Published online Jan 26, 2019. doi: 10.12998/wjcc.v7.i2.191
Peer-review started: October 15, 2018
First decision: December 9, 2018
Revised: December 26, 2018
Accepted: January 3, 2019
Article in press: January 4, 2019
Published online: January 26, 2019
Processing time: 104 Days and 23.9 Hours
Secondary cardiac involvement by lymphoma has received limited attention in the medical literature, despite its grave prognosis. Although chemotherapy improves patients’ survival, a subgroup of treated patients dies suddenly due to myocardial rupture following chemotherapy initiation. Reducing the initial chemotherapy dose with dose escalation to standard doses may be effective in minimizing this risk but the data are limited. We report on the successful management of a patient with disseminated diffuse large B-cell lymphoma (DLBCL) involving the heart using such approach.
An 18-year-old male presented to our hospital with six months history of progressive dyspnea, orthopnea and cough. On physical examination, the patient was found to have a plethoric and mildly edematous face, fixed elevation of the right internal jugular vein, suggestive of superior vena cava obstruction, and a pelvic mass. Investigations during admission including a thoracoabdominal computed tomography (CT) scan with CT guided biopsy of the pelvic mass, echocardiography and cardiac magnetic resonance imaging led to the diagnosis of disseminated DLBCL with cardiac involvement. The patients were successfully treated with chemotherapy dose reduction followed by dose escalation to standard doses, under the guidance of cardiac imaging. The patient completed chemotherapy and underwent a successful bone marrow transplant. He is currently in remission and has a normal left ventricular function.
Imaging-guided chemotherapy dosing may minimize the risk of myocardial rupture in cardiac lymphoma. Data are limited. Management should be individualized.
Core tip: Few Clinicians are comfortable managing a patient with extensive cardiac involvement by lymphoma, due to the limited clinical experience and paucity of data on such cases. Despite the heterogeneity in management, early diagnosis and treatment may lead to remission. Decisions on management should be individualized due to the variability in the pattern and magnitude of cardiac disease. In cases with extensive involvement, reducing the chemotherapy dose with dose escalation to therapeutic levels may be safe and effective management approach. Improvements in imaging technology will likely increase the rate of pre-mortem detection of cardiac lymphoma leading to better understanding of this highly fatal condition and improved patient survival.
- Citation: Al-Mehisen R, Al-Mohaissen M, Yousef H. Cardiac involvement in disseminated diffuse large B-cell lymphoma, successful management with chemotherapy dose reduction guided by cardiac imaging: A case report and review of literature. World J Clin Cases 2019; 7(2): 191-202
- URL: https://www.wjgnet.com/2307-8960/full/v7/i2/191.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i2.191
Although cardiac involvement is relatively common in disseminated lymphoma, it is not well represented in the medical literature and antemortem diagnosis remains suboptimal[1]. This report describes a case of extensive cardiac lymphoma successfully managed with cardiac imaging-guided dosing of chemotherapy. The importance of cardiac imaging in delineating disease extension within the heart, assessing myocardial wall integrity, and healing by fibrosis are delineated.
An 18-year-old immunocompetent male presented to our emergency department with a three-week history of progressive dyspnea, orthopnea, and dry cough.
There was no history of fever, weight loss, or sweating.
The patient’s past medical history was positive for pulmonary tuberculosis that was treated two years previously with a six month course of anti-tuberculous drugs.
He was a non-smoker and there was no history of drug abuse or recent travel. The family history was unremarkable.
On examination, the patient was tachycardic (110 beats/min) and tachypneic (31 breaths/min). His face was mildly edematous and plethoric. Fixed elevation of the right internal jugular vein suggestive of superior vena cava (SVC) obstruction was present. The first and second heart sounds were loud, and murmurs of mitral regurgitation and stenosis were detected. A chest examination revealed reduced air entry at the right base. A pelvic mass was palpable.
The initial laboratory investigations included a complete blood count which reveled leukocytosis [white blood cells: 16.9 × 109/L)] with 22% band neutrophils. He had microcytic hypochromic anemia (hemoglobin: 101 g/L) and a normal platelet count. Serum iron studies showed iron: 5 μmol/L and transferrin 9%. Liver enzymes were elevated; alanine transaminase: 111 U/L, Aspartate transaminase: 100 U/I, Alkaline phosphatase 403 U/L, and Gamma-glutamyl transpeptidase: 414 U/I. Total bilirubin was 55 μmol/L (conjugated: 49 μmol/L) and the international normalized ration was. 1.2 (normal 0.85-1.15). Blood chemistry tests revealed sodium: 131 mmol/L, chloride: 93 mmol/L, potassium: 3.5 mmol/L, bicarbonate: 25 mmol/L, urea 3.2 mmol/L and creatinine 54 μmol/L. The lactate was elevated: 623. Troponin I was negative. The quantiferon-TB gold test was negative. Mycoplasma antibody was negative. Viral hepatitis and HIV serology were negative.
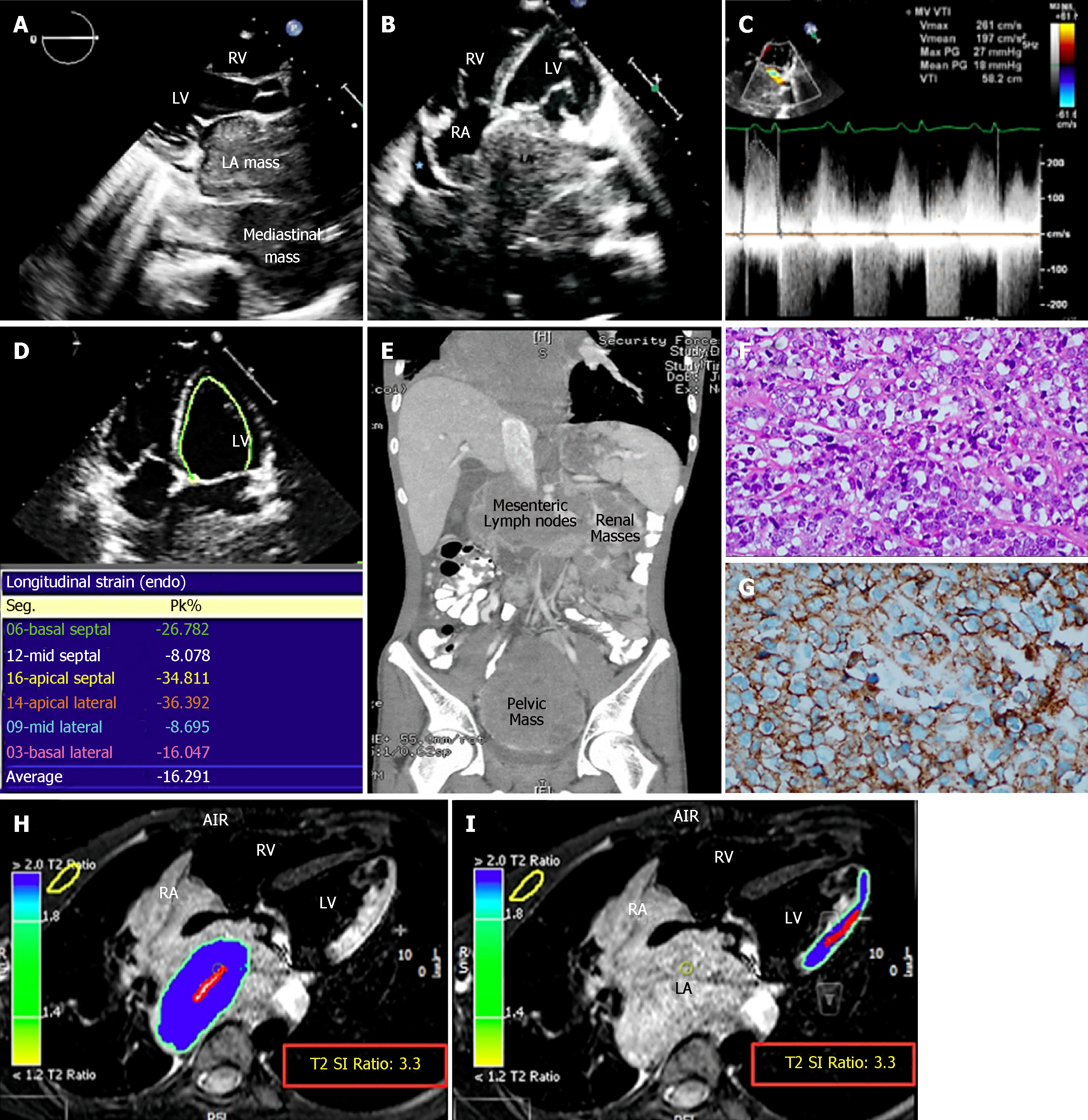
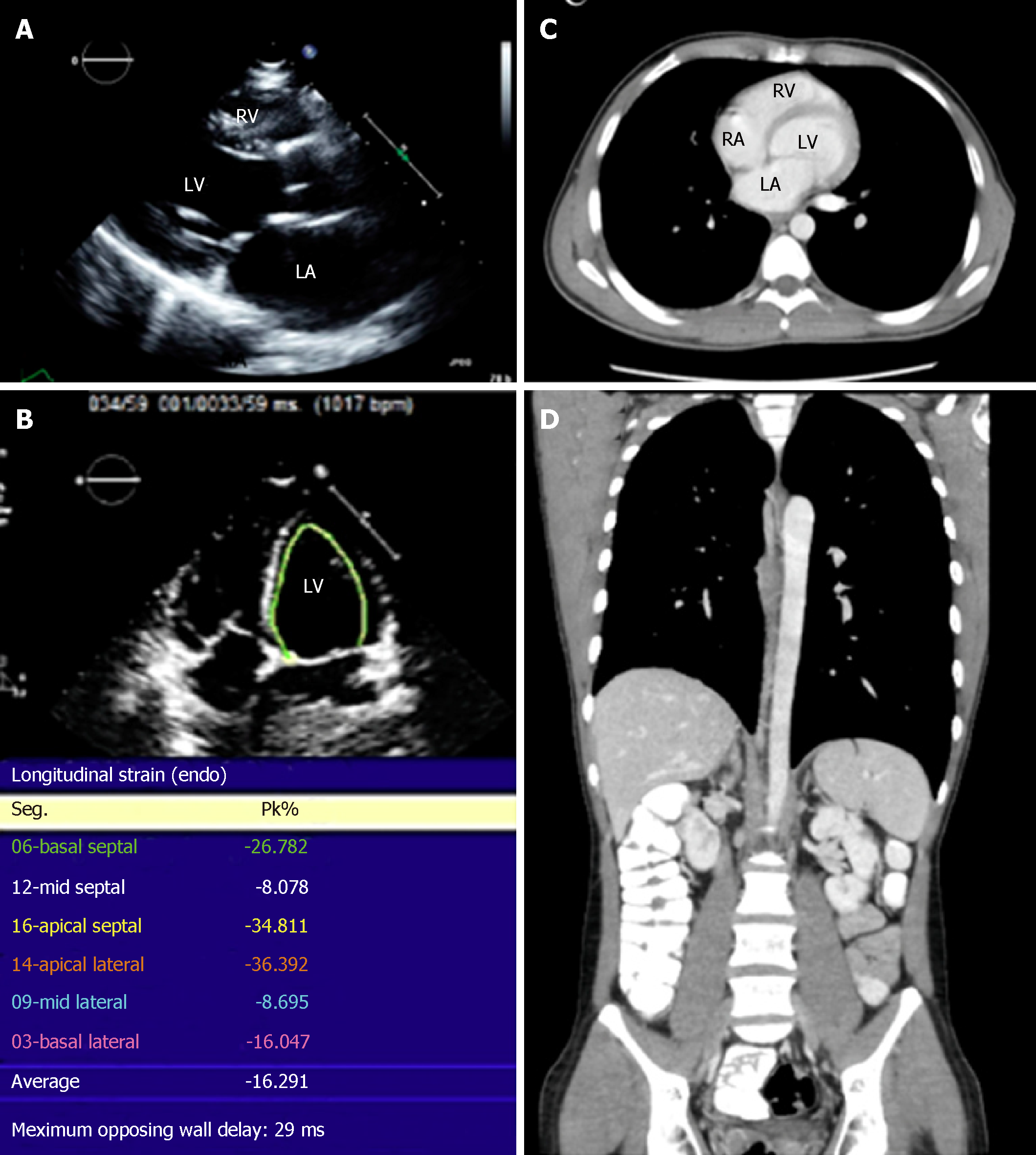
The chest X-ray suggested left atrial (LA) enlargement and a homogenous opacity in the right lung base was noted. A transthoracic echocardiogram (TTE) showed a large LA mass extending from the mediastinum causing functional mitral stenosis and regurgitation. The mass also extended through the interatrial septum (IAS) to involve the right atrium (RA) (Figure 1A-C). There was partial occlusion of the left upper pulmonary vein, occlusion of the remaining three pulmonary veins, and compression of the right pulmonary artery and SVC. The left ventricle (LV) size and ejection fraction were normal. Reduced strain of the lateral LV wall was detected (Figure 1D). A thoracoabdominal contrast-enhanced computed tomography (CT) scan confirmed the echocardiographic findings and showed mesenteric, renal, and pelvic masses (Figure 1E). A CT-guided biopsy of the pelvic mass was consistent with diffuse large B-cell lymphoma (DLBCL). Cytoplasmic clearing was noted in some areas with frequent apoptosis and extensive sclerosis in between groups and individual lymphocytes (Figure 1F). Immunophenotyping showed that these neoplastic lymphocytic cells were strongly positive for CD20, CD45, CD19, CD79A, CD10, and BCL-6 (Figure 1G). Flow cytometry showed the population of CD20+ monotypic (kappa-restricted) B-cells with CD10 co-expression). The B-cells were negative for CD5. DLBCL stage IV-A was diagnosed. CMRI was deferred as the patient could not tolerate the procedure. Lumbar puncture revealed clear cerebrospinal fluid (CSF), CSF white blood count: 1/uL (occasional lymphocytes and monocytes), CSF red blood count: 0/uL, CSF glucose and protein were normal (4.07 mmol/L and 0.4 g/L respectively).
In view of the extensive cardiac involvement and large LA mass, which raised concerns of cardiac rupture or tumor embolization, extra caution in management was taken when administering chemotherapy. We used a less intense, dose-dense protocol, followed by dose escalation[2-4]. The aim was for a slow but persistent reduction in tumor size to allow for the gradual replacement of the lymphoma cells by fibrous tissue and avoid complications. The first cycle of chemotherapy under the cyclophosphamide/doxorubicin/vincristine/prednisolone (CHOP) protocol was divided into three parts administered four to seven days apart. The first part consisted of 50% doxorubicin, 25% cyclophosphamide, and full-dose prednisolone. The second part consisted of 50% vincristine, 25% cyclophosphamide, and full-dose prednisolone. The third part consisted of 50% doxorubicin, 25% vincristine, 50% cyclophosphamide, and full-dose prednisolone. Echocardiography was performed every other day to evaluate the effect of chemotherapy on tumor size, assess the integrity of the LA and LV wall, and evaluate the areas of myocardial thinning. CMRI was performed one day before the third part of the first chemotherapy cycle. T2-weighted short-tau inversion recovery imaging (STIR) was the only sequence that could be obtained due to the patient’s ongoing orthopnea. The tumor was hyperintense on STIR. The LA wall could not be discerned clearly, indicating tumor infiltration. The lateral LV wall was edematous and displayed the same signal intensity as the tumor in keeping with tumor infiltration (Figure 1H and I).
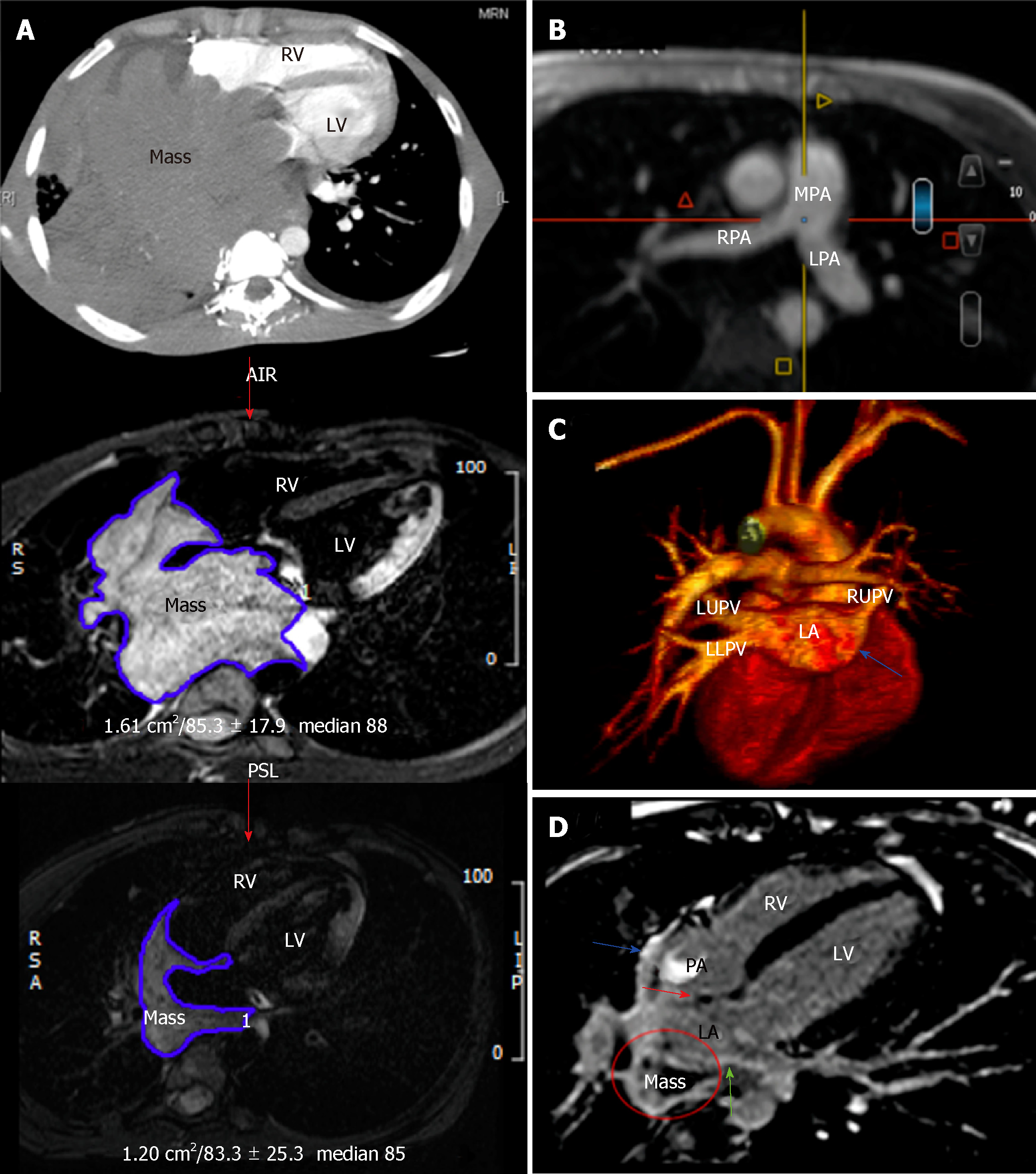
The second cycle of chemotherapy consisted of 50% rituximab-CHOP (R-CHOP) in a single dose and repeated 11 days later. Monitoring with every-other-day echocardiography was continued. When the portion of the mass invading the RA through the IAS vanished completely without creating an atrial septal defect, the chemotherapy dose in the third cycle was increased to 75% of R-CHOP. At this stage, the patient’s orthopnea resolved and the CMRI and CT were repeated, which showed an approximate 80% reduction in the tumor size with no mechanical complications (Figure 2A). The pulmonary arteries became all patent (Figure 2B). The pulmonary veins are now patent except the right lower pulmonary vein (Figure 2C). The CMRI revealed heterogeneous late gadolinium enhancement (LGE) of the already shrunken mass, suggesting tumor necrosis and fibrosis. The LGE signal of the IAS, LA, and RA walls suggested lymphoma resolution by fibrosis (Figure 2D). These findings were indicative of decreased myocardial rupture risk, and full-dose chemotherapy was initiated with etoposide augmentation in the R-CHOP protocol for the remaining cycles.
The patient completed 8 cycles of chemotherapy (CHOP divided into 3 doses, R-CHOP divided into 2 doses, 75% R-CHOP, and 5 full R-CHOP cycles with etoposide augmentation). Six doses of intrathecal chemotherapy were also administered. He underwent successful bone marrow transplant (BMT). The patient is currently in remission and has normal LV function (Figure 3) after more than a year post-BMT.
Disseminated DLBCL involving the myocardium and both atria.
The patient receives imaging-guided chemotherapy with initial dose reduction followed by dose escalation to standard doses. The chemotherapy dosing was guided by multimodality cardiac imaging. He also receives intrathecal chemotherapy, and underwent a successful bone marrow transplantation.
The patient is in remission and has normal left ventricular function one year following bone marrow transplantation. A recent PET scan showed complete metabolic remission.
Cardiac involvement by lymphoma is more commonly secondary to disseminated disease[5-7] as reported in 8.7%-25%[1,8-10]of patients in autopsy studies and accounting for 9% of the total metastasis to the heart[1]. Primary cardiac lymphoma (PCL) is rare, representing < 0.5% of extra-nodal lymphomas and < 2% of resected cardiac tumors[7]. Most cardiac lymphomas (primary or secondary) are of B-cell lineage, of which DLBCL, a subtype of non-Hodgkin lymphoma (NHL), is the most common[7,11]. While PCL commonly involves the right heart, the lymphomatous infiltrates in disseminated lymphoma are typically widespread, affecting the epicardium (61%) and myocardium diffusely. The LV and RA are involved in 55% and 54% of cases, respectively[12].
Involvement of the heart is usually a late manifestation of disseminated lymphoma. It occurs through direct extension from the mediastinal tumor, hematogenous spread, and retrograde lymphatic spread. Direct lymphoma extension predominantly leads to pericardial disease, obliteration of the pericardial space, and compression and invasion of the heart. This route is associated with the greatest cardiac destruction and is usually clinically apparent. The predominant epicardial-adventitial involvement is thought to result from retrograde lymphatic spread. The tumor follows the course of the epicardial coronary arteries, occasionally causing compression. There is no pericardial obliteration, and myocardial involvement maybe mild or non-existent. The diffuse interstitial-perivascular pattern has no predilection for either the epicardial or endocardial aspects of the heart and is attributed to hematogenous spread. The latter two patterns are usually asymptomatic[1].
Cardiac involvement in secondary lymphoma is frequently undetected before death. Cardiac disease may be asymptomatic or the symptoms may be nonspecific, particularly when the metastasis is not extensive[1]. When present, the symptoms include heart failure (34%), chest pain (12%), SVC syndrome, and arrhythmia. Embolic phenomenon[13] and hemodynamic instability may occur[14,15]. The ECG[1,16] and chest x-ray are usually not sensitive or specific for detecting cardiac involvement[16].
Individualized multimodality cardiac imaging is usually necessary for the evaluation of cardiac metastasis[17]. TTE is the initial screening modality of choice[17]. In one retrospective analysis of 29 patients with disseminated lymphoma, TTE had a 60% sensitivity for the detection of cardiac involvement. The pericardium was the most common site involved (41.1%) followed by the right-sided cardiac chambers (34.8%). The valves were the least involved (6.9%)[18]. Transesophageal echocardiography (TEE) had better sensitivity for the detection of lymphomatous involvement, compared to TTE (97% vs 75.9% respectively), in a study involving patients with PCL[16]. The use of strain imaging may improve the sensitivity of two-dimensional TTE for infiltrative lesions but the data are extremely limited. In addition to our reported case, in which the only clue to lateral LV wall involvement was impaired strain, in another reported case, the only hint of LV involvement on echocardiography was reduced two-dimensional and three-dimensional strains[19].
CT has a high spatial and temporal resolution and provides excellent anatomic assessment of both cardiac lymphoma and extracardiac disease. It is an excellent alternative imaging modality in patients with inadequate images from other non-invasive methods or in those with known contraindications to CMRI or require a fast image acquisition time[7]. On CT, cardiac lymphoma usually manifests as multiple iso- to hypo-attenuating masses that enhance heterogeneously. These masses infiltrate the myocardium with a predilection to affect the right atrioventricular groove and extend along the epicardium encasing the coronary arteries[20].
Use of 18F-Fluorodeoxyglucose positron-emission tomography (PET) alone for the diagnosis of cardiac lymphoma is challenging due to its low anatomic resolution and the physiologic accumulation of radiotracer within the myocytes[7]. The combination of PET/CT provides both anatomical and functional imaging at the same position[21] and is superior to each modality alone[20]. It offers superior anatomic resolution compared to PET alone and a higher accuracy in the overall staging of lymphoma in comparison with CT[20]. PET/CT is useful in differentiating DLBCL from other types of cardiac tumors[22]. In case reports, PET/CT permitted early diagnosis and treatment of cardiac lesions and monitoring of response to chemotherapy[23].
CMRI is the preferred imaging modality for the evaluation of the extent of myocardial and pericardial involvement by cardiac lymphoma[7,20]. CMRI has superior tissue characterization and therefore higher sensitivity for the detection of tumor infiltration[5] and a higher correlation with the pathological diagnosis of cardiac masses compared to TTE (77% vs 43%, P < 0.0001)[24]. Cardiac lymphoma typically appears hypointense on T1-weighted images and hyperintense on T2-weighted images (STIR). An isointense signal on either sequence is also possible. Contrast produces minimal or no enhancement[4]. Combined CMRI/18F-FDG-PET provides superior morphologic tumor characterization with simultaneous visualization of tumor metabolism. However, this technique remains a subject of research and is available in limited sites worldwide[25].
Data on the survival of patients with metastatic cardiac lymphoma are scarce with most coming from the from the pre-rituximab era. Available evidence indicates that once the diagnosis of cardiac lymphoma, whether primary or secondary, is made, the prognosis becomes extremely unfavorable[13,26,27]. However, improvements in survival have been observed in more recently reported cases[13].
In a retrospective analysis of 94 cases of both primary and secondary cardiac NHL, median survival was 3 months with worse outcomes in patients with heart failure, T-cell NHL, and aggressive B-cell lymphoma[13]. In another report involving 197 cases of PCL, more than half reported after 1995, median overall survival was one month in patients with LV involvement compared to 22 mo in patients free from LV disease (P = 0.002). Other negative prognostic indicators included immune-compromised status, the presence of extra-cardiac disease, and the absence of arrhythmia. The lack of arrhythmia is thought to delay diagnosis, thereby increasing mortality. The overall response rate to therapy was 84%, with long-term overall survival exceeding 40%[28].
The management strategies for cardiac lymphoma have included chemotherapy, occasionally in combination with radiotherapy, surgery, and autologous stem cell transplantation[7]. Chemotherapy is the mainstay of treatment for cardiac lymphoma, resulting in the prolongation of median survival (18 mo vs 1 mo, P = 0.0003) and durable remissions in patients with B-cell NHL[13]. However, although DLBCL is often sensitive to R-CHOP[29], the early post-chemotherapy period is critical due to the potential occurrence of fatal complications, including ventricular fibrillation, refractory heat failure, massive pulmonary embolism[30], and cardiac rupture resulting from rapid tumor destruction[26]. It is recommended that the standard regimen used for B-cell lymphomas be tailored appropriately for each case, particularly in patients with extensive myocardial infiltration[4,26,31,32]. There is no evidence that surgery improves the prognosis; tumor debulking, however, may be considered as a palliative or lifesaving measure in cases with significant or life-threatening obstructive symptoms[9,15]. In hemodynamically unstable patients, chemotherapy has been administered successfully under extracorporeal membrane oxygenation support[33] or after emergent tumor resection[15,34].
While the incidence of chemotherapy-associated myocardial rupture in cardiac lymphoma is unknown, it is considered a significant threat since it may occur spontaneously in patients with extensive transmural involvement[35]. Attempts to minimize this risk have included chemotherapy dose reduction, using various protocols, which was found to be safe and successful in case reports[4,31,32] (Table 1); although cardiac rupture remains a concern even with reduced chemotherapy doses. In one case, dose reduction was combined with prophylactic application of a bovine pericardial patch[29] to avoid catastrophic rupture (Table 1).
| Ref. | Cardiac involvement | Pathology | Chemotherapy dose | Additional therapy | Cardiac imaging | Follow up |
| Beckwith et al[29], 2000 | PCL; Right atrial mass. | DLBCL | Chemotherapy initiated 2 wk after surgery with 50% CHOP chemotherapy with continuous cardiac monitoring in the intensive care unit; After 2 cycles of CHOP, TEE showed tumor resolution. Two additional cycles of CHOP were given (patient received 90% of planned doses due to cytopenias and gastrointestinal toxicity) | Prophylactic placement of bovine pericardial patch over the involved atrial free wall; cranial irradiation and intrathecal methotrexate | TEE | After 2 mo, intracerebral tumor was detected, treated by cranial irradiation then with intrathecal methotrexate for lymphomatous meningitis. The patient died from the CNS disease |
| Dawson et al[3], 2006 | PCL; Extensive intramyocardial mass involving the right atrium, right ventricular free wall and encircling the pulmonary artery trunk | DLBCL CD20- and CD79a-positive | Dose-dense schedule of R-CHOP every 14 d. 50% reduction of cyclophosphamide and Adriamycin doses in the initial course; Chemotherapy completed with a median of 16 d between cycles | Growth factor support with pegylated granulocyte colony-stimulating factor was administered 24 h following the completion of chemotherapy | TEE, CT, FDG-PET, MRI. MRI tagged short axis slices used to assess the potential for cardiac rupture with chemotherapy | Survived and in remission at 11 mo |
| Shah et al[31], 2014 | PCL; Large right atrial mass resulting in severe tricuspid stenosis and invading the interatrial septum and encasing the aortic root | DLBCL, CD19 and CD20 positive, CD5 and CD10 negative | Rituximab 375 mg/m2 and prednisone 40 mg daily for 10 d; Low dose R-CHOP (cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, vincristine 1 mg, prednisone 60 mg and rituximab 375 mg/m2) 2 cycles, administered every 3 wk; Full dose R-CHOP, every 3 wk for 4 cycles | TTE, TEE, PET, follow up TTE at 3 wk demonstrated no change in tumor size however there was no evidence of ventricular septal perforation | Well and in remission at 12 mo | |
| Cereda et al[32], 2017 (report of 3 patients) | PCL; Lymphoma localized to the right chambers in all patients | DLBCL in all PCL | Pretreatment with steroids and vincristine; 6 cycles of R-COMP (Myocet not pegylated liposomal doxorubicin, rituximab, vincristine, and prednisone) | TTE, FDG-PET/CT scan TTE with tissue Doppler-derived strain and 2D-strain imaging of the RV showed progressive improvement of RV function Following complete resolution by TTE, a second FDG-PET/CT scan confirmed remission | 2 patients were in complete remission at 25 mo. 1patient had extra-cardiac relapse and underwent a salvage therapy and autologous transplantation; 2 patients developed late cardiac toxicity during post-remission surveillance | |
| Almehisen et al. 2019 (present case) | SCL; Large mass invading the left atrium, interatrial septum, right atrium Infiltration of lateral LV wall. Partial occlusion of the left upper pulmonary vein and occlusion of the remaining pulmonary veins. Compression of right pulmonary artery and SVC | DLBCL. Positive for CD20, CD45, CD19, CD79A, CD10, and BCL-6, and negative for CD5 | The first cycle of CHOP was divided into 3 parts administered 4-7 d apart. The first part: 50% doxorubicin, 25% cyclophosphamide, and full-dose prednisolone. The second part: 50% vincristine, 25% cyclophosphamide, and full-dose prednisolone. The third part: 50% doxorubicin, 25% vincristine, 50% cyclophosphamide, and full-dose prednisolone; Chemotherapy consisted of etoposide augmentation in the R-CHOP protocol for the remaining cycles | Six doses of intrathecal chemotherapy; Bone marrow transplant | TTE, Strain, CT, and MRI; MRI demonstrated tumor infiltration of the atrial wall, the infiltrative left ventricular lesion and lymphoma resolution by fibrosis, after which chemotherapy was escalated to full dose | Complete remission with normal LV function at 3 yr |
Cardiac imaging has an integral role in guiding the diagnosis, management and monitoring of this condition[4], particularly CMRI due to its high tissue contrast. CMRI has been used once before to guide chemotherapy. Dawson et al[4] used Cardiac MRI and [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) to assess the potential risk of cardiac rupture with chemotherapy. In their report, the muscular integrity of the atrium and ventricle was assessed using cardiac MRI tagged short axis slices which demonstrated an intact and contractile myocardium[4].In the case presented herein, we used STIR and LGE sequences of CMRI, which we found very useful in depicting the direct infiltration of the myocardium by the mediastinal lymphoma and healing with fibrosis (positive LGE and hyperintensity on STIR indicating lymphoma infiltration and positive LGE without hyperintensity on STIR suggesting fibrosis). Since both lymphomatous infiltrates and myocardial fibrosis are associated with impaired cardiac systolic dysfunction, LGE CMR sequence in combination with other CMR techniques including myocardial longitudinal relaxation time (T1) mapping and STIR are better in that regard[36].
In cases of DLBCL, perforation can affect other hollow organs including the gastrointestinal tract and trachea[37-39]. Although chemotherapy dose reduction/delay followed by the standard full-dose regimen has been used in an attempt to minimize perforation in non-cardiac cases[37], concerns arise as strategy may also decrease treatment intensity leading to reduced therapeutic effects and suboptimal outcomes[40]. The scientific evidence on such management in cases deemed “at risk of perforation” comes at best from retrospective case series[37], and is particularly limited in the case of cardiac involvement (Table 1). The management is therefore largely individualized with consideration the devastating risk of organ perforation (with standard dose of chemotherapy) and failure to achieve remission (with chemo-dose reduction). Knowing the anatomical involvement of the organ with the aid modern imaging techniques[17] and the experience of the attending physician[37] are crucial in the decision making.
This case is unique and of clinical importance. The extensive cardiac infiltration by lymphoma threatened both myocardial rupture and tumor embolization, particularly as the large tumor bulk involved the left heart chambers, and mandated extra caution in management. The use of multimodality cardiac imaging to gauge chemotherapy dosing was a cornerstone in this patient’s management. Every-other-day echocardiography demonstrated slow and safe tumor regression with a less intense dose dense protocol. This dose protocol led to a balance between tumor cell regression and replacement by fibrosis, thereby maintaining myocardial wall integrity. Only when tumor replacement by fibrosis was observed on CMRI was chemotherapy increased to standard doses. Chemotherapy based on cardiac imaging is a more reliable and reproducible indicator of safety and response to therapy.
In conclusion, cardiac lymphoma is associated with high mortality. Although chemotherapy improves survival, the prognosis remains poor, and death from myocardial rupture is a serious treatment complication. Reducing the chemotherapy dose with dose escalation to therapeutic levels may be effective. Decisions on management should be individualized.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gorodetskiy VR, Petix NR S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer. 1982;49:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Lyman GH, Barron RL, Natoli JL, Miller RM. Systematic review of efficacy of dose-dense versus non-dose-dense chemotherapy in breast cancer, non-Hodgkin lymphoma, and non-small cell lung cancer. Crit Rev Oncol Hematol. 2012;81:296-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Citron ML. Dose-Dense Chemotherapy: Principles, Clinical Results and Future Perspectives. Breast Care (Basel). 2008;3:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Dawson MA, Mariani J, Taylor A, Koulouris G, Avery S. The successful treatment of primary cardiac lymphoma with a dose-dense schedule of rituximab plus CHOP. Ann Oncol. 2006;17:176-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lichtenberger JP, Dulberger AR, Gonzales PE, Bueno J, Carter BW. MR Imaging of Cardiac Masses. Top Magn Reson Imaging. 2018;27:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hafeez I, Alai MS, Iqbal K, Aslam K, Lone A, Bhat IA, Samer M. Lymphoma presenting as severe left ventricular systolic dysfunction: a case report. Oman Med J. 2014;29:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Jeudy J, Burke AP, Frazier AA. Cardiac Lymphoma. Radiol Clin North Am. 2016;54:689-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Chinen K, Izumo T. Cardiac involvement by malignant lymphoma: a clinicopathologic study of 25 autopsy cases based on the WHO classification. Ann Hematol. 2005;84:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Gowda RM, Khan IA. Clinical perspectives of primary cardiac lymphoma. Angiology. 2003;54:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Roberts WC, Glancy DL, DeVita VT. Heart in malignant lymphoma (Hodgkin's disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides). A study of 196 autopsy cases. Am J Cardiol. 1968;22:85-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 206] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073-103; quiz 1110-1, 1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 354] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Travis WD BE, Müller-Hermelink HK, Harris C, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press 2004; 282-284. |
| 13. | Gordon MJ, Danilova O, Spurgeon S, Danilov AV. Cardiac non-Hodgkin's lymphoma: clinical characteristics and trends in survival. Eur J Haematol. 2016;97:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Hsueh SC, Chung MT, Fang R, Hsiung MC, Young MS, Lu HF. Primary cardiac lymphoma. J Chin Med Assoc. 2006;69:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Ömeroğlu SN, Balkanay OO, Göksedef D, Öz B, İpek G. The Surgical Treatment of Primary Cardiac B-Cell Lymphoma of Clinically Unstable Patient. Ann Thorac Surg. 2018;105:e215-e217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Faganello G, Belham M, Thaman R, Blundell J, Eller T, Wilde P. A case of primary cardiac lymphoma: analysis of the role of echocardiography in early diagnosis. Echocardiography. 2007;24:889-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Palaskas N, Thompson K, Gladish G, Agha AM, Hassan S, Iliescu C, Kim P, Durand JB, Lopez-Mattei JC. Evaluation and Management of Cardiac Tumors. Curr Treat Options Cardiovasc Med. 2018;20:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathological characteristics of cardiac metastasis in patients with lymphoma. Oncol Rep. 2002;9:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Földeák D, Kalapos A, Domsik P, Sinkó M, Szeleczki N, Bagdi E, Krenács L, Forster T, Borbényi Z, Nemes A. Left ventricular rigid body rotation in a diffuse large B-cell lymphoma patient with cardiac involvement: A case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Rev Port Cardiol. 2017;36:145.e1-145.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 20. | Carter BW, Wu CC, Khorashadi L, Godoy MC, de Groot PM, Abbott GF, Lichtenberger JP. Multimodality imaging of cardiothoracic lymphoma. Eur J Radiol. 2014;83:1470-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Endo K, Oriuchi N, Higuchi T, Iida Y, Hanaoka H, Miyakubo M, Ishikita T, Koyama K. PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int J Clin Oncol. 2006;11:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Kikuchi Y, Oyama-Manabe N, Manabe O, Naya M, Ito YM, Hatanaka KC, Tsutsui H, Terae S, Tamaki N, Shirato H. Imaging characteristics of cardiac dominant diffuse large B-cell lymphoma demonstrated with MDCT and PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Agrawal K, Mittal BR, Manohar K, Kashyap R, Bhattacharya A, Varma S. FDG PET/CT in Detection of Metastatic Involvement of Heart and Treatment Monitoring in Non-Hodgkin's Lymphoma. World J Nucl Med. 2012;11:33-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Patel R, Lim RP, Saric M, Nayar A, Babb J, Ettel M, Axel L, Srichai MB. Diagnostic Performance of Cardiac Magnetic Resonance Imaging and Echocardiography in Evaluation of Cardiac and Paracardiac Masses. Am J Cardiol. 2016;117:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Krumm P, Mangold S, Gatidis S, Nikolaou K, Nensa F, Bamberg F, la Fougère C. Clinical use of cardiac PET/MRI: current state-of-the-art and potential future applications. Jpn J Radiol. 2018;36:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Ban-Hoefen M, Bernstein SH, Bisognano JD, Pryor JG, Friedberg JW. Symptomatic intracardiac diffuse large B-cell lymphoma. Am J Hematol. 2009;84:683-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Jeudy J, Kirsch J, Tavora F, Burke AP, Franks TJ, Mohammed TL, Frazier AA, Galvin JR. From the radiologic pathology archives: cardiac lymphoma: radiologic-pathologic correlation. Radiographics. 2012;32:1369-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Beckwith C, Butera J, Sadaniantz A, King TC, Fingleton J, Rosmarin AG. Diagnosis in oncology. Case 1: primary transmural cardiac lymphoma. J Clin Oncol. 2000;18:1996-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Rolla G, Bertero MT, Pastena G, Tartaglia N, Corradi F, Casabona R, Motta M, Caligaris-Cappio F. Primary lymphoma of the heart. A case report and review of the literature. Leuk Res. 2002;26:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Shah K, Shemisa K. A "low and slow" approach to successful medical treatment of primary cardiac lymphoma. Cardiovasc Diagn Ther. 2014;4:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Cereda AF, Moreo AM, Sormani P, De Chiara B, Casadei F, Zancanella M, Rusconi C, Cairoli R, Giannattasio C. Impact of serial echocardiography in the management of primary cardiac lymphoma. J Saudi Heart Assoc. 2018;30:160-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Allain G, Hajj-Chahine J, Lacroix C, Jayle C. Primary cardiac lymphoma complicated by cardiogenic shock: successful treatment with chemotherapy delivered under extracorporeal membrane oxygenation support. Eur J Cardiothorac Surg. 2015;48:968-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Johri A, Baetz T, Isotalo PA, Nolan RL, Sanfilippo AJ, Ropchan G. Primary cardiac diffuse large B cell lymphoma presenting with superior vena cava syndrome. Can J Cardiol. 2009;25:e210-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Molajo AO, McWilliam L, Ward C, Rahman A. Cardiac lymphoma: an unusual case of myocardial perforation--clinical, echocardiographic, haemodynamic and pathological features. Eur Heart J. 1987;8:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 813] [Cited by in RCA: 749] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 37. | Matsumoto T, Shibata Y, Nakamura N, Nakamura H, Ninomiya S, Kitagawa J, Nannya Y, Shimizu M, Hara T, Tsurumi H. The incidence and the risk factors of bowel perforation due to chemotherapy in gastrointestinal diffuse large B cell lymphoma [abstract]. In:. Blood. 2015;126:1515. |
| 38. | Vaidya R, Habermann TM, Donohue JH, Ristow KM, Maurer MJ, Macon WR, Colgan JP, Inwards DJ, Ansell SM, Porrata LF, Micallef IN, Johnston PB, Markovic SN, Thompson CA, Nowakowski GS, Witzig TE. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Kongpolprom N. Tracheal Perforation following Chemotherapy of Diffuse Large B-Cell Lymphoma. Case Rep Acute Med. 2018;1:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Foote M. The Importance of Planned Dose of Chemotherapy on Time: Do We Need to Change Our Clinical Practice? Oncologist. 1998;3:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |