Published online Jan 26, 2019. doi: 10.12998/wjcc.v7.i2.171
Peer-review started: October 25, 2018
First decision: November 28, 2018
Revised: December 27, 2018
Accepted: January 3, 2019
Article in press: January 3, 2019
Published online: January 26, 2019
Processing time: 93 Days and 22.1 Hours
Exosomes are microvesicles, measuring 30-100 nm in diameter. They are widely distributed in body fluids, including blood, bile, urine and saliva. Cancer-derived exosomes carry a wide variety of DNA, RNA, proteins and lipids, and may serve as novel biomarkers in cancer.
To summarize the performance of exosomal biomarkers in cancer diagnosis and prognosis.
Relevant publications in the literature were identified by search of the “PubMed” database up to September 11, 2018. The quality of the included studies was assessed by QUADAS-2 and REMARK. For assessment of diagnostic biomarkers, 47 biomarkers and 2240 patients from 30 studies were included.
Our results suggested that these exosomal biomarkers had excellent diagnostic ability in various types of cancer, with good sensitivity and specificity. For assessment of prognostic markers, 50 biomarkers and 4797 patients from 42 studies were included. We observed that exosomal biomarkers had prognostic values in overall survival, disease-free survival and recurrence-free survival.
Exosomes can function as potential biomarkers in cancer diagnosis and prognosis.
Core tip: Cancer-derived exosomes carry a wide variety of DNA, RNA, proteins and lipids, which may serve as novel biomarkers in cancer. The current systematic review and meta-analysis summarized the performance of exosomal biomarkers in cancer diagnosis and prognosis. We analyzed 47 diagnostic markers and 50 prognostic markers from 56 studies with various type of cancer. We found that exosomal biomarkers had both diagnostic and prognostic power in many cancers.
- Citation: Wong CH, Chen YC. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases 2019; 7(2): 171-190
- URL: https://www.wjgnet.com/2307-8960/full/v7/i2/171.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i2.171
Cancer is the uncontrolled growth of cells and eventually leads to death. Cancer is the second cause of death, contributing to more than 8.8 million deaths every year[1,2]. Among various types of cancer, lung cancer, gastrointestinal cancers (GI cancer), including liver cancer, pancreatic cancer and colorectal cancer, and breast cancer are the most common cause of cancer-related death[2-4]. Although chemotherapy, targeted therapy, surgical recession and radiotherapy can effectively prolong survival of patients, the survival rate of cancer is still very low, especially in GI cancer, being less than 20%[2]. One of the major reasons is the late diagnosis of cancer, in which patients are already with advanced and metastatic tumors. As a result, no therapies can effectively kill the cancer cells. The situation is even worse in pancreatic cancers at distant stage, with 5-year survival rate of only 3%[2].
Since more than half of the patients present with locally advanced or metastatic stage, early diagnosis and early treatment are fundamentally important for better prognosis. Therefore, many tumor makers have been developed, aiming at accurately detecting various types of cancer and monitoring the disease progression. Blood test of the tumor antigens carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 125 (known as CEA, CA19-9 and CA125 respectively) are commonly used for detection of many cancers, such as GI cancers, ovarian cancer and breast cancer[5-8]. However, the sensitivity of these cancer biomarkers is unsatisfactory[9-12]. Also, the fecal occult blood test of colorectal cancer and the invasion endoscopic detection of gastric and colon cancer represent a great inconvenience to the patients. Therefore, highly sensitive and non-invasive diagnostic markers are urgently needed for early detection of cancer.
Exosomes are microvesicles of 30-100 nm diameter, which are secreted by both normal cells and cancer cells. They are distributed in many body fluids such as blood, saliva and urine, and carry various types of biomolecules, including RNA, proteins and lipids, for inter-cellular communication[13-15]. During cancer development, cancer cells secrete more exosomes, with significant changes in composition[16-18]. These facilitate communication within the tumor environment, acquisition of drug resistance, and metastasis to distant organs[19-21]. Although many potential non-invasive biomarkers have been developed using liquid biopsy, such as serum and urine, studies have found that these biomarkers are commonly located in the exosomes[22,23]. Enriching these exosomal biomarkers could achieve a higher diagnostic and prognostic efficiency[24-26]. Thus, exosomal biomarkers can be novel targets in cancer diagnosis and prognosis.
The objective of this systemic review and meta-analysis is to evaluate the diagnostic and prognostic potential of exosomes in patients with various types of cancer, based on current available data. This information will help in the development of novel non-invasive biomarkers for sensitive and specific diagnosis and prognosis of cancer.
Electronic literature search was performed using the PubMed database, without any language restriction. Articles related to exosomes in cancer from 2010 to September 11, 2018 were identified using the following key words: “exosome” and “cancer” and ““diagnosis” or “prognosis””.
Articles were reviewed by their titles, key words, abstracts and full text to identify eligible studies. Eligible studies were included based on the following inclusion criteria: (1) The original article was related to exosomal diagnostic or prognostic markers in cancer; (2) At least 10 patients and 10 matched controls were enrolled in the study; (3) For diagnostic markers, enough information, such as specificity and sensitivity, was provided to construct 2 × 2 table [true positive (TP), true negative (TN), false positive (FP), false negative (FN)]; and (4) For prognostic markers, enough information was provided to estimate the hazard ratios (HRs) and confidence intervals (CIs). The exclusion criteria were as follows: (1) Duplicate articles; (2) Review articles, abstracts, comments, letters, case-report; (3) Fundamental research or animal study; (4) Diagnostic or prognostic marker that was not specific to exosome; (5) Sample size was less than 10; (6) Performance of the biomarker was not statistically significant; or (7) Incomplete information to estimate diagnostic or prognostic accuracy.
Two reviewers (Chi-Hin Wong and Yang-Chao Chen) independently reviewed and extracted the data from the eligible studies according to the listed criteria. Any disagreement was resolved by consensus among the authors. The following data from included studies were extracted: first author’s name, year of publication, sample size, cancer type, country of origin, source of exosome, isolation method of exosome, and detection method of biomarkers. For diagnostic studies, data for the cut-off value of tested targets, sensitivity, specificity, and area under the receiver operating characteristics curve (ROC) were also extracted. For prognostic studies, data for survival analysis, cut-off value, multivariable HR and its 95%CI were extracted. If odds ratio (OR) was reported, OR was converted to relative risk using the formula introduced by Zhang and Yu[27]. If either OR or HR was not reported, the method introduced by Tierney et al[28] was used to estimate the HR and its 95%CI from a Kaplan-Meier plot.
For diagnostic studies, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to assess the quality of studies for the meta-analysis[29]. Briefly, 14 questions covering the patient selection, patient flow, index test and reference standard test were applied to each study and an answer of “Yes”, “No” or “Unclear” was given to each study. Only answers of “Yes” were given a score.
For prognostic study, the quality of studies was assessed according to reporting recommendations for tumor marker prognostic studies (REMARK)[30]. Briefly, a checklist of 20 items was generated, covering patients’ characteristics, samples’ source and storage, assay methods, statistical analysis, and data interpretation. A score was given when the study fulfilled the requirement of each item.
The statistical analysis of the diagnostic performance of biomarkers was performed using Meta-DiSc 1.4[31]. The 2 × 2 table of each study was used to assess the pooled sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR). Also, the summary receiver operating characteristic (SROC) curve was plotted; the area under the curve (AUC) was calculated and Q* index was estimated to assess the overall performance in cancer diagnosis. An AUC of 0.5 suggested no diagnostic ability; 0.7-0.8 suggested acceptable diagnostic performance; 0.8-0.9 was considered excellent, and 0.9-1.0 suggested outstanding performance[32]. Q* was defined at a point in which sensitivity and specificity are equal. For statistical analysis of the prognostic performance of biomarkers, forest plots were constructed using the HR and its 95%CI of each biomarker to assess the overall prognostic performance of biomarkers on overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS). Graphpad Prism 6 was used in constructing the forest plots. To elevate the heterogeneity between studies, Cochran-Q test and inconsistency index (I2) statistics were calculated[33,34]. P-value of < 0.05 for Cochran-Q test or I2 >50% suggested the presence of heterogeneity.
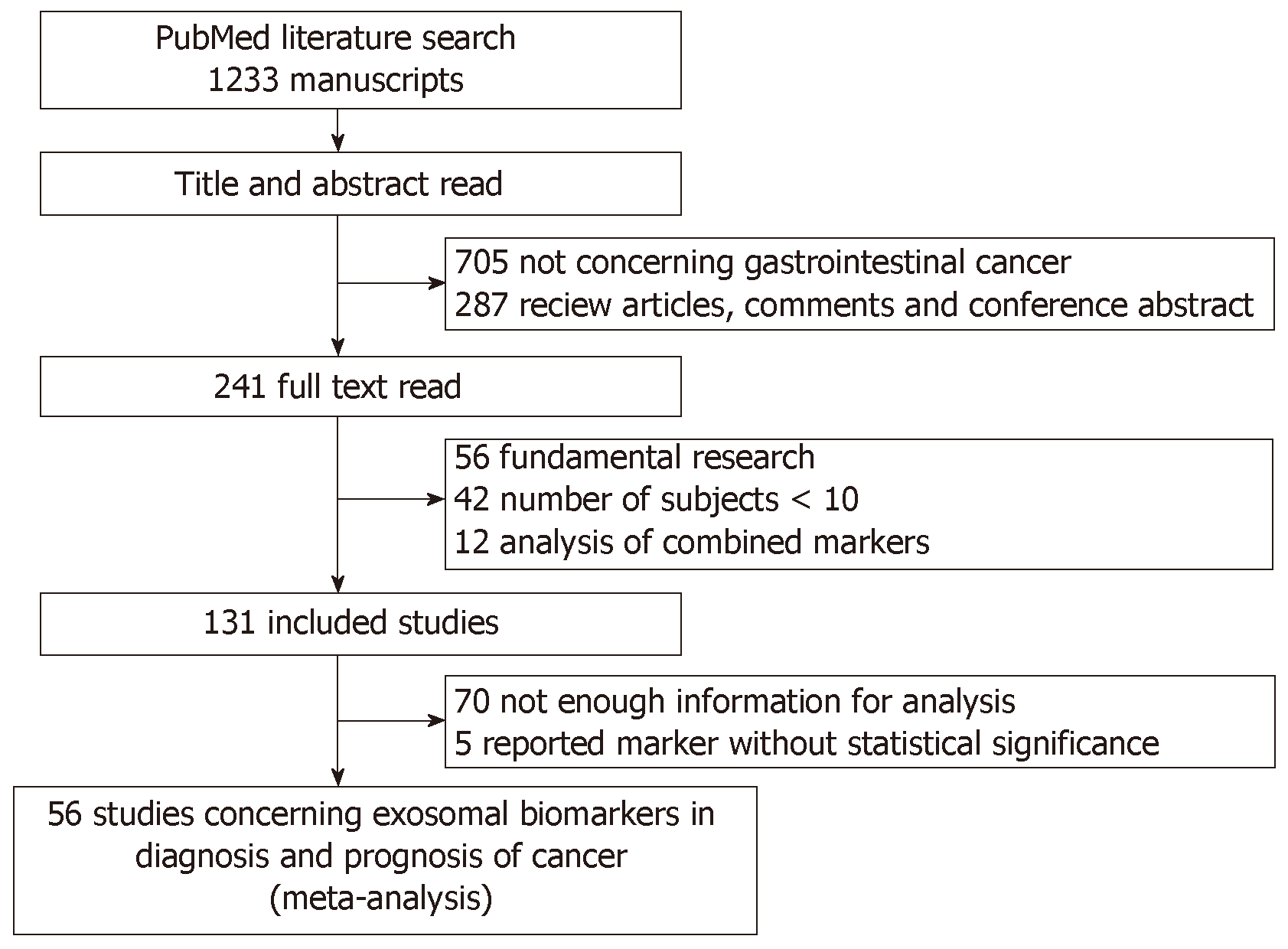
Initially, 1233 articles were identified based on the search strategies. Based on title and abstract screening, 705 were not related to exosome biomarkers in cancer diagnosis or prognosis, and 287 were review articles. Upon further full-text review, 56 studies were basic studies, 42 studies with sample size less than 10 in either group (test group or control group), 12 studies analyzed the performance of combined markers, 70 studies did not provide enough information for analysis, and 5 studies were without statistical significance. Finally, 56 eligible studies were included for systematic review (Figure 1). Of these, 22 candidate studies were related to diagnosis, 34 candidate studies were related to prognosis, and 8 studies were related to both diagnosis and prognosis.
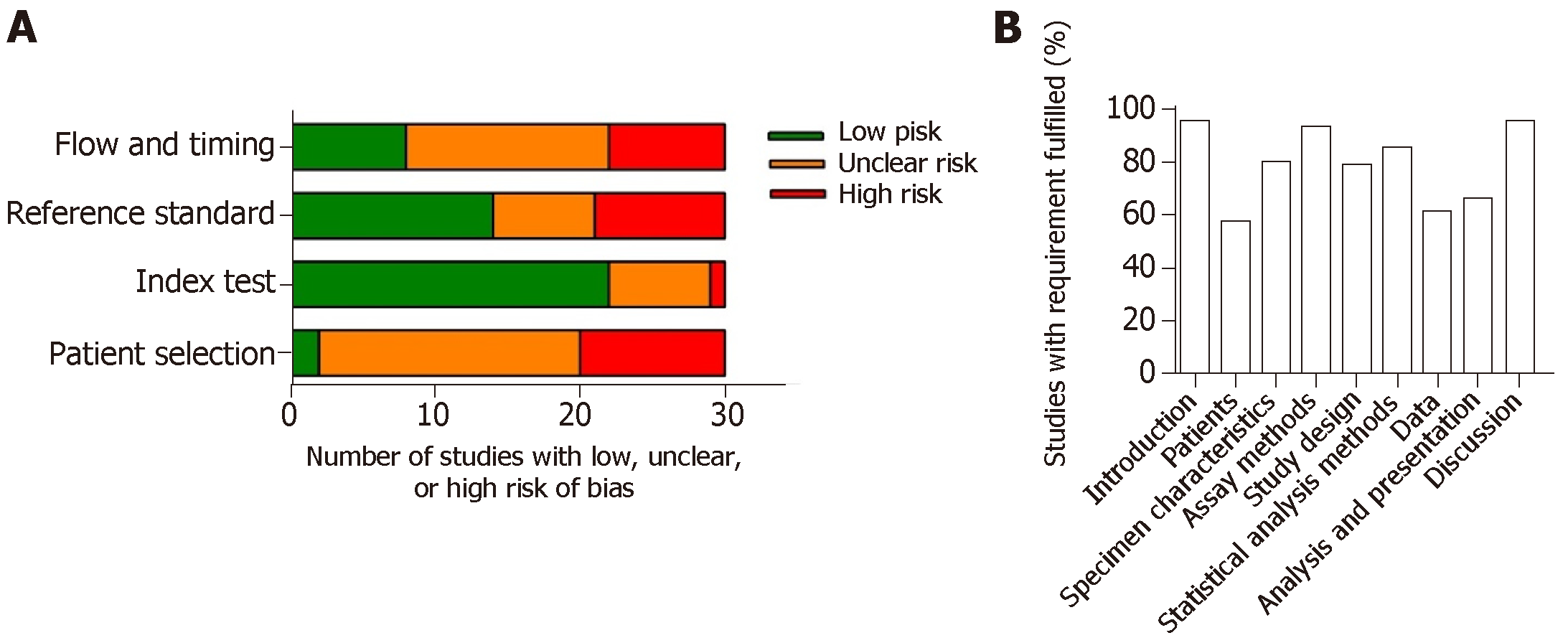
For diagnostic studies, the QUADAS-2 system was used to assess the study quality (Figure 2A). Most of the studies on diagnosis were with moderate-to-high quality, revealed by low risk of publication bias. However, there may be risk of bias in “patient selection” and “flow and timing”. This may due to control-based design in most of the studies. Also, time between the index test and the reference test is poorly reported. Importantly, many studies did not provide enough information on how the patients were selected and classified. Patients excluded from the 2 × 2 table were often observed in some studies.
The REMARK system was used to assess the quality of prognostic studies (Figure 2B). Most of the studies ( > 90%) clearly stated the objective, biomarkers examined, source of exosomes, and methodology of isolation and detection. Also, most of the studies clearly defined the clinical endpoints and the period of the follow-up time. However, details in patient’s characteristics during the follow-up period, such as the use of post-operative adjuvant therapy which significantly affects the OS and DFS, were lacking in most of the studies. Importantly, some studies did not clearly report the clinicpathological characteristics of the patients enrolled. Also, some studies did not show the relationship of the tested biomarkers to prognostic variables, including tumor stages and tumor differentiation. Twelve prognostic marker studies did not perform univariable or multivariable analysis. Twenty-eight of the enrolled studies reported multivariable analysis in prognostic markers, but only five studies clearly stated the adjustment factors.
Diagnostic markers from 30 studies were included in the meta-analysis (Table 1). More than a half of these studies were related to GI cancers (4 studies were about colon cancer; 5 studies were related to liver cancer; 4 studies were about pancreatic or pancreatobiliary tract cancer; and 4 studies were related to gastric cancer). A total of 2240 patients were included in the meta-analysis, with 12 studies having enrolled < 50 patients, 16 studies having enrolled 50-100 patients, and 6 studies having enrolled > 100 patients. There were 47 diagnostic biomarkers analyzed in the meta-analysis. There were 42.6% of the biomarkers as miRNAs, followed by lncRNAs (36.2%) and proteins (19.1%). Notably, 6 studies analyzed the diagnostic performance of exosomal miR-21 in various types of cancer. Also, 61.3%, 16.1%, 12.9%, 3.2% and 3.2% of the biomarkers were detected in serum, plasma, urine, saliva and bile respectively.
| Ref. | Country | Cancer type | Stage | Control | Number of Control | Number of patients | Sample | Isolation method of exosome | Marker | Detection method | Cut-off | TP | TN | FP | FN |
| Sun et al[35] | China | Colorectal | All | Healthy | 32 | 92 | Plasma | UC | CPNE3 | ELISA | 0.143 pg/μg exosome | 62 | 27 | 5 | 30 |
| Ogata-Kawata et al[36] | Japan | Colorectal | All | Healthy | 11 | 88 | Serum | UC | miR-1246 | qRT-PCR | 1.45 | 84 | 10 | 4 | 1 |
| miR-23a | 0.3100 | 81 | 11 | 7 | 0 | ||||||||||
| miR-21 | 1.08 | 54 | 10 | 34 | 1 | ||||||||||
| miR-150 | 0.08 | 49 | 11 | 39 | 0 | ||||||||||
| let-7a | 0.9 | 44 | 10 | 44 | 1 | ||||||||||
| miR-223 | 1.72 | 41 | 10 | 47 | 1 | ||||||||||
| miR-1224-5p | 0.5 | 28 | 11 | 60 | 0 | ||||||||||
| miR-1229 | 0.06 | 20 | 11 | 68 | 0 | ||||||||||
| Liu et al[37] | China | Colorectal | All | Healthy and benign | 320 | 148 | Serum | ExoQuick | CRNDE-h | qRT-PCR | 0.02 | 104 | 302 | 18 | 44 |
| Uratani et al[38] | Japan | Colorectal | NR | Healthy | 47 | 26 | Serum | ExoQuick | miR-21 | qRT-PCR | Youden index | 18 | 38 | 9 | 8 |
| Lin et al[39] | China | Gastric | All | Healthy | 60 | 51 | Plasma | UC | lncUEGC1 | qRT-PCR | NR | 45 | 50 | 10 | 6 |
| lncUEGC2 | NR | 46 | 34 | 26 | 17 | ||||||||||
| Zhao et al[40] | China | Gastric | All | Healthy | 120 | 126 | Serum | NR | HOTTIP | qRT-PCR | 1.72 | 88 | 102 | 18 | 38 |
| Pang et al[41] | China | Gastric | All | Healthy | 37 | 40 | Serum | ExoQuick | ZFAS1 | qRT-PCR | NR | 32 | 28 | 9 | 8 |
| Yang et al[42] | China | Gastric | All | Healthy | 80 | 80 | Serum | ExoQuick | miR-423-5p | qRT-PCR | NR | 65 | 46 | 34 | 15 |
| Goto et al[43] | Japan | Pancreatic | All | Healthy and advanced pancreatic cancer | 22 | 23 | Serum | ExoQuick | miR-191 | qRT-PCR | Distance = (1-sensitivity)2 + (1-specificity)2 in ROC curve | 18 | 17 | 5 | 5 |
| miR-21 | 20 | 18 | 4 | 3 | |||||||||||
| miR-451a | 16 | 18 | 4 | 7 | |||||||||||
| Melo et al[44] | Germany | Pancreatic | All | Healthy | 100 | 190 | Serum | UC | GPC1 | Flow cytometry | Youden index | 190 | 100 | 0 | 0 |
| Que et al[45] | China | Pancreatic | All | Non-PDAC | 27 | 22 | Serum | UC | miR-17-5p | qRT-PCR | 6.826 | 20 | 20 | 7 | 2 |
| miR-21 | 7.693 | 18 | 26 | 1 | 4 | ||||||||||
| Machida et al[46] | Japan | Pancreatobiliary tract | II-IV | Healthy | 13 | 12 | Saliva | Total exosome isolation kit | miR-1246 | qRT-PCR | 13.77 | 8 | 13 | 0 | 4 |
| miR-4644 | -5.205 | 9 | 10 | 3 | 3 | ||||||||||
| Xu et al[47] | China | Liver | All | Chronic hepatitis B | 68 | 88 | Serum | Total exosome isolation kit | hnRNPH1 | qRT-PCR | 0.67 | 75 | 52 | 16 | 13 |
| Sun et al[48] | China | Liver | All | Healthy | 56 | 56 | Serum | Total exosome isolation kit | LINC00161 | qRT-PCR | NR | 42 | 41 | 15 | 14 |
| Xu et al[49] | China | Liver | All | Chronic hepatitis B | 96 | 60 | Serum | Total exosome isolation kit | ENSG00000258332.1 | qRT-PCR | 1.345 | 43 | 80 | 16 | 17 |
| 60 | 55 | ENSG00000258332.1 | 1.366 | 40 | 48 | 12 | 15 | ||||||||
| 96 | 60 | LINC00635 | 1.69 | 46 | 75 | 21 | 14 | ||||||||
| 60 | 55 | LINC00635 | 1.532 | 44 | 45 | 15 | 11 | ||||||||
| Goldvaser et al[50] | Israel | Pan-cancer (not include liver) | Healthy | 45 | 98 | Serum | Total exosome isolation kit | hTERT | qRT-PCR | NR | 61 | 45 | 0 | 37 | |
| Liver | NR | Healthy | 45 | 35 | NR | 21 | 45 | 0 | 14 | ||||||
| Zhang et al[51] | China | Lung | All | Healthy | 30 | 77 | Serum | ExoQuick | MALAT-1 | qRT-PCR | NR | 62 | 21 | 9 | 15 |
| Sun et al[52] | China | Lung | All | Healthy | 15 | 15 | Plasma | UC | 14-3-3ζ | ELISA | 9 | 12 | 3 | 6 | |
| Li et al[53] | NR | Ovarian | Benign | 21 | 50 | Serum | UC | ephrinA2 | ELISA | 20.4 ng/L | 44 | 17 | 4 | 6 | |
| Meng et al[54] | NR | Ovarian | All | Benign | 20 | 163 | Serum | Total exosome isolation kit | miR-200a | PCR+ qRT-PCR | Youden index | 135 | 18 | 2 | 28 |
| miR-200b | 86 | 20 | 0 | 77 | |||||||||||
| miR-200c | 51 | 20 | 0 | 112 | |||||||||||
| Pan et al[55] | Germany | Ovarian | All | Healthy | 29 | 106 | Plasma | ExoQuick | miR-21 | PCR+ qRT-PCR | Youden index | 65 | 24 | 5 | 41 |
| miR-100 | 66 | 21 | 8 | 40 | |||||||||||
| miR-200b | 68 | 25 | 4 | 38 | |||||||||||
| miR-320 | 59 | 20 | 9 | 47 | |||||||||||
| Bryzgunova et al[56] | Russia | Prostate | All | Healthy | 20 | 14 | Urine | UC | miR-125 | qRT-PCR | NR | 12 | 13 | 7 | 2 |
| miR-19b | NR | 11 | 19 | 1 | 3 | ||||||||||
| Wang et al[57] | China | Prostate | II-IV | Healthy | 30 | 34 | Plasma | Total exosome isolation kit | SAP30L-AS1 | qRT-PCR | NR | 21 | 25 | 5 | 13 |
| SChLAP1 | NR | 30 | 23 | 7 | 4 | ||||||||||
| Øverbye et al[58] | NR | Prostate | All | Healthy | 15 | 16 | Urine | UC | ADIRF | Mass spectrometry | Youden index | 12 | 16 | 0 | 3 |
| TMEM256 | 14 | 16 | 0 | 1 | |||||||||||
| Işın et al[59] | NR | Prostate | All | BPH | 49 | 30 | Urine | Urine Exosome RNA Isolation Kit | LincRNA-p21 | qRT-PCR | 0.181 | 20 | 31 | 18 | 10 |
| Wang et al[60] | China | Laryngeal | All | Vocal cord polyps | 49 | 52 | Serum | ExoQuick | miR-21 | qRT-PCR | 0.043 | 36 | 40 | 9 | 16 |
| HOTAIR | 0.032 | 48 | 28 | 21 | 4 | ||||||||||
| Alegre et al[61] | NR | Melanoma | NR | Healthy | 25 | 53 | Serum | ExoQuick | exo-MIA | ELISA | 1.4 μg/L | 42 | 20 | 5 | 11 |
| exo-S100B | ELISA | 0.015 μg/L | 42 | 20 | 5 | 11 | |||||||||
| Manterola et al[62] | France | GBM | NR | Healthy | 30 | 50 | Serum | ExoQuick | RNU6 | qRT-PCR | 0.372 | 33 | 20 | 10 | 17 |
| Chen et al[63] | Taiwan | Bladder | All | hernia | 81 | 140 | Urine | UC | TACSTD2 | ELISA | 2.47 ng/mL | 103 | 62 | 19 | 37 |
| Ge et al[64] | China | Cholangiocarcinoma | All | Biliary obstruction | 56 | 35 | Bile | UC | ENST00000588480.1 | qRT-PCR | NR | 22 | 41 | 15 | 13 |
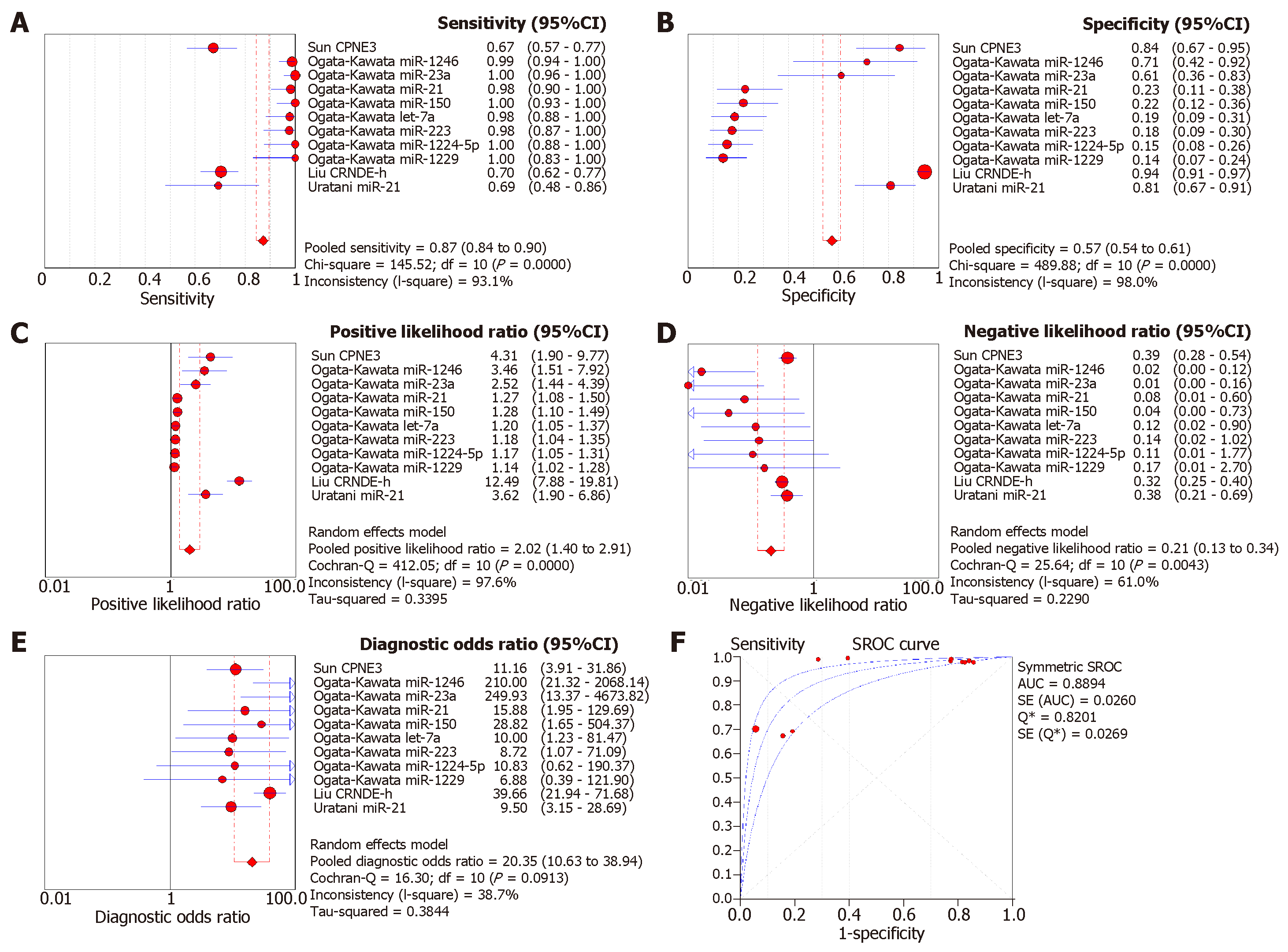
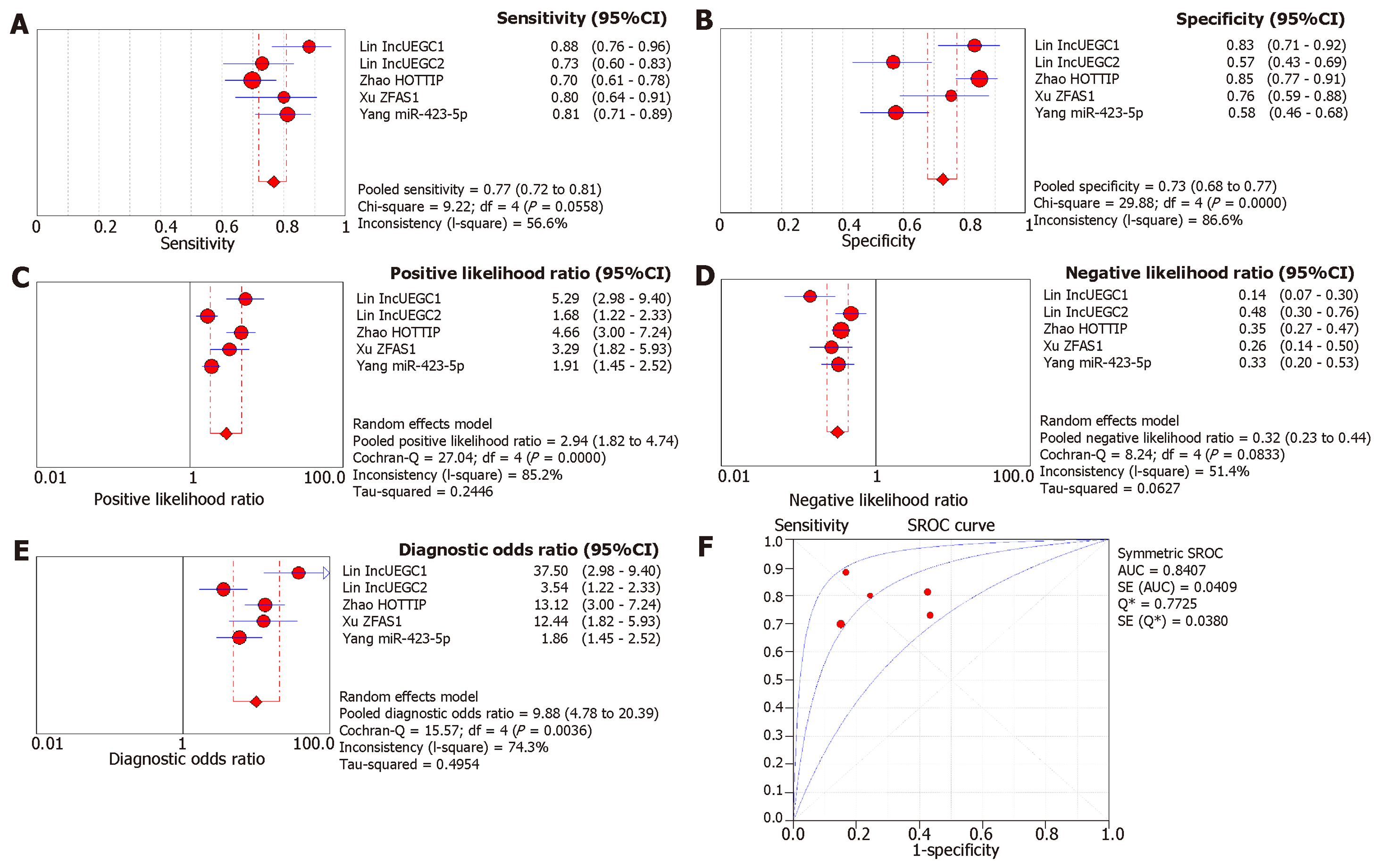
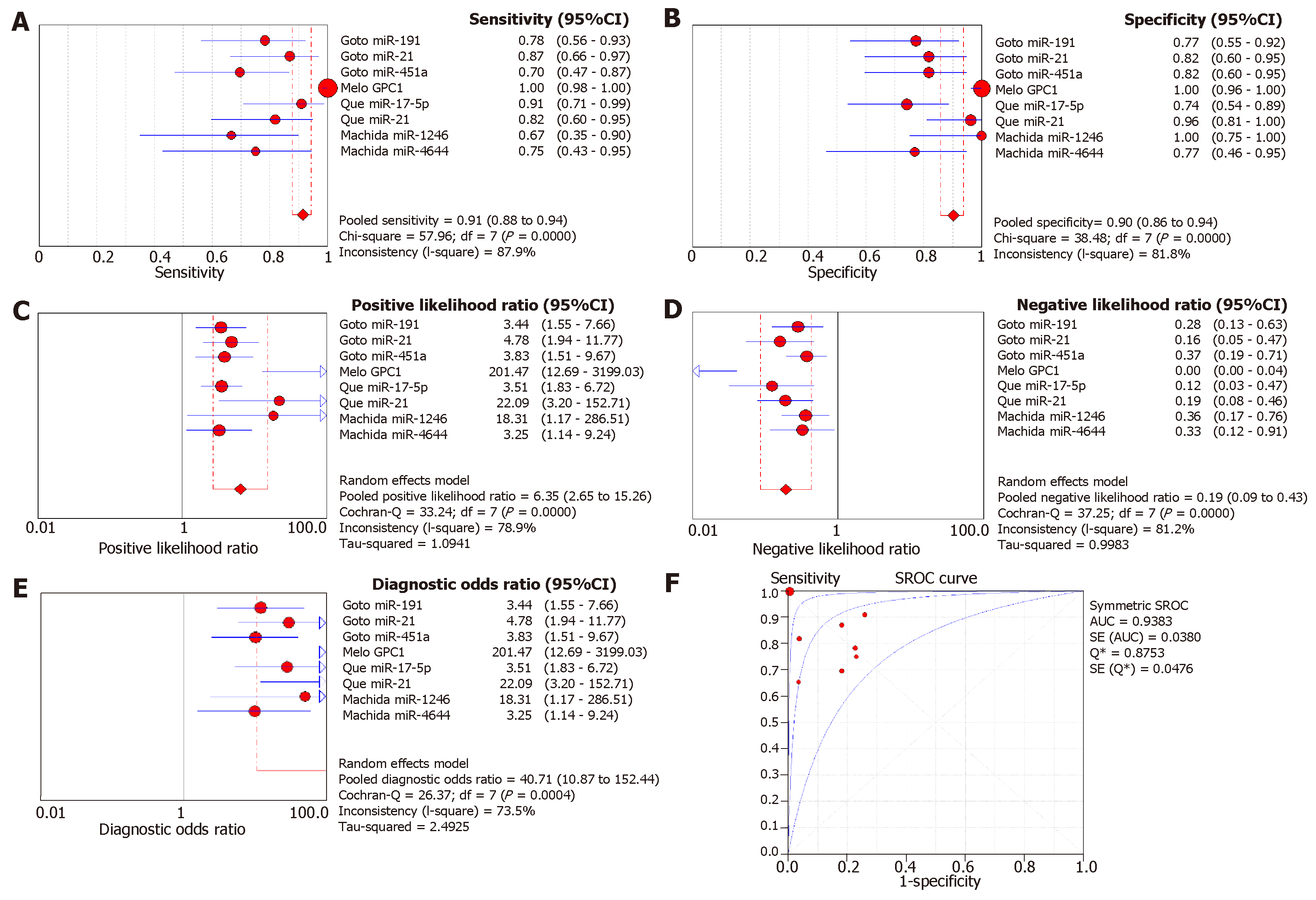
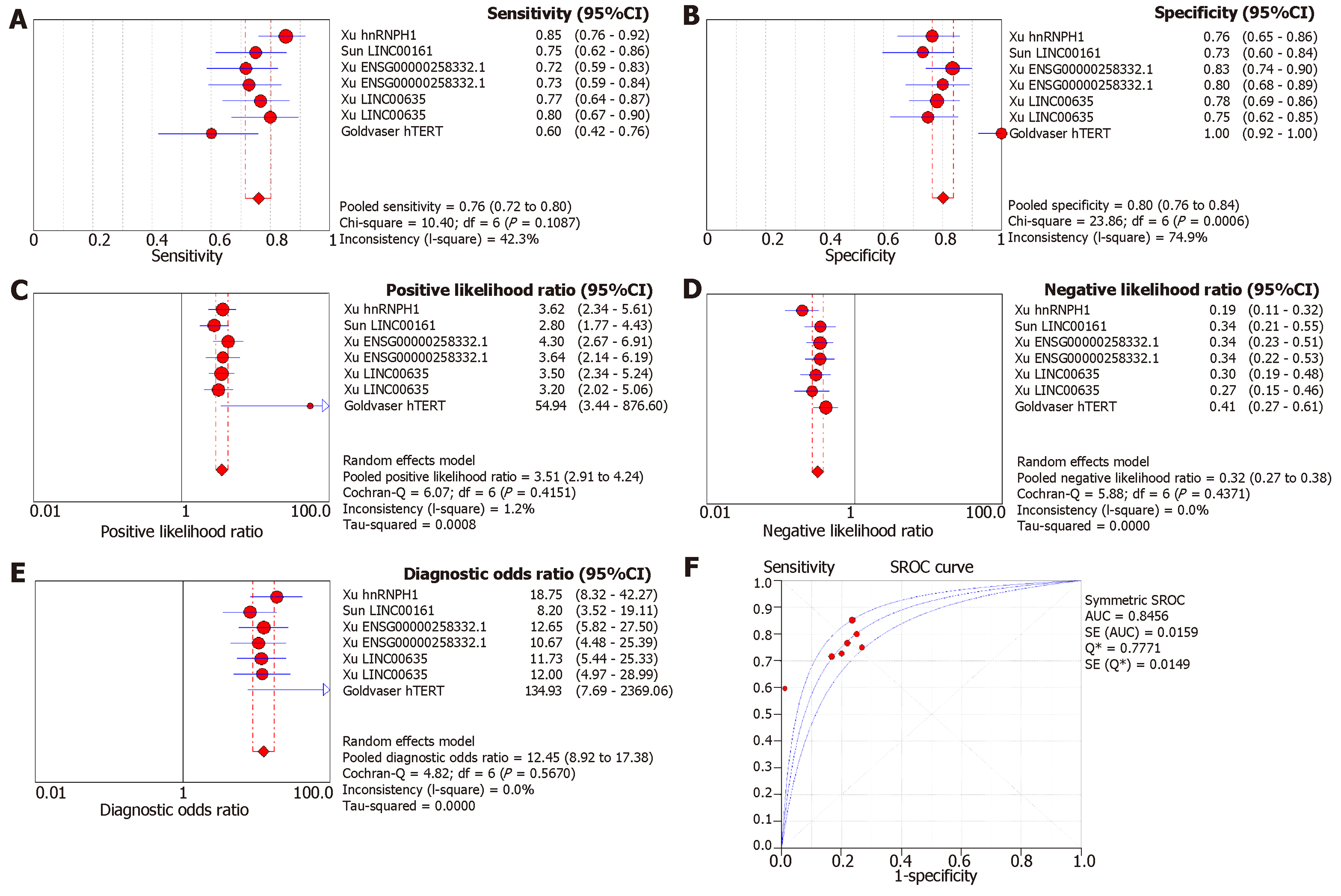
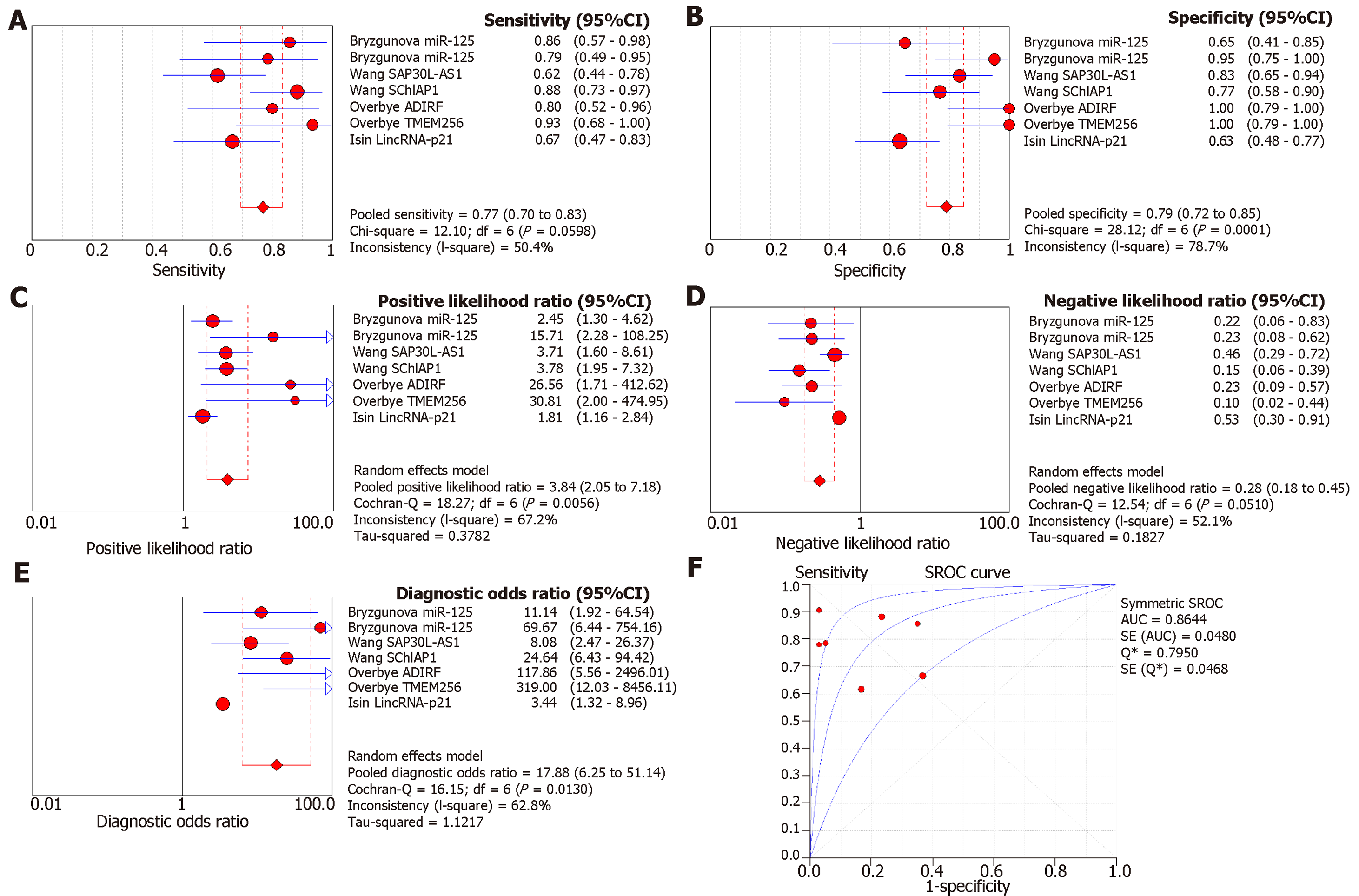
Since a wide range of cancers was studied by different groups, we separated the diagnostic biomarkers according to cancer types and meta-analyzed cancer types with more than three biomarkers studied. Therefore, we focused on colorectal cancer (4 studies with 11 biomarkers), gastric cancer (4 studies with 5 biomarkers), pancreatic cancer (4 studies with 8 biomarkers), liver cancer (4 studies with 7 biomarkers), and prostate cancer (4 studies with 7 biomarkers (Figures 3-7). We observed that the pooled biomarkers had a good specificity of 0.87 but poor sensitivity of 0.57 in colorectal cancer diagnosis (Figure 3A and B). The PLR and NLR were 2.02 and 0.21 respectively (Figure 3C and D). The diagnostic OR was 20.35 (Figure 3E). Importantly, the AUC of the SROC curve was 0.89 and the Q* was 0.82 (Figure 3F). In diagnosis of gastric cancer, we observed that the pooled biomarkers had a good sensitivity of 0.77 and specificity of 0.73 with PLR, NLR, AUC of the SROC curve and Q* of 2.94, 0.32, 9.88, 0.84 and 0.77 respectively (Figure 4). For diagnosis of pancreatic cancer, we also observed the pooled biomarkers had an excellent sensitivity of 0.91 and specificity of 0.90 with PLR, NLR, AUC of the SROC curve and Q* of 6.35, 0.19, 40.71, 0.94 and 0.88 respectively (Figure 5). In liver cancer, the pooled biomarkers had a good diagnostic sensitivity of 0.76 and specificity of 0.80 with PLR, NLR, AUC of the SROC curve and Q* of 3.51, 0.32, 12.45, 0.85 and 0.78 respectively (Figure 6). The pooled biomarkers also had a good sensitivity of 0.77 and specificity of 0.79 in detecting prostate cancer with PLR, NLR, AUC of the SROC curve and Q* of 3.84, 0.28, 17.88, 0.88 and 0.80 respectively (Figure 7). The high sensitivity, specificity and Q* demonstrated that the pooled biomarkers could effectively discriminate cancer patients from healthy people or non-cancer patients.
Prognostic biomarkers from 42 studies were included in the systematic review (Table 2). In total, 4797 patients were represented among the studies, with 7 studies having enrolled < 50 patients, 15 studies having enrolled 50-100 patients, and 20 studies having enrolled > 100 patients. There were 50 prognostic biomarkers analyzed in the systematic review, with 60% of the biomarkers being miRNAs, followed by lncRNAs (18%) and proteins (16%). Also, 50%, 43%, 2.4%, 2.4% and 2.4% of the biomarkers were detected in serum, plasma, bile, ascetic fluid and cell-free effusion supernatant respectively. For the included studies, 92.9%, 26.2% and 9.5% used OS, DFS and RFS respectively as the primary endpoints. In addition, a wide range of cancers was studied by the different groups. More than one-half of the included studies were related to GI cancers (11 studies were about colorectal or colon cancer, 5 studies were related to liver cancer, 5 studies were about pancreatic cancer, and 4 studies were related to gastric cancer). In this meta-analysis, we separated studies according to clinical endpoints and focused on cancer types with more than three biomarkers studied.
| Ref. | Period | Country | Sample Size | Cancer Type | Stage | Sample | Isolation method of exosome | Marker | Detection method | Cut-off value | Survival analysis | HR (95%CI) |
| Peng et al[65] | 2008-2014 | China | 108 | Colorectal | All | Serum | Total exosome isolation kit | miR-548c-5p | qRT-PCR | NR | OS | 3.40 (1.02‐11.27) |
| Sun et al[35] | 2012-2017 | China | 92 | Colorectal | All | Plasma | UC | CPNE3 | ELISA | ≥ 0.143 pg/μg exosome | OS | 3.0 (1.0-8.9) |
| ≥ 0.143 pg/μg exosome | DFS | 2.5 (1.1-5.5) | ||||||||||
| Tsukamoto et al[66] | 2002-2012 | Japan | 326 | Colorectal | II-IV | Plasma | UC | miR-21 | qRT-PCR | > median | OS | 2.28 (1.81-5.74) |
| DFS | 2.34 (1.87- 4.60) | |||||||||||
| Liu et al[37] | 2007-2010 | China | 148 | Colorectal | All | Serum | ExoQuick | CRNDE-h | qRT-PCR | > 0.02 | OS | 2.000 (1.269-3.154) |
| Liu et al[67] | 2006-2011 | United States | 84 | Colorectal | II-III | Serum | ExoQuick | miR-4772-3p | qRT-PCR | ≥ 27.88 | OS | 6.19 (1.50-25.5) |
| ≥ 27.88 | RFS | 5.48 (2.49-12.1) | ||||||||||
| Liu et al[24] | 2013-2014 | China | 158 | Colorectal | All | Plasma | UC | lncRNA GAS5 | qRT-PCR | NR | OS | 0.265 (0.082 -0.844) |
| RFS | 0.449 (0.194- 0.909) | |||||||||||
| miR-221 | qRT-PCR | NR | OS | 2.141 (1.368-3.054) | ||||||||
| RFS | 1.600 (1.162-2.007) | |||||||||||
| Gao et al[68] | 2011-2014 | China | 108 | Colorectal | All | Serum | ExoQuick | 91H | qRT-PCR | ≥ 0.85 | RFS | 7.14 (1.23-21.35) |
| Yan et al[69] | NR | NR | 168 | Colorectal | All | Serum | Total Exosome Isolation kit | miR-6803 | qRT-PCR | NR | OS | 2.93 (1.35-6.37) |
| DFS | 3.26 (1.56-6.81) | |||||||||||
| Li et al[70] | 2013-2015 | China | 85 | Colorectal | III | Plasma | ExoCapTM | GPC1 | Flow cytometry | > mean | OS | 1.89 (1.23-2.89) |
| Silva et al[71] | 2003-2009 | Spain | 91 | Colorectal | All | Plasma | UC | Exosome | Flow cytometry of EpCAM | High | OS | 0.87 (0.57-1.32) |
| Matsumura et al[72] | 1992-2007 | Japan | 209 | Colorectal | All | Serum | UC | miR-19 | qRT-PCR | > mean | O | 2.49 (1.12-6.61) |
| DFS | 2.49 (1.12-6.61) | |||||||||||
| Yan et al[73] | 2012-2015 | China | 142 | Colorectal | All | Serum | Total Exosome Isolation kit | miR-6869-5p | qRT-PCR | < mean | OS | 2.32 (1.08-4.99) |
| Santasusagna et al[25] | 2009-2013 | Spain | 32 | Colon | I-III | Plasma | UC | miR-141 | qRT-PCR | High | OS | 1.89 (0.93-3.83) |
| Zhao et al[40] | 2011-2012 | China | 126 | Gastric | All | Serum | NR | HOTTIP | qRT-PCR | > 1.72 | OS | 2.037 (1.085-3.823) |
| Liu et al[74] | 2012-2017 | China | 76 | Gastric | All | Serum | Total Exosome Isolation kit | miR-451 | qRT-PCR | > median | 5yr-OS | 4.344 (2.853‐5.721) |
| Yang et al[42] | NR | China | 80 | Gastric | All | Serum | ExoQuick | miR-423-5p | qRT-PCR | > median | DFS | 1.93 (1.25-2.99) |
| OS | 1.42 (0.92-2.20) | |||||||||||
| Kumata et al[75] | 2006-2013 | Japan | 232 | Gastric | All | Plasma | UC | miR23b | qRT-PCR | > 0.78 | OS | 0.57 (0.370.78) |
| DFS | 0.64 (0.410.91) | |||||||||||
| Zhou et al[76] | 2010-2014 | China | 152 | Pancreatic | All | Plasma | ExoQuick | miR-125b-5p | qRT-PCR | < median | OS | 0.285 (0.108-0.75) |
| Li et al[77] | 2012-2016 | China | 87 | Pancreatic | All | Plasma | NR | circPDE8A | qRT-PCR | > median | OS | 1.764 (1.064-2.925) |
| Goto et al[43] | 2013-2015 | Japan | 32 | Pancreatic | All | Serum | ExoQuick | miR-21 | qRT-PCR | > median | OS | 4.071 (1.832-11.996) |
| Takahasi et al[78] | 2013-2017 | Japan | 50 | Pancreatic | I-II | Plasma | UC | miR-451a | qRT-PCR | > 1.75 | OS | 3.20 (1.07-11.94) |
| DFS | 2.87 (1.23-7.23) | |||||||||||
| Xu et al[49] | 2012-2016 | China | 60 | Liver | All | Serum | Total Exosome Isolation kit | ENSG00000258332.1 | qRT-PCR | > 1.845 | OS | 2.22 (1.34-3.68) |
| LINC00635 | qRT-PCR | > 2.100 | OS | 1.46 (0.88-2.43) | ||||||||
| Shi et al[79] | 2008-2011 | China | 126 | Liver | All | Serum | Total Exosome Isolation kit | miR-638 | qRT-PCR | NR | 3yr-OS | 3.52 (1.37-6.02) |
| 5yr-OS | 2.80 (1.24-4.31) | |||||||||||
| Liu et al[26] | 2012 | China | 128 | Liver | All | Serum | ExoQuick | miR-125b | qRT-PCR | < median | RFS | 0.14 (0.07-0.29) |
| OS | 0.36 (0.18-0.74) | |||||||||||
| Xue et al[80] | 2015-2017 | China | 85 | Liver | All | Serum | Total Exosome Isolation kit | miR-93 | qRT-PCR | NR | OS | 1.47 (0.96-2.25) |
| Liu et al[81] | 2008-2013 | China | 32 | Hepatoblastoma (children) | All | Serum | ExoQuick | miR-21 | qRT-PCR | NR | EFS | 1.434 (1.257-2.766) |
| Matsumoto et al[82] | 2011-2012 | Japan | 66 | Esophageal | All | Plasma | Total Exosome Isolation kit | exosome | AChE activity | < 600 x 108/mL | OS | 2.177 (1.085-3.605) |
| Lu et al[83] | 2007-2015 | China | 110 | Nasopharyngeal | All | Plasma | UC | miR-9 | qRT-PCR | NR | OS | 1.5 (1.03-2.18) |
| Ye et al[84] | 2011-2013 | China | 83 | Nasopharyngeal | II-IV | Serum | UC | protein concentration | BCA assay | > 11 μg/mL | DFS | 214.22 (139.27-329.49) |
| Huang et al[85] | NR | NR | 23 | Prostate | All | Plasma | ExoQuick | miR-1290 | qRT-PCR | > mean | OS | 1.79(1.30-2.48) |
| miR-375 | qRT-PCR | > mean | OS | 2.69(1.52-4.77) | ||||||||
| Tang et al[86] | NR | NR | 35 | Ovarian | All | Ascitic fluid | UC | E-cadherin | NR | > 10 μg/mL | OS | 1.82 (0.53-3.58) |
| Vaksman et al[87] | 1998-2003 | 86 | Ovarian | III-IV | Effusion supernatant | ExoQuick | miR-21 | qRT-PCR | > median | OS | 1.70 (1.1-2.59) | |
| Kanaoka et al[88] | 2012-2017 | Japan | 285 | Lung | I-III | Plasma | UC | miR-451a | qRT-PCR | > 1.45 | OS | 6.06 (2.61-15.94) |
| DFS | 2.55 (1.44-4.65) | |||||||||||
| Liu et al[89] | 2012-2014 | China | 196 | Lung | All | Plasma | ExoQuick | miR-23b-3p | qRT-PCR | High | OS | 2.42 (1.45-4.04) |
| miR-21-5p | qRT-PCR | OS | 2.12(1.28-3.49) | |||||||||
| miR-10b-5p | qRT-PCR | OS | 2.22 (1.18-4.16) | |||||||||
| Liu et al[90] | 2012-2014 | China | 208 | Lung | All | Plasma | ExoQuick | Exosome | AChE activity | OS | 1.72 (1.05-2.83) | |
| Sandfeld-Paulsen et al[91] | 2011-2014 | Denmark | 276 | Lung | All | Plasma | / | CD171 | ELISA | NR | OS | 0.56 (0.41-0.79) |
| Flotilin1 | ELISA | NR | OS | 0.63 (0.46-0.86) | ||||||||
| HER3 | ELISA | NR | OS | 0.63 (0.46-0.86) | ||||||||
| GRP78 | ELISA | NR | OS | 0.69 (0.51-0.91) | ||||||||
| Manier et al[92] | 2006-2008 | France | 156 | Multiple myeloma | All | Plasma | ExoQuick | let-7b | qRT-PCR | < median | OS | 2.83 (1.07-7.50) |
| let-7b | qRT-PCR | < median | DFS | 1.90 (1.22-2.94) | ||||||||
| let-7e | qRT-PCR | < median | DFS | 2.01 (1.30-3.11) | ||||||||
| miR-106a | qRT-PCR | < median | DFS | 2.34 (1.52-3.61) | ||||||||
| miR-106b | qRT-PCR | < median | DFS | 3.54 (2.21-5.68) | ||||||||
| miR-155 | qRT-PCR | < median | OS | 2.41 (0.96-6.05) | ||||||||
| miR-155 | qRT-PCR | < median | DFS | 1.76 (1.15-2.69) | ||||||||
| miR-16 | qRT-PCR | < median | DFS | 2.21 (1.41-3.47) | ||||||||
| miR-17 | qRT-PCR | < median | DFS | 2.29 (1.48-3.55) | ||||||||
| miR-18a | qRT-PCR | < median | DFS | 4.52 (1.57-12.98) | ||||||||
| miR-18a | qRT-PCR | < median | OS | 2.76 (1.79-4.26) | ||||||||
| miR-20a | qRT-PCR | < median | DFS | 2.31 (1.52-3.53) | ||||||||
| Alegre et al[61] | NR | NR | 53 | Melanoma | NR | Serum | ExoQuick | MIA | ELISA | 2.5 μg/L | OS | 1.28 (0.65-2.51) |
| Lan et al[93] | 2011-2012 | China | 60 | Glioma | All | Serum | ExoQuick | miR-301a | qRT-PCR | >median | OS | 4.4 (3.1-9.6) |
| Ge et al[64] | NR | China | 35 | Cholangiocarcinoma | All | Bile | UC | ENST00000588480.1 | qRT-PCR | > median | OS | 2.40 (1.24-4.66) |
| ENST00000517758.1 | qRT-PCR | OS | 1.55 (0.80-3.01) | |||||||||
| Fujii et al[94] | 2005-2014 | Japan | 108 | Renal cell | I-III | Serum | Total Exosome Isolation kit | miR-224 | qRT-PCR | > median | OS | 9.1 (1.8-166.1) |
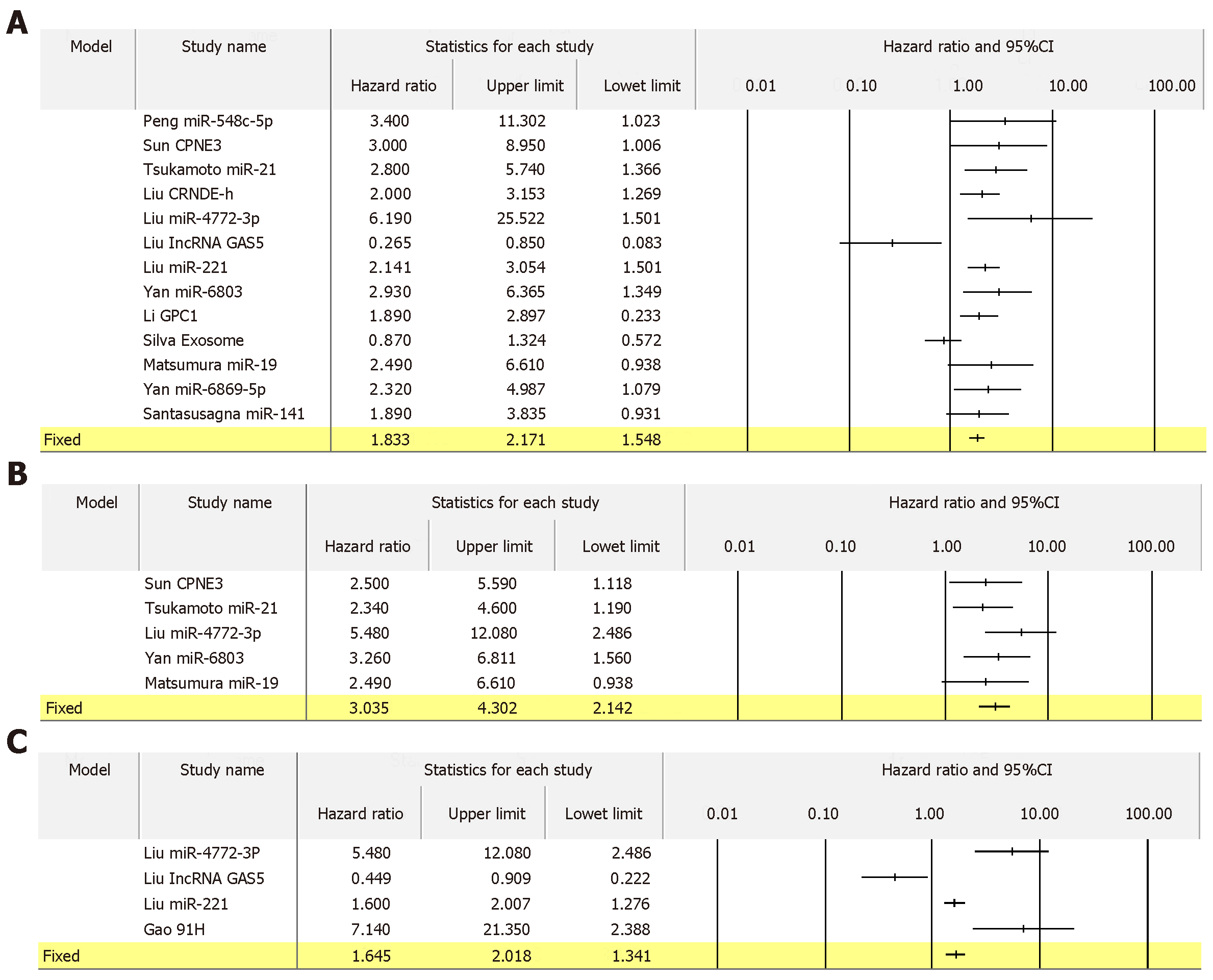
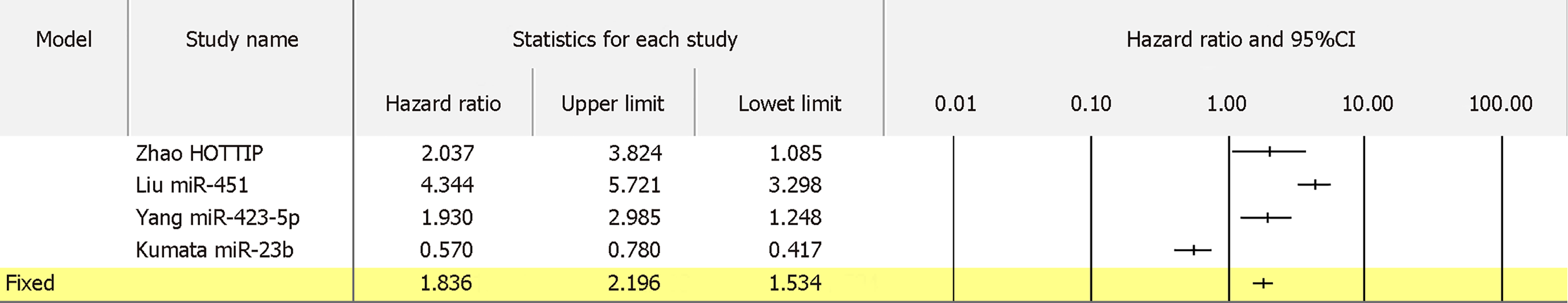
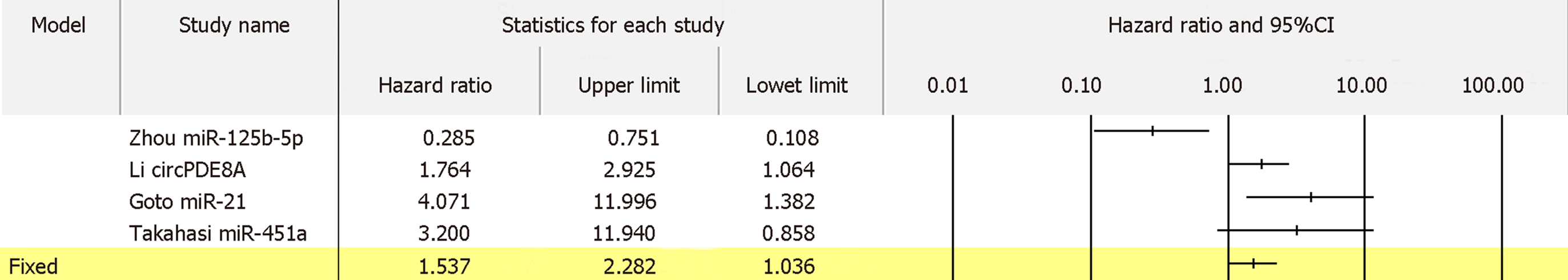
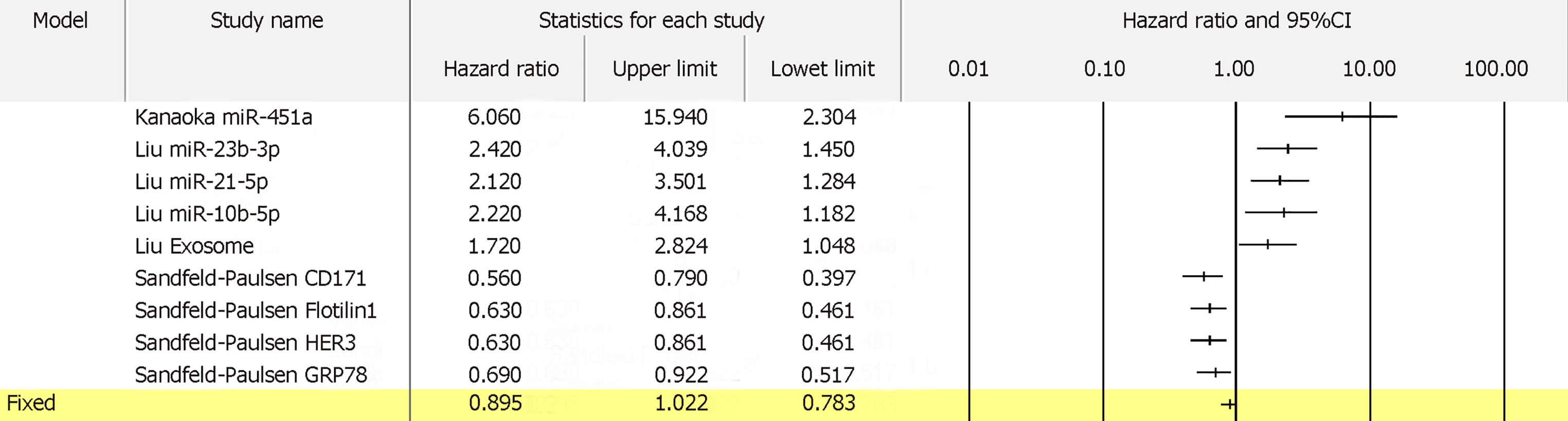
For 13 biomarkers with OS reported in colon cancer, the pooled HR was 1.833 with I2 of 62.14% and P = 0.002 (Figure 8A). Also, for 5 biomarkers with DFS reported in colon cancer, the pooled HR was 3.035 with I2 of 0.00% and P = 0.536 (Figure 8B). Furthermore, for 4 biomarkers with RFS reported in colon cancer, the pooled HR was 1.645 with I2 of 89.61% and P = 0.000 (Figure 8C). Apart from colon cancer, for the 4 biomarkers with OS reported in gastric cancer, the pooled HR was 1.836 with I2 of 96.71 and P = 0.000 (Figure 9). In addition, for the 4 biomarkers with OS reported in pancreatic cancer, the pooled HR was 1.537 with I2 of 81.50 and P = 0.001 (Figure 10). For 5 biomarkers, the pooled HR was 1.828, I2 of 84.48% and P = 0.000 for prognosing OS in liver cancer (Figure 11). Also, 9 biomarkers with the pooled HR of 0.895, I2 of 89.50% and P = 0.000 were reported to function as prognostic biomarkers of OS in lung cancer (Figure 12). These results demonstrated that exosomes were associated with OS, DFS and RFS in various types of cancer.
Exosomes play important roles in cancer development via intercellular communication, promoting cell metastasis and developing drug resistance[19-21]. Importantly, exosomes are frequently secreted by the cancers and are widely distributed in many body fluids. Therefore, they can be detected in blood, saliva and urine. Exosomal biomarkers have better performance in cancer diagnosis and prognosis than liquid biopsy used alone[24-26]. However, the methods of isolating exosomes from liquid biopsy varies between studies. Ultracentrifugation or the use of commercial isolation kits are common methods in extracting exosomes. Ultracentrifugation gives highly pure exosomes but the isolation efficiency is relatively low; whereas, the use of commercial kits maximizes the efficiency with the loss of purity[95,96]. Therefore, a standardized protocol of detecting exosomal biomarkers is greatly needed.
There are some limitations of our meta-analysis. We excluded studies that utilized combined biomarkers because this cannot tell the performance of individual biomarkers[97,98]. For example, a six-microRNA panel was developed for diagnosis of lung cancer but miR-409-3p, miR-425-5p and miR-584-5p were not significantly dysregulated in patients’ exosomes[98]. This may reduce the diagnostic performance of other biomarkers in the same panel. Since many of the individual biomarkers in the panel were significantly differentially expressed in cancer exosomes, further studies may be needed to explore the correlation of these potential biomarkers with patients’ characteristics and their performances in cancer diagnosis and prognosis.
A further limitation is that we focused on exosomal markers only in cancer diagnosis and prognosis and excluded tissue-based biomarkers from this meta-analysis. In fact, many studies have reported that expression levels in exosomes and in tissues are highly associated[35,66]. This suggests that many exosomal markers can reflect the situation in cancer cells, and this notion has been developed for potential biomarkers in various cancers. Importantly, this strong association may also suggest that many tissue-based biomarkers can be developed into non-invasive exosomal biomarkers in cancer diagnosis.
Notably, most of the included studies are retrospective, having been performed on stored samples. However, the main disadvantage of the retrospective study is its lack of complete clinicpathological information[30], which lowers the quality of study. Despite the above limitations, our meta-analysis indicates that exosomes can be potential biomarkers in cancer diagnosis and prognosis. Further large prospective studies are greatly needed to clarify the performance of exosomal biomarkers in cancer diagnosis and prognosis.
Exosomes, which are widely distributed in body fluids, including blood, bile, urine and saliva, are microvesicles of 30-100 nm diameter in size. Cancer-derived exosomes carry a wide variety of DNA, RNA, proteins and lipids, and may serve as novel biomarkers in cancer.
Exosomes may function as exosomal biomarkers in cancer diagnosis and prognosis.
To summarize the performance of exosomal biomarkers in cancer diagnosis and prognosis.
Relevant studies in the literature were identified using the PubMed database. QUADAS-2 and REMARK were used to assess the quality of the included studies. For diagnostic biomarkers, 47 biomarkers and 2240 patients from 30 studies were included.
These exosomal biomarkers had excellent diagnostic ability in various types of cancer, with good sensitivity and specificity. A total of 50 biomarkers and 4797 patients from 42 studies were included for the prognostic markers. We observed that exosomal biomarkers had prognostic values in overall survival, disease-free survival and recurrence-free survival.
Exosomes could be potential biomarkers in cancer diagnosis and prognosis.
Further large prospective studies are needed to clarity the performance of exosomal biomarkers in cancer diagnosis and prognosis, through exosomes can be potential biomarkers in cancer diagnosis and prognosis.
The work described in this paper was supported by grants from the General Research Fund, Research Grants Council of Hong Kong (CUHK462713, 14102714, 14136416 and 14171217), National Natural Science Foundation of China (8142730 and 81672323) and Direct Grant from CUHK to YC.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Balaban YH, Treeprasertsuk S S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Bernard WS, Christopher PW. World Cancer Report. Geneva: World Health Organization 2014; . |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13144] [Article Influence: 1877.7] [Reference Citation Analysis (4)] |
| 3. | Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 703] [Article Influence: 100.4] [Reference Citation Analysis (2)] |
| 4. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3652] [Article Influence: 304.3] [Reference Citation Analysis (2)] |
| 5. | Duffy MJ. Tumor markers in clinical practice: a review focusing on common solid cancers. Med Princ Pract. 2013;22:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1110] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 7. | He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival - a review of the epidemiological literature. J Ovarian Res. 2009;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Acharya A, Markar SR, Matar M, Ni M, Hanna GB. Use of Tumor Markers in Gastrointestinal Cancers: Surgeon Perceptions and Cost-Benefit Trade-Off Analysis. Ann Surg Oncol. 2017;24:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Sørensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence - A systematic review. Int J Surg. 2016;25:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Shinkins B, Nicholson BD, Primrose J, Perera R, James T, Pugh S, Mant D. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: Results from the FACS trial. PLoS One. 2017;12:e0171810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Chang CY, Huang SP, Chiu HM, Lee YC, Chen MF, Lin JT. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology. 2006;53:1-4. [PubMed] |
| 13. | Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. 2014;369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 15. | Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer. 2014;50:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 17. | Palazzolo G, Albanese NN, DI Cara G, Gygax D, Vittorelli ML, Pucci-Minafra I. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res. 2012;32:847-860. [PubMed] |
| 18. | Sinha A, Ignatchenko V, Ignatchenko A, Mejia-Guerrero S, Kislinger T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem Biophys Res Commun. 2014;445:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 554] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 20. | Li K, Chen Y, Li A, Tan C, Liu X. Exosomes play roles in sequential processes of tumor metastasis. Int J Cancer. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Yu DD, Wu Y, Shen HY, Lv MM, Chen WX, Zhang XH, Zhong SL, Tang JH, Zhao JH. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106:959-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 857] [Article Influence: 65.9] [Reference Citation Analysis (2)] |
| 23. | Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1745] [Article Influence: 174.5] [Reference Citation Analysis (0)] |
| 24. | Liu L, Meng T, Yang XH, Sayim P, Lei C, Jin B, Ge L, Wang HJ. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. 2018;22:283-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Santasusagna S, Moreno I, Navarro A, Martinez Rodenas F, Hernández R, Castellano JJ, Muñoz C, Monzo M. Prognostic Impact of miR-200 Family Members in Plasma and Exosomes from Tumor-Draining versus Peripheral Veins of Colon Cancer Patients. Oncology. 2018;95:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Liu W, Hu J, Zhou K, Chen F, Wang Z, Liao B, Dai Z, Cao Y, Fan J, Zhou J. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco Targets Ther. 2017;10:3843-3851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690-1691. [PubMed] |
| 28. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4941] [Article Influence: 274.5] [Reference Citation Analysis (0)] |
| 29. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9507] [Article Influence: 679.1] [Reference Citation Analysis (0)] |
| 30. | Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 632] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 31. | Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1571] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 32. | Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 2532] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 33. | Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2053] [Cited by in RCA: 2623] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 34. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46285] [Article Influence: 2103.9] [Reference Citation Analysis (3)] |
| 35. | Sun B, Li Y, Zhou Y, Ng TK, Zhao C, Gan Q, Gu X, Xiang J. Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J Cell Physiol. 2019;234:1416-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H, Watanabe M, Nakagama H, Yokota J, Kohno T, Tsuchiya N. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9:e92921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 643] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 37. | Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551-85563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 38. | Uratani R, Toiyama Y, Kitajima T, Kawamura M, Hiro J, Kobayashi M, Tanaka K, Inoue Y, Mohri Y, Mori T, Kato T, Goel A, Kusunoki M. Diagnostic Potential of Cell-Free and Exosomal MicroRNAs in the Identification of Patients with High-Risk Colorectal Adenomas. PLoS One. 2016;11:e0160722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Lin LY, Yang L, Zeng Q, Wang L, Chen ML, Zhao ZH, Ye GD, Luo QC, Lv PY, Guo QW, Li BA, Cai JC, Cai WY. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 40. | Zhao R, Zhang Y, Zhang X, Yang Y, Zheng X, Li X, Liu Y, Zhang Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 41. | Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 42. | Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, Wang M, Sun Z, Qian H, Xu W. Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol Carcinog. 2018;57:1223-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 43. | Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, Utsumi T, Sato H, Iwama T, Ijiri M, Sakatani A, Tanaka K, Nomura Y, Ueno N, Kashima S, Moriichi K, Mizukami Y, Kohgo Y, Okumura T. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. 2018;18:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 44. | Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2108] [Cited by in RCA: 2186] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 45. | Que R, Ding G, Chen J, Cao L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol. 2013;11:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 46. | Machida T, Tomofuji T, Maruyama T, Yoneda T, Ekuni D, Azuma T, Miyai H, Mizuno H, Kato H, Tsutsumi K, Uchida D, Takaki A, Okada H, Morita M. miR1246 and miR4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol Rep. 2016;36:2375-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Xu H, Dong X, Chen Y, Wang X. Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma. Clin Chem Lab Med. 2018;56:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 48. | Sun L, Su Y, Liu X, Xu M, Chen X, Zhu Y, Guo Z, Bai T, Dong L, Wei C, Cai X, He B, Pan Y, Sun H, Wang S. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J Cancer. 2018;9:2631-2639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 49. | Xu H, Chen Y, Dong X, Wang X. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2018;27:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 50. | Goldvaser H, Gutkin A, Beery E, Edel Y, Nordenberg J, Wolach O, Rabizadeh E, Uziel O, Lahav M. Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: a potential pan-cancer marker. Br J Cancer. 2017;117:353-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Zhang R, Xia Y, Wang Z, Zheng J, Chen Y, Li X, Wang Y, Ming H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 52. | Sun N, Sun SG, Lu ZL, He J. Diagnostic value of protein markers in plasma exosomes of lung squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2018;40:418-421. [PubMed] |
| 53. | Li S, Zhao Y, Chen W, Yin L, Zhu J, Zhang H, Cai C, Li P, Huang L, Ma P. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J Cancer. 2018;9:2659-2665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Meng X, Müller V, Milde-Langosch K, Trillsch F, Pantel K, Schwarzenbach H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget. 2016;7:16923-16935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 55. | Pan C, Stevic I, Müller V, Ni Q, Oliveira-Ferrer L, Pantel K, Schwarzenbach H. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12:1935-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 56. | Bryzgunova OE, Zaripov MM, Skvortsova TE, Lekchnov EA, Grigor'eva AE, Zaporozhchenko IA, Morozkin ES, Ryabchikova EI, Yurchenko YB, Voitsitskiy VE, Laktionov PP. Comparative Study of Extracellular Vesicles from the Urine of Healthy Individuals and Prostate Cancer Patients. PLoS One. 2016;11:e0157566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 57. | Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, Luo CL, Zhang WW, Wang FB, Zhang XL. Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell Physiol Biochem. 2018;46:532-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Øverbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, Sandvig K, Llorente A. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357-30376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 59. | Işın M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel ÖB, Gezer U, Dalay N. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 60. | Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, Tian L. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31:148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 61. | Alegre E, Zubiri L, Perez-Gracia JL, González-Cao M, Soria L, Martín-Algarra S, González A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin Chim Acta. 2016;454:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 62. | Manterola L, Guruceaga E, Gállego Pérez-Larraya J, González-Huarriz M, Jauregui P, Tejada S, Diez-Valle R, Segura V, Samprón N, Barrena C, Ruiz I, Agirre A, Ayuso A, Rodríguez J, González A, Xipell E, Matheu A, López de Munain A, Tuñón T, Zazpe I, García-Foncillas J, Paris S, Delattre JY, Alonso MM. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 63. | Chen CL, Lai YF, Tang P, Chien KY, Yu JS, Tsai CH, Chen HW, Wu CC, Chung T, Hsu CW, Chen CD, Chang YS, Chang PL, Chen YT. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J Proteome Res. 2012;11:5611-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 64. | Ge X, Wang Y, Nie J, Li Q, Tang L, Deng X, Wang F, Xu B, Wu X, Zhang X, You Q, Miao L. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget. 2017;8:69995-70005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Peng ZY, Gu RH, Yan B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Tsukamoto M, Iinuma H, Yagi T, Matsuda K, Hashiguchi Y. Circulating Exosomal MicroRNA-21 as a Biomarker in Each Tumor Stage of Colorectal Cancer. Oncology. 2017;92:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 67. | Liu C, Eng C, Shen J, Lu Y, Takata Y, Mehdizadeh A, Chang GJ, Rodriguez-Bigas MA, Li Y, Chang P, Mao Y, Hassan MM, Wang F, Li D. Serum exosomal miR-4772-3p is a predictor of tumor recurrence in stage II and III colon cancer. Oncotarget. 2016;7:76250-76260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 68. | Gao T, Liu X, He B, Nie Z, Zhu C, Zhang P, Wang S. Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression. Cancer Cell Int. 2018;18:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 69. | Yan S, Jiang Y, Liang C, Cheng M, Jin C, Duan Q, Xu D, Yang L, Zhang X, Ren B, Jin P. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J Cell Biochem. 2018;119:4113-4119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Li J, Li B, Ren C, Chen Y, Guo X, Zhou L, Peng Z, Tang Y, Chen Y, Liu W, Zhu B, Wang L, Liu X, Shi X, Peng Z. The clinical significance of circulating GPC1 positive exosomes and its regulative miRNAs in colon cancer patients. Oncotarget. 2017;8:101189-101202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, Peña C, Herrera M, Diaz R, Mohammed N, Bonilla F. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409-418. [PubMed] |
| 72. | Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano Y, Shinden Y, Eguchi H, Yamamoto H, Doki Y, Mori M, Ochiya T, Mimori K. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 73. | Yan S, Liu G, Jin C, Wang Z, Duan Q, Xu J, Xu D. MicroRNA-6869-5p acts as a tumor suppressor via targeting TLR4/NF-κB signaling pathway in colorectal cancer. J Cell Physiol. 2018;233:6660-6668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 74. | Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018;109:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Kumata Y, Iinuma H, Suzuki Y, Tsukahara D, Midorikawa H, Igarashi Y, Soeda N, Kiyokawa T, Horikawa M, Fukushima R. Exosomeencapsulated microRNA23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage. Oncol Rep. 2018;40:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Zhou X, Lu Z, Wang T, Huang Z, Zhu W, Miao Y. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis. Gene. 2018;673:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K, Zhao X, Zhang Y, Zhen P, Zhu J, Li X. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 78. | Takahasi K, Iinuma H, Wada K, Minezaki S, Kawamura S, Kainuma M, Ikeda Y, Shibuya M, Miura F, Sano K. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2018;25:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 79. | Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem. 2018;119:4711-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 80. | Xue X, Wang X, Zhao Y, Hu R, Qin L. Exosomal miR-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting TIMP2/TP53INP1/CDKN1A. Biochem Biophys Res Commun. 2018;502:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 81. | Liu W, Chen S, Liu B. Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study. Pediatr Surg Int. 2016;32:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Matsumoto Y, Kano M, Akutsu Y, Hanari N, Hoshino I, Murakami K, Usui A, Suito H, Takahashi M, Otsuka R, Xin H, Komatsu A, Iida K, Matsubara H. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2016;36:2535-2543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 83. | Lu J, Liu QH, Wang F, Tan JJ, Deng YQ, Peng XH, Liu X, Zhang B, Xu X, Li XP. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2018;37:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 84. | Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439-5452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 85. | Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 505] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 86. | Tang MKS, Yue PYK, Ip PP, Huang RL, Lai HC, Cheung ANY, Tse KY, Ngan HYS, Wong AST. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9:2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 87. | Vaksman O, Tropé C, Davidson B, Reich R. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis. 2014;35:2113-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 88. | Kanaoka R, Iinuma H, Dejima H, Sakai T, Uehara H, Matsutani N, Kawamura M. Usefulness of Plasma Exosomal MicroRNA-451a as a Noninvasive Biomarker for Early Prediction of Recurrence and Prognosis of Non-Small Cell Lung Cancer. Oncology. 2018;94:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 89. | Liu Q, Yu Z, Yuan S, Xie W, Li C, Hu Z, Xiang Y, Wu N, Wu L, Bai L, Li Y. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget. 2017;8:13048-13058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 90. | Liu Q, Xiang Y, Yuan S, Xie W, Li C, Hu Z, Wu N, Wu L, Yu Z, Bai L, Li Y. Plasma exosome levels in non-small-cell lung cancer: Correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018;22:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, Jakobsen KR, Meldgaard P, Folkersen BH, Rasmussen TR, Varming K, Jørgensen MM, Sorensen BS. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol. 2016;10:1595-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 92. | Manier S, Liu CJ, Avet-Loiseau H, Park J, Shi J, Campigotto F, Salem KZ, Huynh D, Glavey SV, Rivotto B, Sacco A, Roccaro AM, Bouyssou J, Minvielle S, Moreau P, Facon T, Leleu X, Weller E, Trippa L, Ghobrial IM. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129:2429-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 93. | Lan F, Qing Q, Pan Q, Hu M, Yu H, Yue X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol (Dordr). 2018;41:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 94. | Fujii N, Hirata H, Ueno K, Mori J, Oka S, Shimizu K, Kawai Y, Inoue R, Yamamoto Y, Matsumoto H, Shimabukuro T, Udoh K, Hoshii Y, Dahiya R, Matsuyama H. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017;8:109877-109888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One. 2017;12:e0170628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 475] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 96. | Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, Xu Y, Wu YS, Hu XM, Ping BH, Wang Q. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 97. | Zhou X, Wen W, Zhu J, Huang Z, Zhang L, Zhang H, Qi LW, Shan X, Wang T, Cheng W, Zhu D, Yin Y, Chen Y, Zhu W, Shu Y, Liu P. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8:34468-34480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 98. | Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R, Cheng W, Wang F, Qi LW, Chen Y, Huang Z, Wang T, Zhu D, Liu P, Shu Y. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget. 2017;8:6513-6525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |