Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3120
Peer-review started: July 1, 2019
First decision: July 31, 2019
Revised: August 24, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 91 Days and 19.3 Hours
Supernumerary phantom limb (SPL) caused by spinal cord injury (SCI) has previously been reported in several studies. However, the mechanisms and management of SPL in SCI patients are still not fully understood. Herein, we report a rare case of SPL in a patient with incomplete SCI.
A 46-year-old man complained of four hands 7 d after SCI. He was diagnosed with SPL complicated with actual limb neuropathic pain. Following a period of treatment with neurotrophic agents and Chinese traditional and analgesic medications, SPL symptoms and actual limb pain did not improve. However, his symptoms gradually lessened after combined treatment with high-frequency repetitive transcranial magnetic stimulation (rTMS), a promising neuromodulation technique, over the M1 cortex and visual feedback. After 7 wk of this treatment, SPL disappeared completely and actual limb pain was significantly relieved.
Cerebral plasticity changes may be a mechanism underlying the occurrence of non-painful SPL in SCI patients, and high-frequency rTMS applied to the M1 cortex could be a promising treatment method for SPL.
Core tip: Supernumerary phantom limb (SPL) is rare in spinal cord injury (SCI). We report a rare case of painless SPL in a patient with incomplete SCI. Repetitive transcranial magnetic stimulation (rTMS) was first used in the treatment of SPL. The combination of rTMS and visual feedback showed positive effects on the recovery of SPL. This case indicates that the pathogenesis of painless SPL in SCI could include cerebral plasticity and some of the mechanisms assumed in amputees. Furthermore, it demonstrated that high-frequency rTMS applied to the M1 cortex is a promising method for modulating SPL in SCI patients.
- Citation: Lu YS, Tong P, Guo TC, Ding XH, Zhang S, Zhang XJ. Effects of combined rTMS and visual feedback on the rehabilitation of supernumerary phantom limbs in a patient with spinal cord injury: A case report. World J Clin Cases 2019; 7(19): 3120-3125
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3120.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3120
Supernumerary phantom limb (SPL) designates the awareness of an illusory extra limb in addition to the actual existence of two arms and two legs[1]. SPL is manifested in many different patterns as follows[2-6]: (1) Being painful or non-painful; (2) Having one, two, or several phantom limbs simultaneously; (3) Involving only part of a limb, such as the hand, forearm, middle of the arm, and upper arm; (4) Being visual and/or movable; (5) Existing occasionally or continuously; and (6) Accompanying corresponding physical limb pain or not. SPL has been reported in various neurologic disorders and clinical contexts, such as stroke, epileptic seizure, spinal cord injury (SCI), multiple sclerosis, and continuous theta-burst stimulation[2-6].
SPL is rare in SCI, and all reported cases have occurred following cervical cord injury[1,3,7,8]. The underlying mechanisms of SPL after SCI are unknown, and effective therapeutic methods remain to be developed. Here, we report the diagnosis and treatment of SPL in a patient with incomplete SCI complicated with neuropathic pain.
A 46-year-old Chinese man complained of four hands 7 d after SCI. The two supernumerary hands were painless but complicated with actual limb pain.
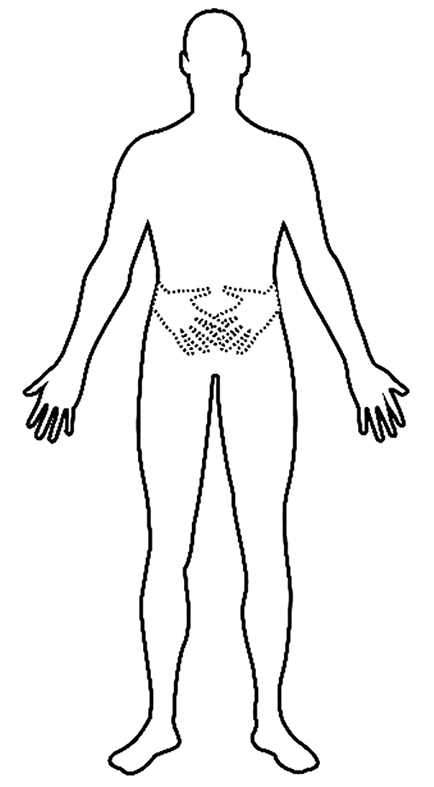
The patient suffered from tetraplegia caused by an accident on April 9, 2018. He underwent major surgery consisting of cervical posterior unilateral open-door expansive laminoplasty on 16 April 2018. Seven days after the accident, the patient felt the presence of an additional pair of hands that originated at the wrist joints and extended medially, with equal length to the paralyzed hands. He complained that he could feel but could not see the additional limbs. According to the patient’s description, the two supernumerary hands that were placed across his abdomen were not painful and persisted throughout the day (Figure 1). However, he felt a burning-like pain on both actual forearms, which measured 7 points (right side) and 5 points (left side) on the visual analogue scale. He experienced a more intense feeling of the existence and movement of the supernumerary hands, and stronger actual limb pain when he tried to control his limbs or someone touched his body.
The patient had no significant medical history, psychiatric history, and history of substance misuse, except for type 2 diabetes mellitus, which was diagnosed 5 years previously and treated with regular injections of insulin.
According to the American Spinal Injury Association (ASIA) standards for neurological classification of SCI, the patient was classified as having an incomplete lesion (ASIA impairment scale B) with a neurologic level at C4. The ASIA evaluation for neurological function was performed 18 d after injury, and the motor score for the upper and lower limbs was 0, and the total score for light touch and pin prick, for both sides, was 31. Bulbocavernosus reflex was positive.
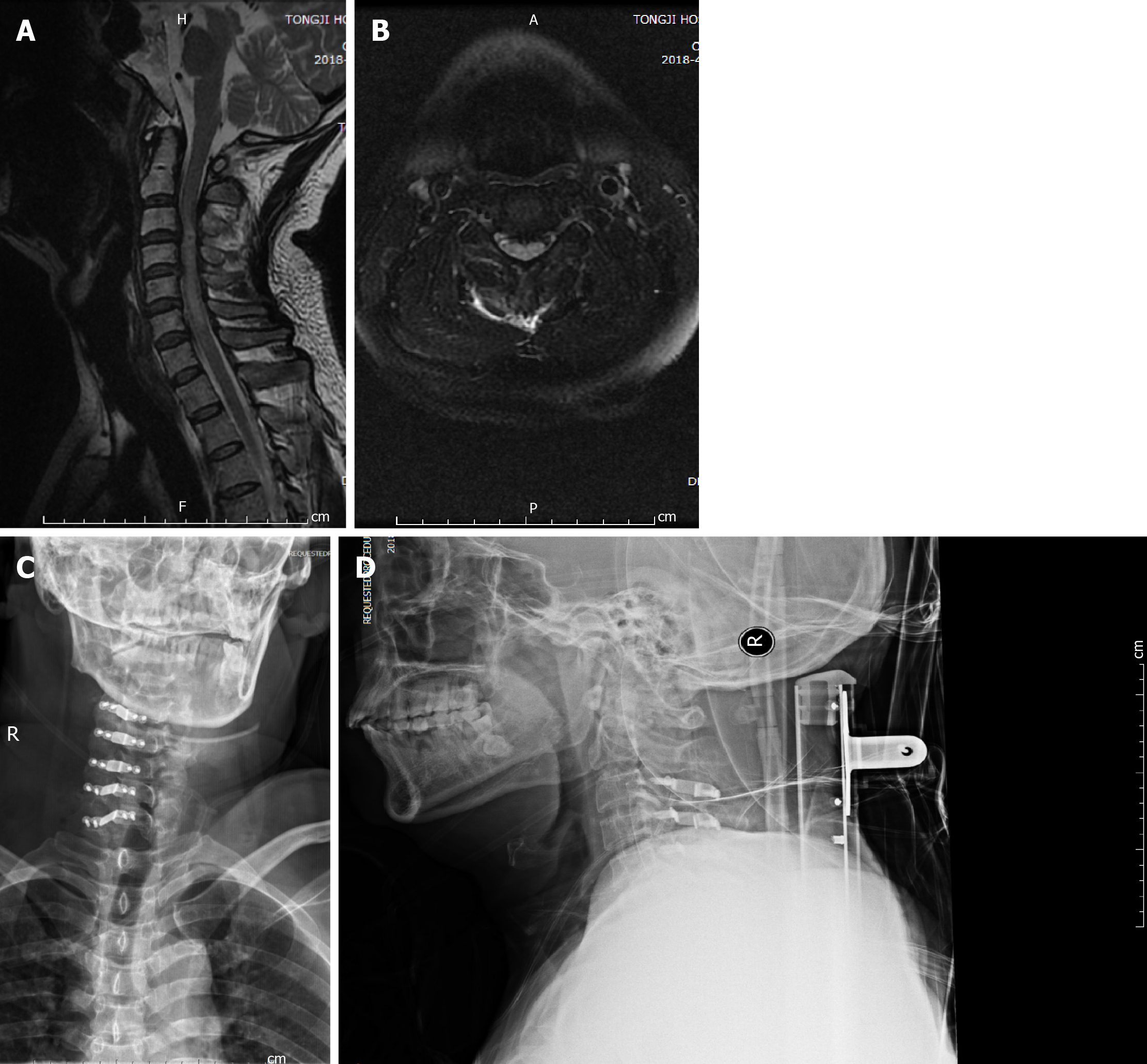
Computed tomography scan of the cervical spine on the day of the accident did not show vertebral body fracture or SCI. Three days later, magnetic resonance imaging showed an abnormal signal at C3-6 cervical spinal cord on T2-weighted magnetic resonance imaging (Figure 2A and B). No concomitant brain injury was observed on head computed tomography scans, and all cognitive evaluations suggested no abnormalities. Follow-up cervical spine X-ray was performed (Figure 2C and D).
The final diagnosis in this patient was painless SPL complicated by actual limb neuropathic pain following SCI.
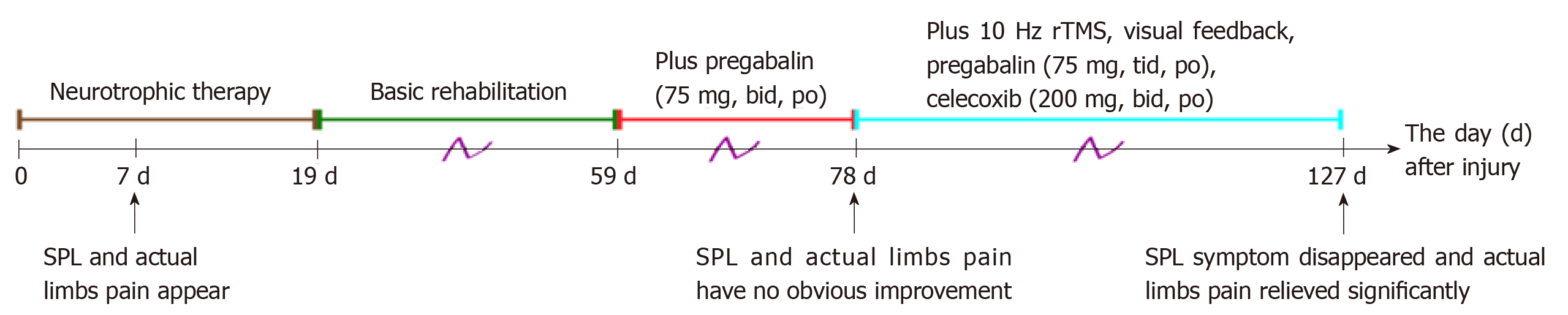
As shown in Figure 3, the therapeutic process was as follows: The day after injury, rehabilitation interventions including passive range of motion were administered in the Department of Orthopedics. Nineteen days after injury, the patient was referred to the Department of Rehabilitation Medicine and received combinations of medications and rehabilitation therapy. Basic rehabilitation therapies included physical therapy (electrical stimulation of the bladder, passive joint motion training, and spastic muscle stretching training), acupuncture, and hyperbaric oxygen. Ganglioside and nerve growth factor were also administered. For the management of SPL and pain, oral pregabalin (75 mg, twice a d) was added to the therapeutic schedule 59 d after injury. However, the symptoms of SPL and neuropathic pain did not improve 78 d after injury. Repetitive transcranial magnetic stimulation (rTMS) was then delivered alternately over the M1 region of the left and right hemisphere with a TMS stimulator (YRD CCY-I, 6.0 T; Yiruide Medical Equipment Co., Ltd., Wuhan, China), with 104 trains of 1.5 s each (intertrain interval 10 s), intensity equal to 40% of maximal output, and a frequency of 10 Hz, once daily, six times a week.
At the same time, the dosage of oral pregabalin was modified to 75 mg three times/d and oral celecoxib was added at a dose of 200 mg twice/d. Furthermore, we suggested that the patient should look at his hands more frequently to increase visual input when he was thinking about his hands or trying to move his hands.
The patient adhered to the systematic rehabilitation as above at our department for 6 weeks. SPL eventually disappeared and to date has not reappeared, and the pain at both sides measured 3 points on the visual analogue scale. Moreover, the motor scores for the upper limbs improved to 6. The total score for light touch and total pin prick was unchanged up to discharge. Pregabalin, rTMS, visual feedback, and other therapies were continued until he was transferred to another hospital.
Phantom limb mainly occurs after amputation and can make patients almost forget that their limbs have been removed, and they can even feel the movement or pain of amputated limbs[9]. Phantom limb has also been reported in SCI patients and has been defined as SPL[3]. In addition to their actual limbs, these patients complain of having extra limbs. Some patients do not realize the existence of extra limbs, and their symptoms may have disappeared before they were even aware of SPL. Furthermore, some patients might be afraid of being considered unusual or “crazy” and did not report SPL[10]. Therefore, some cases may have been ignored in the clinic.
In addition, some SCI patients may experience phantom sensations below the level of the lesion, such as pressure, tingling, electrical sensation, or positional sensations[11]. In the present case, the patient began to complain of two hands moving on his abdomen when he tried to move his hands, while his actual hands were paralyzed, 1 week after cervical cord injury. The two extra hands were not painful, but an obvious burning pain was felt in both actual upper extremities. Initially, we thought that the unusual feeling of extra limbs could be just a simple sense of pressure or paresthesia or the result of cognitive or psychological issues. However, the phantom sensation in this patient was a more complex sensation, which was related to his brain’s commands, and the SPL was movable. Thus, this patient was finally diagnosed with painless SPL complicated by actual limb neuropathic pain following SCI. Meanwhile, the differential diagnosis between SPL and other simple phantom sensations or paresthesia in SCI patients may warrant further investigation.
Research has demonstrated that virtual feedback has therapeutic effects on the PL following amputation and neurological injury[3,8,12]. Thus, we prescribed for our patient with virtual feedback. Some researchers reported phantom limb pain in an amputee, which was relieved by low-frequency rTMS over the unaffected/ipsilateral hemisphere[13,14]. Moreover, a recent study showed that application of high-frequency rTMS to the M1 cortex contralateral to the amputated limb markedly reduced phantom limb pain[9]. Thus, modulation of M1 cortex excitability may have contributed to the rehabilitation of phantom limb sensation. Moreover, rTMS is a safe and noninvasive neuromodulation technique. Hence, we tried to manage SPL in our patient using this method. Due to the bilateral sensation of SPL in this patient, high-frequency rTMS was applied at the M1 cortex in the left and right hemisphere alternately. After a period of visual feedback combined with rTMS, the SPL sensation disappeared and did not recur. Hence, it is assumed that SPL symptoms in SCI patients might be caused by maladaptive plasticity and some pathological changes similar to that in amputees.
It has been found that when delivered to the posterior parietal cortex or primary sensory cortex of SCI patients with non-painful phantom sensation, high-frequency rTMS reversed the symptoms of phantom sensation. However, rTMS applied to the M1 cortex had no therapeutic effect[11]. On the contrary, our findings suggested that applying rTMS at the M1 cortex relieved painless SPL. With regard to treatment, the mechanism of painless SPL could be different from that of other phantom sensations after SCI.
To date, the mechanisms underlying SPL caused by SCI are unclear. Based on a review of relevant literature, there are four major theories. The first theory is that the primary somatosensory cortex is reorganized and that the regular cerebral schema that was gained by the interaction between sensory feedback from the periphery and image formulation originating from the brain, subsequently deteriorated[15]. Secondly, central command is moved and is mismatched with sensory feedback from the periphery, which is caused by impairment of sensory roots resulting from SCI and dysfunction that failed to integrate motor information to generate normal physical experience[3]. Thirdly, cortical or subcortical brain reorganization occurs, which includes a change in brain excitability[16,17]. Fourth, there is conflict between visuomotor and proprioceptive sensations caused by excessive activation of the motor area[7]. Following a comprehensive analysis of the present case, including symptoms and the recovery process, we conclude that the mechanisms of SPL in SCI may involve the second and third hypotheses as described above.
In the present case, the patient endured neuropathic pain in both actual upper limbs at the same time, which was mainly modulated by drug therapy. It has been proposed that rTMS is an effective therapy for the treatment of neuropathic pain[18,19]. The reduction in pain observed in this case may have been the result of the combined effects of drug therapy and rTMS. SPL was not relieved following a long period of drug therapy, and it has been rarely reported that the drugs used in this case have any effect on SPL recovery. This inspired us to choose rTMS as a good alternative therapy for SPL complicated by neuropathic pain of actual limbs in our SCI patient.
Several significant points in this case deserve attention. First, it is noteworthy that painless SPL after SCI may be confused with a simple phantom sensation or paresthesia. Second, in similar cases, rTMS was first used in the treatment of painless SPL. The combination of rTMS and visual feedback has positive effects on the recovery of SPL, which indicates that the pathogenesis of painless SPL in SCI could include cerebral plasticity and some of the mechanisms assumed in amputees. Third, the mechanism of painless SPL may differ from that of other phantom sensations in SCI. Furthermore, this case demonstrated that high-frequency rTMS applied to the M1 cortex is a promising method for modulating SPL in SCI patients. However, the efficacy, optimal stimulation parameters, and mechanisms of rTMS for SPL in SCI deserve further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elgafy H S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Davis R. Pain and suffering following spinal cord injury. Clin Orthop Relat Res. 1975;76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 2. | Bourlon C, Urbanski M, Quentin R, Duret C, Bardinet E, Bartolomeo P, Bourgeois A. Cortico-thalamic disconnection in a patient with supernumerary phantom limb. Exp Brain Res. 2017;235:3163-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Choi JY, Kim HI, Lee KC, Han ZA. Atypical supernumerary phantom limb and phantom limb pain in a patient with spinal cord injury: case report. Ann Rehabil Med. 2013;37:901-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Mayeux R, Benson DF. Phantom limb and multiple sclerosis. Neurology. 1979;29:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Millonig A, Bodner T, Donnemiller E, Wolf E, Unterberger I. Supernumerary phantom limb as a rare symptom of epileptic seizures--case report and literature review. Epilepsia. 2011;52:e97-e100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Dieguez S, Kaeser M, Roux C, Cottet J, Annoni JM, Schmidlin E. Birth and death of a phantom. Ann Clin Transl Neurol. 2017;5:98-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Curt A, Yengue CN, Hilti LM, Brugger P. Supernumerary phantom limbs in spinal cord injury. Spinal Cord. 2011;49:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Katayama O, Iki H, Sawa S, Osumi M, Morioka S. The effect of virtual visual feedback on supernumerary phantom limb pain in a patient with high cervical cord injury: a single-case design study. Neurocase. 2015;21:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Malavera A, Silva FA, Fregni F, Carrillo S, Garcia RG. Repetitive Transcranial Magnetic Stimulation for Phantom Limb Pain in Land Mine Victims: A Double-Blinded, Randomized, Sham-Controlled Trial. J Pain. 2016;17:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Antoniello D, Kluger BM, Sahlein DH, Heilman KM. Phantom limb after stroke: an underreported phenomenon. Cortex. 2010;46:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nardone R, Langthaler PB, Höller Y, Bathke A, Frey VN, Brigo F, Trinka E. Modulation of non-painful phantom sensation in subjects with spinal cord injury by means of rTMS. Brain Res Bull. 2015;118:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Herrador Colmenero L, Perez Marmol JM, Martí-García C, Querol Zaldivar MLÁ, Tapia Haro RM, Castro Sánchez AM, Aguilar-Ferrándiz ME. Effectiveness of mirror therapy, motor imagery, and virtual feedback on phantom limb pain following amputation: A systematic review. Prosthet Orthot Int. 2018;42:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Di Rollo A, Pallanti S. Phantom limb pain: low frequency repetitive transcranial magnetic stimulation in unaffected hemisphere. Case Rep Med. 2011;2011:130751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Grammer GG, Williams-Joseph S, Cesar A, Adkinson DK, Spevak C. Significant reduction in phantom limb pain after low-frequency repetitive transcranial magnetic stimulation to the primary sensory cortex. Mil Med. 2015;180:e126-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Khateb A, Simon SR, Dieguez S, Lazeyras F, Momjian-Mayor I, Blanke O, Landis T, Pegna AJ, Annoni JM. Seeing the phantom: a functional magnetic resonance imaging study of a supernumerary phantom limb. Ann Neurol. 2009;65:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Flor H, Elbert T, Mühlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 1998;119:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Flor H, Mühlnickel W, Karl A, Denke C, Grüsser S, Kurth R, Taub E. A neural substrate for nonpainful phantom limb phenomena. Neuroreport. 2000;11:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Gao F, Chu H, Li J, Yang M, DU L, Li J, Chen L, Yang D, Zhang H, Chan C. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: a systematic review and meta-analysis. J Neurosurg Sci. 2017;61:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schönfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1372] [Article Influence: 124.7] [Reference Citation Analysis (0)] |