 | 35 | 36 | 40 | 33 | 0.04 | 0.85 | |
| Minocycline | 23 | 28 | 31 | 57 | 19 | 45 | 13 | 58 | 12 | 61 | 17.53 | < 0.01 | |
| Escherichia coli | Cefoperazone/sulbactam | 3 | 51 | 2 | 37 | 12 | 45 | 4 | 44 | 13 | 26 | 11.30 | < 0.01 |
| Imipenem | 0 | 54 | 4 | 35 | 3 | 54 | 0 | 48 | 5 | 34 | 3.21 | 0.07 | |
| Tigecycline | - | - | 3 | 36 | 0 | 57 | 0 | 48 | 0 | 39 | |||
| Amikacin | 3 | 51 | 1 | 38 | 1 | 56 | 1 | 47 | 7 | 32 | 3.20 | 0.07 | |
| Ciprofloxacin | 35 | 19 | 25 | 14 | 34 | 23 | 28 | 20 | 27 | 12 | 0.00 | 0.98 | |
| Minocycline | 19 | 35 | 7 | 32 | 4 | 53 | 9 | 39 | 4 | 35 | 8.41 | < 0.01 | |
Staphylococcus aureus is the top one of the Gram-positive bacteria isolated from clinical examination specimens of emergency department patients. As shown in Table 2, the resistance rates of Staphylococcus aureus to levofloxacin, penicillin G, and tetracycline were high, but the resistance rates to penicillin G and tetracycline showed a downward trend (P < 0.01). Moreover, the resistance rates to vancomycin and tigecycline were always zero.
| Gram-positive bacteria | Drugs | 2013 | 2014 | 2015 | 2016 | 2017 | CATT | P value | |||||
| R | I/S | R | I/S | R | I/S | R | I/S | R | I/S | ||||
| Staphylococcus aureus | Vancomycin | 0 | 149 | 0 | 142 | 0 | 58 | 0 | 44 | 0 | 43 | - | - |
| Levofloxacin | 131 | 18 | 130 | 12 | 38 | 20 | 25 | 19 | 21 | 22 | 53.68 | < 0.01 | |
| Linezolid | 1 | 148 | 4 | 138 | 0 | 58 | 0 | 44 | 0 | 43 | 0.75 | 0.39 | |
| Tigecycline | 0 | 149 | 0 | 142 | 0 | 58 | 0 | 44 | 0 | 43 | - | - | |
| PenicillinG | 146 | 3 | 140 | 2 | 55 | 3 | 41 | 3 | 41 | 2 | 3.45 | 0.06 | |
| Tetracycline | 123 | 26 | 131 | 11 | 35 | 23 | 26 | 18 | 25 | 18 | 28.27 | < 0.01 | |
As mentioned above, the isolation rates of common pathogens decreased year after year except Klebsiella pneumoniae. We then further analyzed the clinical data of all patients infected with Klebsiella pneumoniae (Table 3). There were 347 patients with Klebsiella pneumoniae infection aged between 18 and 96 years, with an average age of 73.35 ± 14.60 years. Notably, we found that the percentage of patients with deep venous catheterization or retention catheterization was much higher than that of patients without both operations.
| Influence factors | Cases | Percentage (%) | Influence factors | Cases | Percentage (%) | ||
| Gender | Male | 175 | 53.19 | Deep vein catheterization | Yes | 224 | 68.09 |
| Female | 154 | 46.81 | No | 105 | 31.91 | ||
| Age (yr) | ≥ 60 | 277 | 84.19 | Mechanical ventilation | Yes | 93 | 28.27 |
| < 60 | 52 | 15.81 | No | 236 | 71.73 | ||
| Tracheotomy | Yes | 9 | 2.74 | Retention catheterization | Yes | 241 | 73.25 |
| No | 320 | 97.26 | No | 88 | 26.75 | ||
This study showed that the top six pathogens isolated from emergency patients in our hospital from 2013 to 2017 were Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans, consistent with other reports in the emergency department of the top three hospitals in the same area. Besides, the detection rates of drug-resistant bacteria were high, showing the severe situation of antibiotic resistance. Therefore, these findings provided a good basis for the early delivery of empirical medication for critically ill patients.
With the emergence of antibacterial drugs, the rate of bacterial resistance remains high[6]. In this study, we found that Staphylococcus aureus had high resistance rates to levofloxacin, penicillin G, and tetracycline, but the resistance rates to vancomycin and tigecycline were always zero. Besides, five linezolid-insensitive strains of Staphylococcus aureus were discovered, consistent with the previous report[7]. Therefore, vancomycin should be given priority in the clinical treatment of severe patients with Staphylococcus aureus infection. However, in case of adverse reactions, such as hyperpyrexia and hypersensitivity after vancomycin administration, tigecycline may be selected and targeted drugs may be given after drug sensitivity testing[8,9]. Hussein et al indicated that a high MRSA prevalence was found amongst healthcare workers (HCWs) in Kurdistan Region, Iraq. Twenty-two point five percent of HCWs were Staphylococcus aureus carriers compared with 18.7% of non-HCWs. 61.0% of S. aureus strains isolated from HCWs were MRSA compared with 21.6% from non-HCWs. The mean working years of MRSA carriers was significantly higher than that of MRSA non-carriers. Basic infection control measures, a screening programme, and treatment of MRSA-positive HCWs can help as an effective measure to control MRSA infections[10]. In addition, it was shown that among 13 patients infected with vancomycin-resistant Enterococcus, most patients had lower limb wound infection[11]. Therefore, for patients with lower limb infection of vancomycin-resistant enterococcus, strict observation and active treatment are required to prevent multiple infections.
Acinetobacter baumannii is a common colonized pathogen in hospital, and it is also a relatively common pathogen of nosocomial acquired pneumonia[12]. In this study, its isolation rate ranked first for many years, and it was an extensively drug-resistant strain. Acinetobacter baumannii in the emergency department is mostly due to nosocomial infection during hospitalization[13,14], and there are few reports that Acinetobacter baumannii causes community infection.
Klebsiella pneumoniae ranked second in the number of Gram-negative strains isolated in the emergency department. Recently, it has been reported that the drug resistance rate of carbapenems resistant enterobacteriaceae (CRE) shows a steady increasing trend, among which Klebsiella bacteria account for the largest proportion[15]. In this study, Klebsiella pneumoniae showed higher resistance to imipenem, cefoperazone/sulbactam, amikacin, and ciprofloxacin. The resistance of Klebsiella pneumoniae to carbapenem is mainly due to the production of carbapenemases, and few antibacterial drugs are available for the strains resistant to carbapenem, making clinical treatment difficult. A study in southern Europe showed a higher incidence of inappropriate empirical treatment for the multidrug-resistant Klebsiella pneumoniae bloodstream infection, resulting in a more than two-fold increase in patient mortality[16]. However, it has also been demonstrated that patients who received carbapenem monotherapy or combination therapy within the first five days after blood culture positive for β-lactamase-producing Klebsiella pneumoniae infection had a significantly lower mortality than those who received non-carbapenem antibiotics[17]. Therefore, carbapenems are still recommended as the treatment of choice for patients with severe infections, and compound agents containing β-lactamase inhibitors can be considered for patients with mild to moderate infections. Besides, due to the increasing reports of carbapenem-resistant Klebsiella pneumoniae in recent years, high doses of carbapenem can be given for treatment[18], and tigecycline can also be selected, owing to its good in vitro antibacterial activity[19].
The positive rate of β-lactamase-producing Escherichia coli from emergency sources should be low, but our study revealed that Escherichia coli had high resistance to ciprofloxacin and cefoperazone/sulbactam, indicating that its resistance to quinolones and cephalosporins is still very prominent. In addition, the resistance rate of Escherichia coli to imipenem was low, which was consistent with the previous study[20].
As mentioned above, Klebsiella pneumoniae has been the only bacteria with the increasing detection rate among the most common bacteria at the emergency department for the past five years, and the resistance to antibiotics such as meropenem and imipenem was also gradually increasing. These findings were in agreement with previous study[21], bringing great difficulties for clinical anti-infective treatment. Therefore, we further analyzed the clinical data of all patients infected with Klebsiella pneumoniae and found that the proportion of patients with deep venous catheterization or retention catheterization was much higher than that of patients without both operations, indicating that indwelling deep venous catheter or urinary catheter is an independent risk factor for bloodstream infection with Klebsiella pneumoniae[22]. Long-term indwelling urethral catheter causes bloodstream infection likely due to the colonization of Klebsiella pneumoniae in damaged urethral mucosa during intubation and its regular release into the blood[23]. Whereas the central venous catheter provides a direct way for bacteria to invade into the bloodstream, inducing the catheter-related bloodstream infection. Further, because the catheter is left in the blood vessel, the pathogenic bacteria in the blood can easily attach the front end of the catheter to gradually form a biofilm that is difficult to remove, which becomes a secondary infection source and aggravates the severity of infection. Therefore, in patients with clinically diagnosed bloodstream infections, when anti-infective drug therapy fails, the possibility of catheter-associated bacteremia should be considered. The ureter or central venous catheter should be removed timely. Other reports show that the use of antibiotics, especially cephalosporins and quinolones, is a risk factor for Klebsiella pneumoniae bloodstream infection[23,24]. Besides, the total amount and days of antibiotic use were also found to be significantly associated with the development of Klebsiella pneumoniae infection[25]. Hence, short-course antibiotics may be useful in reducing drug-resistant bacteria[26].
There is a certain course of treatment with antibiotics, which should be administered on time once adopted to maintain sufficient concentration of drugs in patients to reduce the generation of drug-resistant strains[27,28]. Some recent studies offer some hope for tackling bacterial resistance[29]. Bacteriophages have many advantages over antibiotics in their use to treat and prevent infection by drug-resistant bacteria. Their therapeutic effects are significantly different from those of antibiotics, making them still sensitive to multidrug-resistant bacteria[30]. Therefore, phages are currently seen as a potential effective treatment for many multidrug-resistant bacteria[31]. However, at the present stage, it is still of great significance to strengthen the pathogenic examination and monitoring in the emergency department and understand the distribution and drug resistance trends of the prevalent strains, so as to guide the rational use of drugs, reduce the production of multidrug-resistant bacteria, reduce the hospital infection rate and improve the success rate of patient treatment.
In summary, the pathogenic bacteria isolated from the emergency department were mainly Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans, with high detection rates of drug-resistant bacteria. When critically ill patients are admitted to the emergency department, initial antibiotic treatment should be selected empirically according to the distribution characteristics of bacteria in this area while bacteriological examination should be conducted on the clinical samples as soon as possible, and the later drug regimen should be adjusted timely according to the results of pathogen culture and drug sensitivity. For patients with extremely serious infections and life risk at any time, multi-drug regimens can be considered to achieve early control of the disease.
Antibiotic resistance has become a global threat for human health, calling for rational use of antibiotics.
The premise of rational drug use is to understand the distribution of pathogens and the trends and characteristics of drug resistance.
In this study, we analyzed the distribution characteristics and drug resistance to different antibiotics of the bacteria isolated from the emergency department specimens in our hospital from 2013 to 2017. This study is of great significance for the selection of drugs for initial empirical treatment in the emergency department and the careful selection of antibiotics to reduce the generation of drug-resistant strains.
The isolation media including China-blue plate and blood agar plate were purchased from Oxoid, United Kingdom. The bacterial identification was then performed using VITEK 2 Compact automatic microorganism analysis system (bioMerieux, France) and BD-Bruker MALDI Biotyper microorganism mass spectrometry rapid identification system (BD, United Kingdom). Data were analyzed using the R 3.5.1 software. The rate was expressed as a percentage. The Cochran-Armitage trend test was used to analyze the change trend of drug resistance rates with time. P < 0.05 was considered to be statistically significant.
The top six bacteria isolated from emergency patients were Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. From 2013 to 2017, the isolation rates of common pathogens in the emergency department decreased, including Acinetobacter baumannii (25.5% to 17.4%), Staphylococcus aureus (21. 0% to 7.1%), Pseudomonas aeruginosa (12.3% to 10.3. %), and Escherichia coli (7.6% to 6.5%). However, the isolation rate of Klebsiella pneumoniae increased from 7.1% to 12.1%. The drug with the lowest resistance rate of Acinetobacter baumannii to common clinical antibiotics was tigecycline, followed by minocycline, but the sensitivity to the two drugs has been also decreasing each year (P < 0.01). The resistance rates of Pseudomonas aeruginosa to cefoperazone/sulbactam and imipenem were higher, with the resistance to cefoperazone/sulbactam increasing year over year (P < 0.01). But its resistance rate to amikacin was below 10%. Klebsiella pneumoniae had higher resistance rates to imipenem, cefoperazone/sulbactam, amikacin, and ciprofloxacin, with the lowest resistance rate to tigecycline. The resistance rate to minocycline was also high but it decreased year over year (P < 0.01). For Escherichia coli, the resistance to ciprofloxacin was high, and the resistance rate to cefoperazone/sulbactam showed an upward trend year after year (P < 0.01). the resistance rates of Staphylococcus aureus to levofloxacin, penicillin G, and tetracycline were high, but the resistance rates to penicillin G and tetracycline showed a downward trend (P < 0.01).
The pathogenic bacteria isolated from the emergency department were mainly Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans, with high detection rates of drug-resistant bacteria. When critically ill patients are admitted to the emergency department, initial antibiotic treatment should be selected empirically according to the distribution characteristics of bacteria in this area while bacteriological examination should be conducted on the clinical samples as soon as possible, and the later drug regimen should be adjusted timely according to the results of pathogen culture and drug sensitivity. For patients with extremely serious infections and life risk at any time, multi-drug regimens can be considered to achieve early control of the disease. These findings provided a good basis for the early delivery of empirical medication for critically ill patients. There is a certain course of treatment with antibiotics, which should be administered on time once adopted to maintain sufficient concentration of drugs in patients to reduce the generation of drug-resistant strains. Some recent studies offer some hope for tackling bacterial resistance. Bacteriophages have many advantages over antibiotics in their use to treat and prevent infection by drug-resistant bacteria. Their therapeutic effects are significantly different from those of antibiotics, making them still sensitive to multidrug-resistant bacteria. A total of 2048 critically ill patients were enrolled. The clinical examination specimens of these patients were collected, including deep sputum retained after adequate gargling (secretions from deep respiratory tract in patients with tracheotomy or intubation), urine, localized secretions, and blood from the apical segment of the catheter in patients with central venous catheterization. As mentioned above, Klebsiella pneumoniae has been the only bacteria with the increasing detection rate among the most common bacteria at the emergency department for the past five years, and the resistance to antibiotics such as meropenem and imipenem was also gradually increasing. The pathogenic bacteria isolated from the emergency department were mainly Acinetobacter baumannii, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans, with high detection rates of drug-resistant bacteria. Phages are currently seen as a potential effective treatment for many multidrug-resistant bacteria. However, at the present stage, it is still of great significance to strengthen the pathogenic examination and monitoring in the emergency department and understand the distribution and drug resistance trends of the prevalent strains, so as to guide the rational use of drugs, reduce the production of multidrug-resistant bacteria, reduce the hospital infection rate and improve the success rate of patient treatment.
There may be bias in data collection of retrospective studies. The future research direction is the rational use of antibiotics.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hussein NR S-Editor: Ma RY L-Editor: Filipodia E-Editor: Qi LL
| 1. | Christensen A, Scheel O, Urwitz K, Bergh K. Outbreak of methicillin-resistant Staphylococcus aureus in a Norwegian hospital. Scand J Infect Dis. 2001;33:663-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 2. | Leroy S, Marc E, Bavoux F, Tréluyer JM, Gendrel D, Bréart G, Pons G, Chalumeau M. Hospitalization for severe bacterial infections in children after exposure to NSAIDs: a prospective adverse drug reaction reporting study. Clin Drug Investig. 2010;30:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Ostrowsky B, Steinberg JT, Farr B, Sohn AH, Sinkowitz-Cochran RL, Jarvis WR. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol. 2001;22:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Pallin DJ, Camargo CA, Schuur JD. Skin infections and antibiotic stewardship: analysis of emergency department prescribing practices, 2007-2010. West J Emerg Med. 2014;15:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Lin YB, Gardiner MF. Fingernail-induced corneal abrasions: case series from an ophthalmology emergency department. Cornea. 2014;33:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Magalhães M, Doherty C, Govan JR, Vandamme P. Polyclonal outbreak of Burkholderia cepacia complex bacteraemia in haemodialysis patients. J Hosp Infect. 2003;54:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Mendes RE, Flamm RK, Hogan PA, Ross JE, Jones RN. Summary of linezolid activity and resistance mechanisms detected during the 2012 LEADER surveillance program for the United States. Antimicrob Agents Chemother. 2014;58:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Vandijck DM, Blot SI, Decruyenaere JM. Update on the management of infection in patients with severe sepsis. Dimens Crit Care Nurs. 2008;27:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Díaz A, Barria P, Niederman M, Restrepo MI, Dreyse J, Fuentes G, Couble B, Saldias F. Etiology of community-acquired pneumonia in hospitalized patients in chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007;131:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Miyaji R, Asano K, Matsubara S. Induction of Axial Chirality in 8-Arylquinolines through Halogenation Reactions Using Bifunctional Organocatalysts. Chemistry. 2017;23:9996-10000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Öksüz L, Gürler N. [Serotype distribution and antibiotic resistance of Streptococcus pneumoniae strains isolated from the adult patients in a Turkish üniversity hospital]. Mikrobiyol Bul. 2017;51:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Falcone M, Russo A, Giannella M, Cangemi R, Scarpellini MG, Bertazzoni G, Alarcón JM, Taliani G, Palange P, Farcomeni A, Vestri A, Bouza E, Violi F, Venditti M. Individualizing risk of multidrug-resistant pathogens in community-onset pneumonia. PLoS One. 2015;10:e0119528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Chan J, Wong J, Saginur R, Forster AJ, van Walraven C. Epidemiology and outcomes of bloodstream infections in patients discharged from the emergency department. CJEM. 2015;17:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Arbex MA, Pereira LA, Carvalho-Oliveira R, Saldiva PH, Braga AL. The effect of air pollution on pneumonia-related emergency department visits in a region of extensive sugar cane plantations: a 30-month time-series study. J Epidemiol Community Health. 2014;68:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Xu Y, Gu B, Huang M, Liu H, Xu T, Xia W, Wang T. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J Thorac Dis. 2015;7:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 16. | Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto F, Cristini F, Trapani F, Gaibani P, Viale P. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore). 2014;93:298-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 404] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Giannella M, Trecarichi EM, Giacobbe DR, De Rosa FG, Bassetti M, Bartoloni A, Bartoletti M, Losito AR, Del Bono V, Corcione S, Tedeschi S, Raffaelli F, Saffioti C, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viscoli C, Lewis RE, Viale P, Tumbarello M; Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva (ISGRI-SITA). Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2018;51:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Geng TT, Xu X, Huang M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A retrospective cohort study. Medicine (Baltimore). 2018;97:e9961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Bou-Antoun S, Davies J, Guy R, Johnson AP, Sheridan EA, Hope RJ. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Tian L, Tan R, Chen Y, Sun J, Liu J, Qu H, Wang X. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Bilavsky E, Temkin E, Lerman Y, Rabinovich A, Salomon J, Lawrence C, Rossini A, Salvia A, Samso JV, Fierro J, Paul M, Hart J, Gniadkowski M, Hochman M, Kazma M, Klein A, Adler A, Schwaber MJ, Carmeli Y; MOSAR WP5 study team. Risk factors for colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae on admission to rehabilitation centres. Clin Microbiol Infect. 2014;20:O804-O810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Freeman JT, Nimmo J, Gregory E, Tiong A, De Almeida M, McAuliffe GN, Roberts SA. Predictors of hospital surface contamination with Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: patient and organism factors. Antimicrob Resist Infect Control. 2014;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Wener KM, Schechner V, Gold HS, Wright SB, Carmeli Y. Treatment with fluoroquinolones or with beta-lactam-beta-lactamase inhibitor combinations is a risk factor for isolation of extended-spectrum-beta-lactamase-producing Klebsiella species in hospitalized patients. Antimicrob Agents Chemother. 2010;54:2010-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Shanthi M, Sekar U. Extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. J Assoc Physicians India. 2010;58 Suppl:41-44. [PubMed] |
| 26. | Qadir MI. Review: phage therapy: a modern tool to control bacterial infections. Pak J Pharm Sci. 2015;28:265-270. [PubMed] |
| 27. | Angus DC. Management of sepsis: a 47-year-old woman with an indwelling intravenous catheter and sepsis. JAMA. 2011;305:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med. 2004;30:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Viertel TM, Ritter K, Horz HP. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother. 2014;69:2326-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Chanishvili N. Bacteriophages as Therapeutic and Prophylactic Means: Summary of the Soviet and Post Soviet Experiences. Curr Drug Deliv. 2016;13:309-323. [PubMed] |
| 31. | Macía-Rodríguez C, Alende-Castro V, Vazquez-Ledo L, Novo-Veleiro I, González-Quintela A. Skin and soft-tissue infections: Factors associated with mortality and re-admissions. Enferm Infecc Microbiol Clin. 2017;35:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |