Published online Jan 26, 2019. doi: 10.12998/wjcc.v7.i2.236
Peer-review started: October 16, 2018
First decision: November 22, 2018
Revised: December 4, 2018
Accepted: December 7, 2018
Article in press: December 8, 2018
Published online: January 26, 2019
Processing time: 102 Days and 3 Hours
Sarcomatoid carcinoma of the pancreas (SCP) is a rare and aggressive epithelial tumor that has both epithelial and mesenchymal features. It is characterized by sarcomatous elements with evidence of epithelial differentiation. And the term “sarcomatoid carcinoma” is often confused with “carcinosarcoma”.
We present a case of SCP with lymph node metastasis in a 59-year-old male patient. He had experienced darkening of the urine, scleral icterus, and fatigue for 4 weeks. Computed tomography and magnetic resonance imaging revealed a mass in the pancreatic head, and laboratory tests revealed elevated serum bilirubin levels. The patient underwent pancreaticoduodenectomy after biliary decompression. Histologically, spindle cells with marked nuclear atypia and brisk mitotic activity arranged in a storiform or fascicular pattern were present in the bulk of the tumor. Immunohistochemical analysis found that the spindle cells exhibited strong diffuse positivity for epithelial markers, indicative of epithelial differentiation. Accordingly, the pathologic diagnosis of the pancreatic neoplasm was SCP.
Although sarcomatoid carcinomas and carcinosarcomas have different pathologic features, both have epithelial origin.
Core tip: Sarcomatoid carcinoma of the pancreas is a rare and aggressive epithelial tumor with a sarcoma-like element. The term “sarcomatoid carcinoma” is often confused with “carcinosarcoma”. In the present study, we report a case of sarcomatoid carcinoma arising in the pancreas and discuss the similarities and differences between sarcomatoid carcinomas and carcinosarcomas.
- Citation: Zhou DK, Gao BQ, Zhang W, Qian XH, Ying LX, Wang WL. Sarcomatoid carcinoma of the pancreas: A case report. World J Clin Cases 2019; 7(2): 236-241
- URL: https://www.wjgnet.com/2307-8960/full/v7/i2/236.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i2.236
Sarcomatoid carcinoma of the pancreas (SCP) is a rare and aggressive epithelial tumor with a sarcoma-like element, which exhibits epithelial markers and epithelial ultrastructural features. This could be considered as a stable intermediate stage of the epithelial–mesenchymal transition (EMT)[1,2]. As a variant of conventional pancreatic carcinoma, it has similar clinical features but shows a worse prognosis, with an average survival after diagnosis of 5 mo[3]. According to the World Health Organization (WHO) histological classification, it is grouped as an undifferentiated (anaplastic) carcinoma of the pancreas, together with anaplastic giant cell carcinoma and carcinosarcoma[3]. However, the terms “sarcomatoid carcinoma” and “carcinosarcoma” have been used interchangeably, and their definitions vary among authors. Herein, we report a case of sarcomatoid carcinoma arising in the pancreas and discuss the similarities and differences between sarcomatoid carcinomas and carcinosarcomas.
A 59-year-old male patient had experienced darkening of the urine, scleral icterus, and fatigue for 4 wk.
Biliary decompression by placing stents via endoscopic retrograde cholangiopancreatography had been performed at a different hospital a few days prior, because of elevated serum bilirubin levels and an ampullary tumor revealed by computed tomography (CT). The patient was admitted to our hospital for further evaluation and treatment.
He was a smoker but not a drinker of alcohol.
His medical history and family history were unremarkable, with no diabetes or chronic pancreatitis.
A physical examination revealed scleral icterus, cutaneous jaundice, but no palpable abdominal mass.
Laboratory tests yielded the following results: total bilirubin 44 μmol/L (reference < 21 μmol/L), direct bilirubin 31 μmol/L (reference < 5 μmol/L), alanine aminotransferase 97 U/L (reference < 40 U/L), and carbohydrate antigen 19-9 14.6 U/L (reference < 37 U/L).
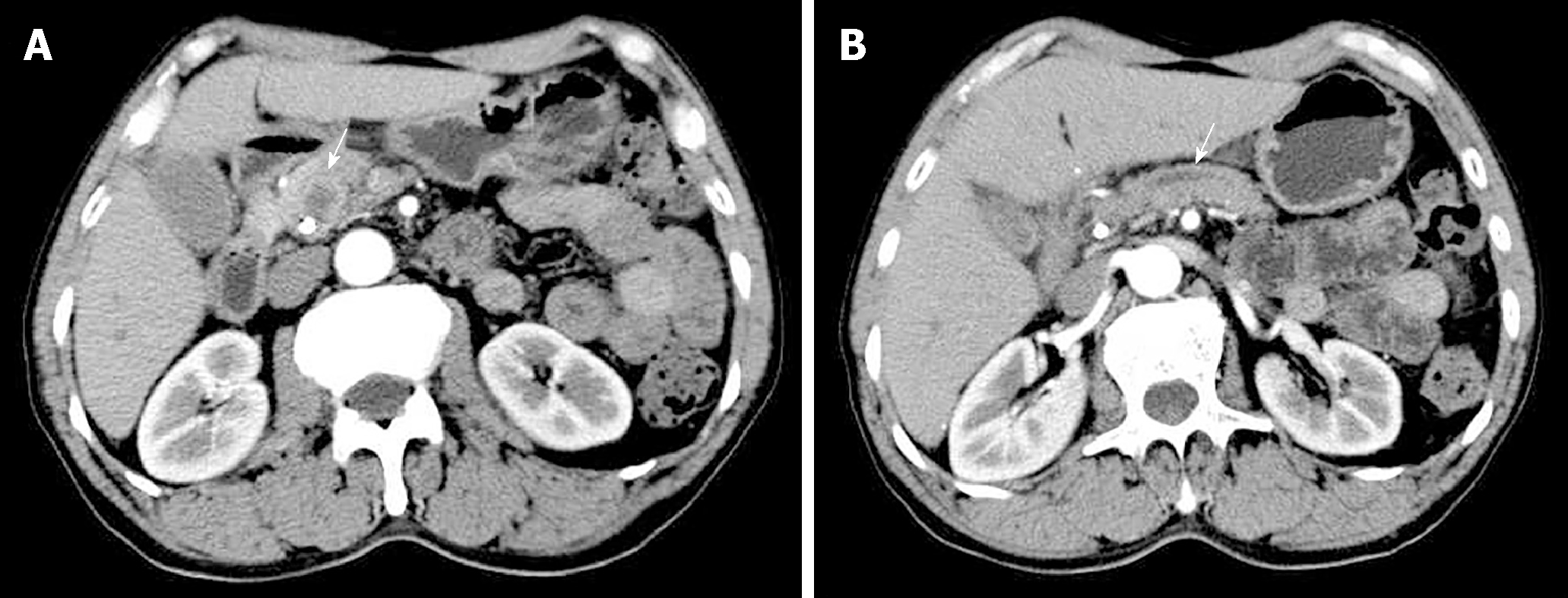
Contrast-enhanced CT revealed a low-density round mass measuring about 1.5 cm × 1.1 cm in the pancreatic head, which was slightly enhanced after intravenous administration of contrast material (Figure 1A). The pancreatic duct, extrahepatic bile duct, and intrahepatic ducts upstream of the obstruction were dilated (Figure 1B). Magnetic resonance imaging revealed an irregular bulky region in the head of the pancreas and a sheet-like lesion in the main pancreatic duct, with an iso-T1 and a long T2 signal.
After his bilirubin levels returned to normal range, the patient underwent a laparotomy due to a suspected pancreatic tumor. During surgery, a firm tumor was palpated in the head of the pancreas. No direct invasion of the surrounding pancreatic tissue or adjacent organs, including the duodenum, stomach, liver, and peritoneum, was found. Subsequently, a pancreaticoduodenectomy was performed and regional lymph nodes were removed.
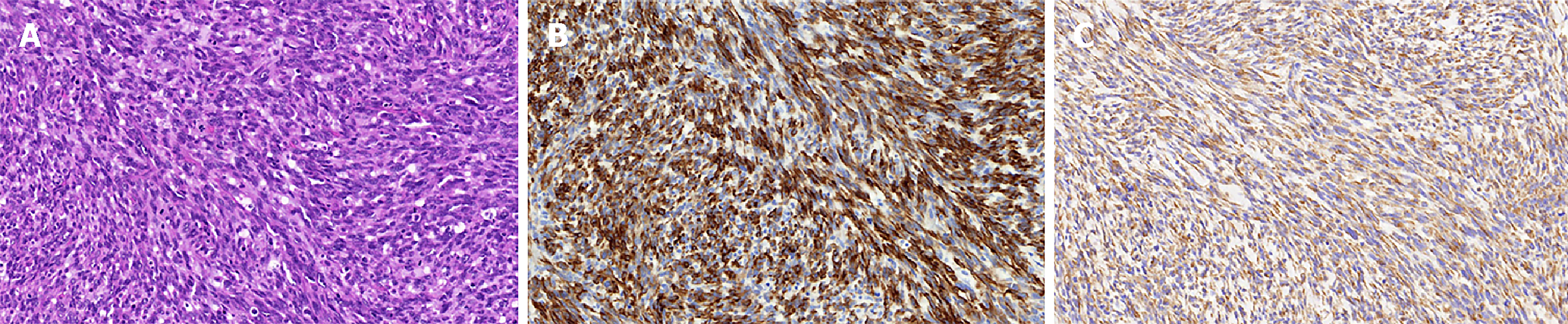
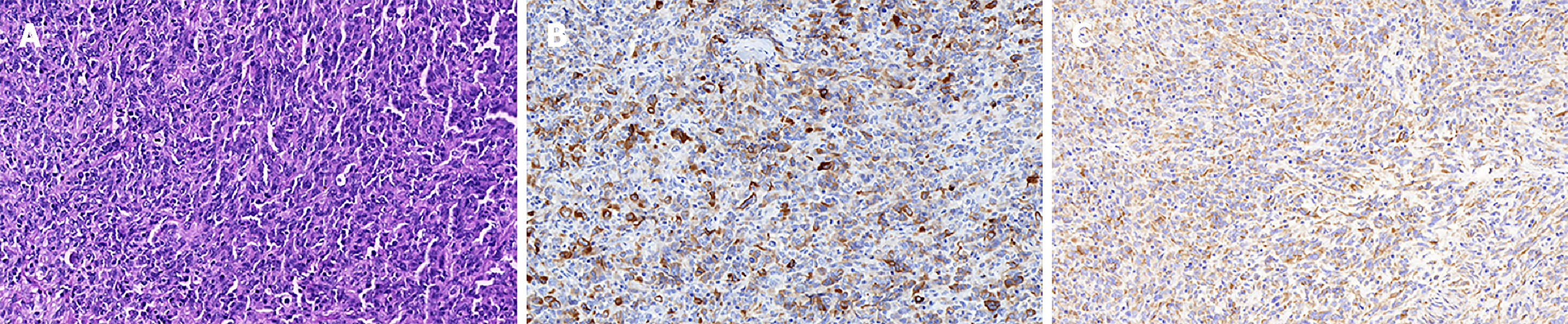
The gross pathology revealed a mass (2.5 cm × 2.5 cm × 2.0 cm) located mainly in the pancreatic head with extension into the main pancreatic duct. Microscopically, spindle cells with marked nuclear atypia and brisk mitotic activity arranged in a storiform or fascicular pattern were present in the bulk of the tumor (Figure 2A). The resection margins of the bile duct, stomach, and duodenum were free of tumor cells, but 3 of the 23 lymph nodes were positive for metastasis. An immunohistochemical examination was performed to identify the sarcomatous elements. The tumor did not express cluster of differentiation (CD) 34, CD117, soluble protein-100, smooth muscle actin, human melanoma black 45, and anaplastic lymphoma kinase, but exhibited strong diffuse positivity for cytokeratin 19 (Figure 2B) and vimentin (Figure 2C). More than 50% of the malignant cells expressed Ki-67. The metastatic lymph nodes exhibited similar histological and immunohistochemical results (Figure 3). Accordingly, the pathologic diagnosis of the pancreatic neoplasm was SCP with TNM stage IIB (T2N1M0).
The patient was discharged from the hospital on the eleventh postoperative day and died of liver metastasis and peritoneal metastasis 6 mo later.
Sarcomatoid carcinomas and carcinosarcomas are rare aggressive malignancies that can develop at various sites of the body, including the genitourinary tract, respiratory tract, digestive tract, breast and thyroid glands, among others[1,4]. So far, 23 cases of sarcomatoid carcinomas or carcinosarcomas arising in the pancreas have been reported[5]. The use of the terms “sarcomatoid carcinoma” and “carcinosarcoma” is unclear and inconsistent both within and across organs, causing confusion for both pathologists and clinicians. For example, according to the WHO histological classification, carcinosarcoma is a hyponym of sarcomatoid carcinoma in lung tumors[6], while they, together with anaplastic giant cell carcinoma, are grouped as undifferentiated (anaplastic) carcinomas of the pancreas[3]. Anaplastic giant cell carcinoma is a relatively common type composed of pleomorphic mononuclear cells and bizarre-appearing giant cells[3], and the latter can be further divided into pleomorphic giant cells and osteoclast-like giant cells[7]. The definitions of pancreatic sarcomatoid carcinoma and carcinosarcoma vary among authors. Based on the histological, ultrastructural, and immunohistochemical evidence, it is undisputable that both sarcomatoid carcinoma and carcinosarcoma of the pancreas have epithelial and mesenchymal features.
Sarcomatoid carcinomas can exhibit a monophasic or biphasic appearance. The monophasic pattern, often referred to as spindle cell carcinoma, is akin to a soft tissue sarcoma without epithelioid areas. The biphasic pattern, the more frequent type, features a mixture of mesenchymal-like and epithelial-like cells with a transition zone[8]. The sarcomatous tissue of both biphasic and monophasic tumors shows evidence of epithelial differentiation, such as epithelial markers and epithelial ultrastructural features, rather than a specific line of mesenchymal differentiation[8,9]. SCP appear to be tumors at a stable intermediate stage of the EMT, as they retain many epithelial characteristics but have a mesenchymal morphology[1,2]. Transforming growth factor-β1 may induce the EMT in pancreatic cells and promote the formation of SCP[10].
Carcinosarcomas are considered to be truly biphasic neoplasms composed of intermingled carcinomatous and sarcomatous components, which have epithelial and mesenchymal differentiation, respectively, according to their pathomorphological and immunohistochemical features[1]. These two components are typically separated without a transition zone[11]. The carcinomatous component expresses epithelial markers and exists as a variety of pathologic types; e.g., pancreatic ductal adenocarcinoma, mucinous cystadenocarcinoma, and intraductal papillary mucinous neoplasm. The sarcomatous component is sub-classified into homologous tissues (mostly malignant spindle cell proliferations) and heterologous tissues (such as osteosarcoma and rhabdomyosarcoma)[1]. The heterologous tissues are defined as those not native to the primary tumor site and show specific mesenchymal differentiation[12]. On immunohistochemical analysis, the sarcomatous components are positive for mesenchymal markers and negative for epithelial markers, indicative of mesenchymal differentiation. The classification of cases with weak or focal positivity for cytokeratin is controversial, and most researchers classify them as carcinosarcomas rather than sarcomatoid carcinomas[1,13,14]. While there is substantial evidence that both carcinosarcomas and sarcomatoid carcinomas have epithelial origin, carcinosarcomas show a more complete EMT of the sarcomatoid component compared to SCP[1].
As variants of conventional pancreatic carcinoma, sarcomatoid carcinoma and carcinosarcoma of the pancreas share similar clinical features. They are found more frequently in the head of the pancreas, and can infiltrate adjacent tissues including the duodenum, stomach, and peripheral nerves. Regional lymph node metastasis and distant metastasis can also occur. The tumors are predominantly found in older persons, and strike both genders with a similar frequency[3,5]. The presenting signs and symptoms include abdominal pain, jaundice, nausea/vomiting, and weight loss[1]. The recommended treatments for sarcomatoid carcinoma and carcinosarcoma mirror those of conventional pancreatic carcinoma[1,15]. Almost all patients undergo surgical treatment, the standard of which is pancreaticoduodenectomy. If needed, postoperative adjuvant chemotherapy with gemcitabine can be applied[1]. Although the tumor is related with the EMT, agents that block or reverse the EMT are at a very early stage of development[15]. Irrespective of the treatment provided, patients have an extremely poor prognosis, with an average survival after diagnosis of 5 mo[3]. According to a report by Shi et al[16], the median OS for T2N1M0 pancreatic ductal adenocarcinoma was 19 mo. In our case, by contrast, the patient survived 6 mo after surgery.
In summary, we present a case of pancreatic sarcomatoid carcinoma and describe its histologic and immunohistochemical features. Although sarcomatoid carcinomas and carcinosarcomas have different pathologic features, both can be interpreted as more malignant variants of conventional pancreatic carcinoma at different stages of the EMT. Therefore, the terms “sarcomatoid carcinoma” and “carcinosarcoma” can be used interchangeably for practical diagnostic purposes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mesa H S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Ruess DA, Kayser C, Neubauer J, Fichtner-Feigl S, Hopt UT, Wittel UA. Carcinosarcoma of the Pancreas: Case Report With Comprehensive Literature Review. Pancreas. 2017;46:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Lu BC, Wang C, Yu JH, Shen ZH, Yang JH. A huge adenosquamous carcinoma of the pancreas with sarcomatoid change: an unusual case report. World J Gastroenterol. 2014;20:16381-16386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Fukushima N, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Shimizu M, Terris B, Bosman FT, Carneiro F, Hruban RH, Theise ND. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. WHO classification of tumours of the digestive system. 4th ed. Bosman FT, Carneiro F, Hruban RH, Theise ND. Lyon: International Agency for Research on Cancer 2010; 294-295. |
| 4. | Rakul NK, Roshni S, Attokaran LL, Rari PM. Sarcomatoid carcinoma of pancreas with liver metastases – A case report with review of literature. J Med Therap. 2017;1:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Xie Y, Xiang Y, Zhang D, Yao X, Sheng J, Yang Y, Zhang X. Sarcomatoid carcinoma of the pancreas: A case report and review of the literature. Mol Med Rep. 2018;18:4716-4724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3099] [Article Influence: 344.3] [Reference Citation Analysis (0)] |
| 7. | Layfield LJ, Bentz J. Giant-cell containing neoplasms of the pancreas: an aspiration cytology study. Diagn Cytopathol. 2008;36:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Guarino M, Tricomi P, Giordano F, Cristofori E. Sarcomatoid carcinomas: pathological and histopathogenetic considerations. Pathology. 1996;28:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Kane JR, Laskin WB, Matkowskyj KA, Villa C, Yeldandi AV. Sarcomatoid (spindle cell) carcinoma of the pancreas: A case report and review of the literature. Oncol Lett. 2014;7:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Ren CL, Jin P, Han CX, Xiao Q, Wang DR, Shi L, Wang DX, Chen H. Unusual early-stage pancreatic sarcomatoid carcinoma. World J Gastroenterol. 2013;19:7820-7824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Shi HY, Xie J, Miao F. Pancreatic carcinosarcoma: first literature report on computed tomography imaging. World J Gastroenterol. 2015;21:1357-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Giordano G, Berretta R, Silini E. Primary pure spindle cell carcinoma (sarcomatoid carcinoma) of the ovary: A case report with immunohistochemical study. Diagn Pathol. 2016;11:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Darvishian F, Sullivan J, Teichberg S, Basham K. Carcinosarcoma of the pancreas: a case report and review of the literature. Arch Pathol Lab Med. 2002;126:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Nakano T, Sonobe H, Usui T, Yamanaka K, Ishizuka T, Nishimura E, Hanazaki K. Immunohistochemistry and K-ras sequence of pancreatic carcinosarcoma. Pathol Int. 2008;58:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Pang A, Carbini M, Moreira AL, Maki RG. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. J Clin Oncol. 2018;36:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, Ni Q, Yu X. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |