Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3090
Peer-review started: March 26, 2019
First decision: July 30, 2019
Revised: August 6, 2019
Accepted: August 25, 2019
Article in press: August 25, 2019
Published online: October 6, 2019
Processing time: 189 Days and 21.7 Hours
Kaposi’s sarcoma (KS) is one of the most common cancers in human immunodeficiency virus (HIV)-positive patients and leads to a high prevalence of morbidity and mortality. It usually appears as cutaneous or mucous lesions. Patients with visceral KS are asymptomatic and clinically silent. As the disease advances, patients may progress from a normal condition to exhibiting severe symptoms.
A 27-year-old man presented with a 2-mo history of fever, bearing-down pain, and rectal bleeding. His hepatitis B virus DNA level was 2.7 ×107 IU/mL. Abdominal computed tomography (CT) indicated liver cirrhosis. Before he was admitted to our hospital, he was diagnosed with HIV infection. His CD4 count was 24 cells/μL. Pelvic cavity CT suggested a thickened rectum wall accompanied by multiple enlarged lymph nodes. The patient was initially treated as having haemorrhoidal varices with bleeding, telbivudine for anti-hepatitis B virus treatment, and antibiotics for anti-infection. After half a month of treatment, the patient felt that his lower lumbus ache and bearing-down pain had not improved, and a colonoscopy was conducted. The result revealed a rectal mass that was histologically confirmed as KS with rectal spindle cells that were positive for cluster of differentiation 117 (CD117), CD34, human herpes virus 8, and CD31. He was administered systemic chemotherapy with 36 mg/d liposomal doxorubicin six times. The patient experienced no sign of lower gastrointestinal bleeding again.
This case highlights the diagnosis of primary KS with lower gastrointestinal bleeding in HIV-positive patients, which means visceral KS could not be excluded. The gold standard relies on colonoscopy and biopsy findings.
Core tip: Human immunodeficiency virus (HIV)-related Kaposi’s sarcoma (KS) is the most common malignancy affecting HIV-positive patients. It usually appears as cutaneous or mucous lesions; however, KS in the viscera cannot be excluded. Patients may progress from a normal condition to exhibiting severe symptoms. We present herein, a rare case of HIV/hepatitis B virus co-infection that manifested as fever, bearing-down pain, and rectal bleeding. This case highlights that primary KS without a cutaneous manifestation should not be ignored when physicians make infectious consultation. Emphasis should be placed on colonoscopy and biopsies.
- Citation: Zhou QH, Guo YZ, Dai XH, Zhu B. Kaposi’s sarcoma manifested as lower gastrointestinal bleeding in a HIV/HBV-co-infected liver cirrhosis patient: A case report. World J Clin Cases 2019; 7(19): 3090-3097
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3090
Kaposi’s sarcoma (KS) is a low-degree tumour consisting of multicentric vascular nodules. Human herpes virus type 8 (HHV8), also known as Kaposi's sarcoma-associated herpes virus (KSHV), is an aetiological agent of KS[1]. KSHV infects characteristic spindle cells of endothelial origin, B cells, and keratinocytes and expresses a wide range of genes encoding proliferation and angiogenesis, thus leading to pathogenesis of KS[1-3]. Because of the human immunodeficiency virus (HIV)-1 tat gene product, HIV-1 and KSHV can accelerate replication reciprocally. In the meantime, upregulated T-helper type-1 cytokines induce reactivation of KSHV and growth of the disease[3-5]. It usually presents as cutaneous or mucous lesions undergoing three stages: patch, plaque, and nodular[2]. Pain, oedema, and ulceration may occur when lymphatic obstruction involves subcutaneous tissue[4]. Visceral KS only accounts for a small part, which can be life threatening. It is symptomless at the beginning followed by exacerbation when it advances[1]. Although combination anti-retroviral therapy is still the mainstay of treatment for acquired immune deficiency syndrome-related KS, tumour-specific therapies including cytotoxic agents and anti-angiogenic agents are necessary for aggressive type[3].
We present herein, a patient with HIV/hepatitis B virus (HBV) co-infected liver cirrhosis that manifested as lower gastrointestinal (GI) bleeding. This case shows that essential colonoscopy and pathological diagnosis should not be forgotten for visceral KS with GI bleeding. We used “Kaposi’s Sarcoma”, “HIV”, “gastrointestinal bleeding”, and pertinent words to search the articles on PubMed.
A 27-year-old man presented with a 2-mo history of fever, bearing-down pain, and rectal bleeding.
Patient’s symptoms started 2 mo ago; he previously visited another hospital to undergo a thorough examination. Based on the results of laboratory examinations and imaging examinations, he was primarily diagnosed with haemorrhoidal varices bleeding due to liver cirrhosis by another physician. He was treated with piperacillin/tazobactam and meropenem for anti-infection therapy, with somatostatin and octreotide to lower the portal pressure and telbivudine for anti-HBV therapy. Three days before he was admitted to our hospital, he was diagnosed with HIV infection. He was transferred to our ward for specific treatments. He still complained of fever and rectal bleeding.
The patient suffered from chronic hepatitis B since he was born. Abdominal computed tomography (CT) indicated liver cirrhosis as well.
On examination, the patient’s temperature was 39.3 °C, heart rate was 96 beats per min, respiratory rate was 16 breaths per min, blood pressure was 105/73 mmHg, and oxygen saturation in room air was 98%. The patient did not show any rashes, but was pale and had an abdominal bulge.
A routine blood test showed mild anaemia with a haemoglobin level of 11.7 g/dL, leucopoenia with a leucocyte count of 1900/µL, thrombocytopenia with a platelet count of 83000/µL, and an albumin level of 29.8 g/L. Coagulation function tests showed an activated partial thromboplastin time of 56.2 s and a prothrombin time of 17 s. His CD4 count was 24 cells/µL. The stool test was positive. The G test, GM test, and latex agglutination test were all negative.
Pelvic cavity CT suggested a thickened rectum wall accompanied by multiple enlarged lymph nodes. Abdominal CT showed diffuse adenopathy surrounding the hepatic portal and the posterior peritoneum.
During hospitalisation, because of his history of hepatitis B-related cirrhosis, the patient was initially treated as having haemorrhoidal varices with bleeding. Octreotide was administered to stop the bleeding. Telbivudine was added to the anti-HBV treatment. The patient developed a fever before being admitted to our hospital; the fever did not improve during the first days of hospitalisation. He was given antibiotics, including piperacillin sodium and sulbactam sodium, empirically. On the 5th day of admission, the blood culture results showed that Staphylococcus aureus was still present. Considering his symptoms, we concluded that the patient had septicaemia. The abovementioned antibiotics were discontinued, and biapenem was added to his treatments. Due to the absolute CD4 T-lymphocyte counts along with the mucosa blaze in the patient’s mouth, the antifungal agent voriconazole was used.
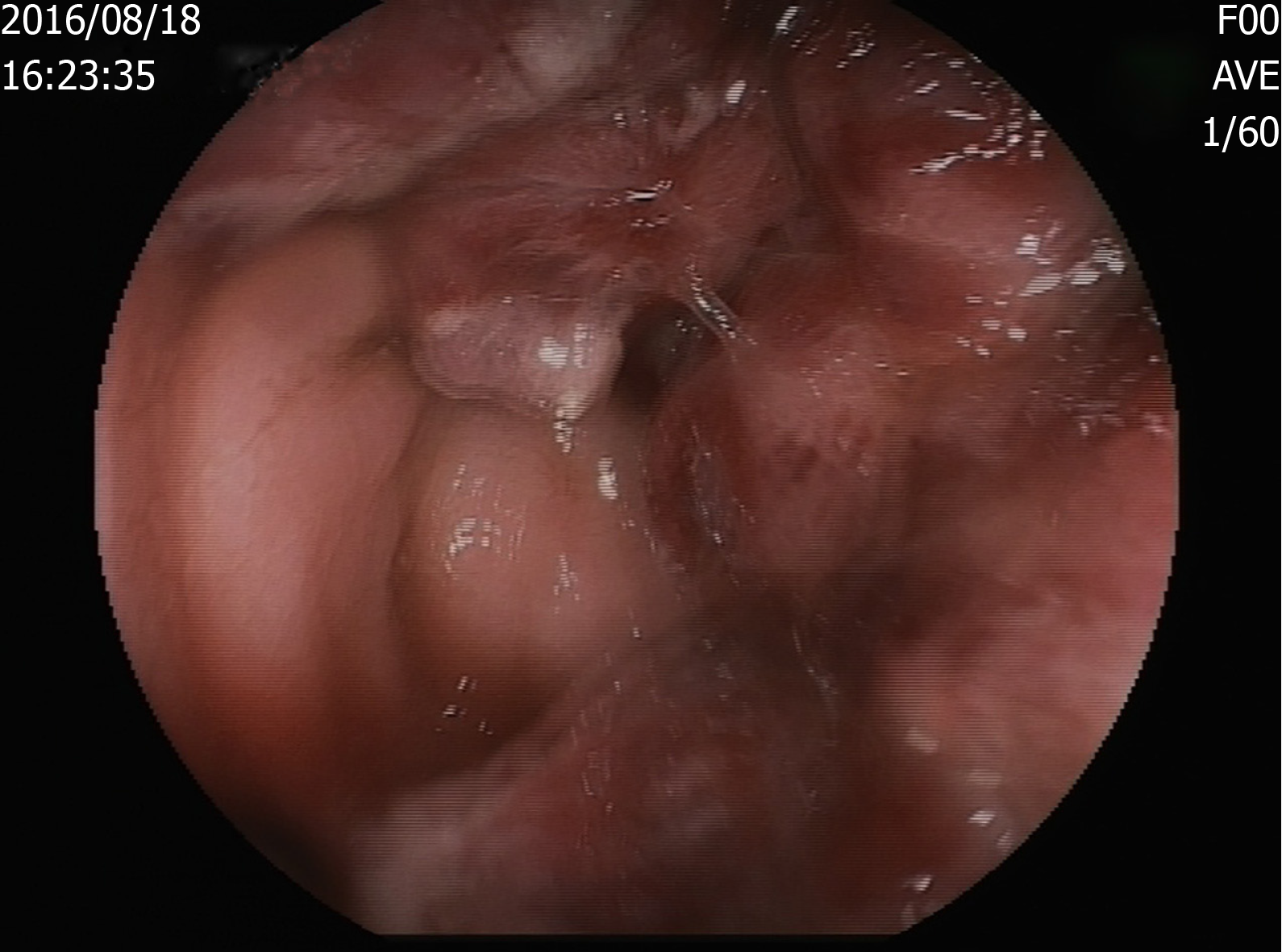
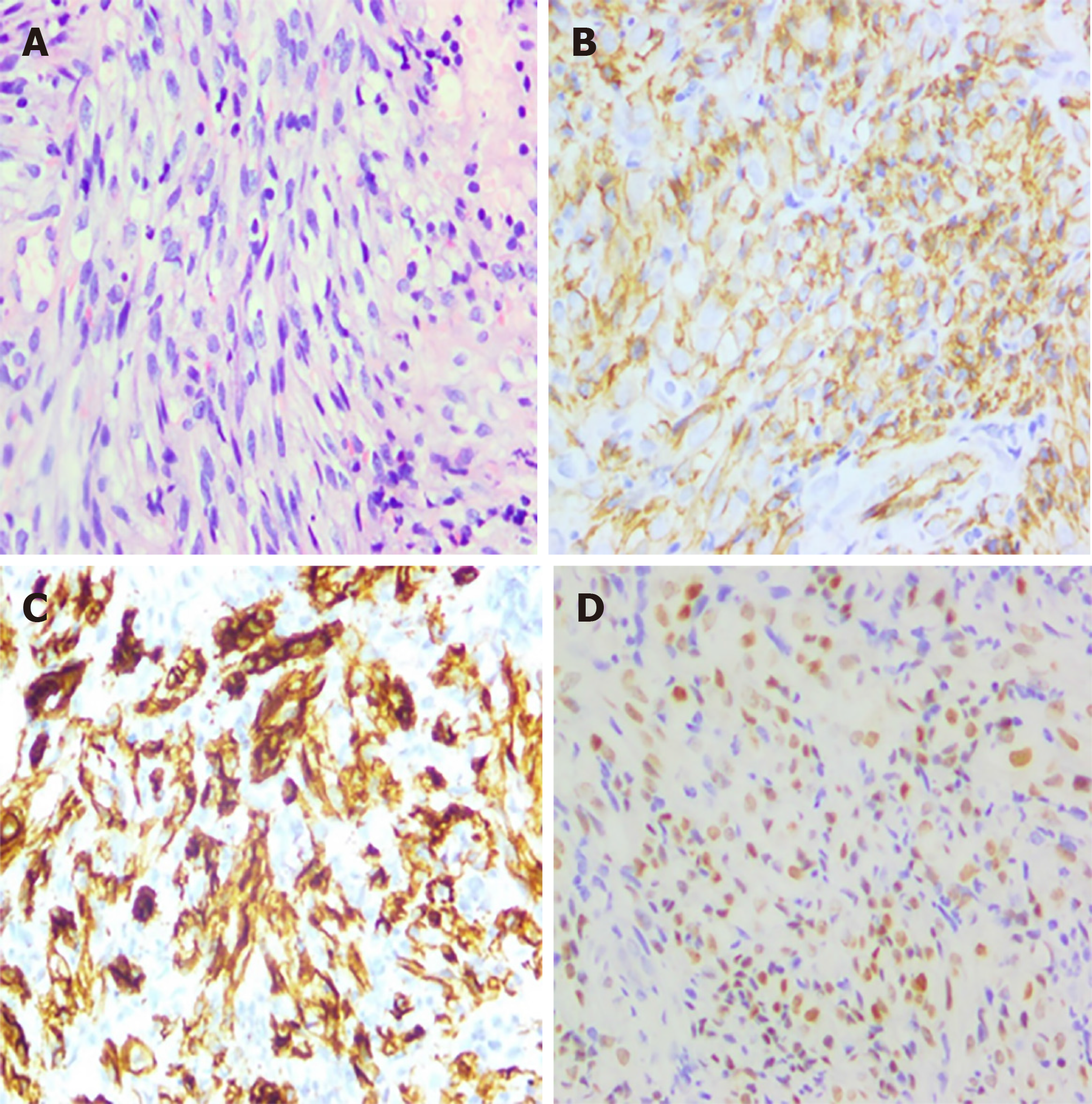
After half a month of treatment, the patient felt that his lower lumbus ache and bearing-down pain had not improved, and a colonoscopy was conducted. The result revealed a rectal mass (Figure 1) that was histologically confirmed as KS with rectal spindle cells that were positive for cluster of differentiation 117 (CD117), CD34, HHV8, CD31, and Ki-67 (30%) and negative for soluble protein-100, smooth muscle actin, and desmin (Figure 2).
Based on the pathological and immunohistochemistry results, when the patient was clinically stable, he was given highly active antiretroviral therapy (HAART) consisting of lamivudine, efavirenz, lopinavir, and ritonavir. He started systemic chemotherapy with 36 mg/d liposomal doxorubicin on August 26, 2016. At that time, his local bleeding was resolved, and he was discharged from the hospital. Then the patient was readmitted to the hospital for chemotherapy with liposomal doxorubicin at the same dosage on a monthly basis.
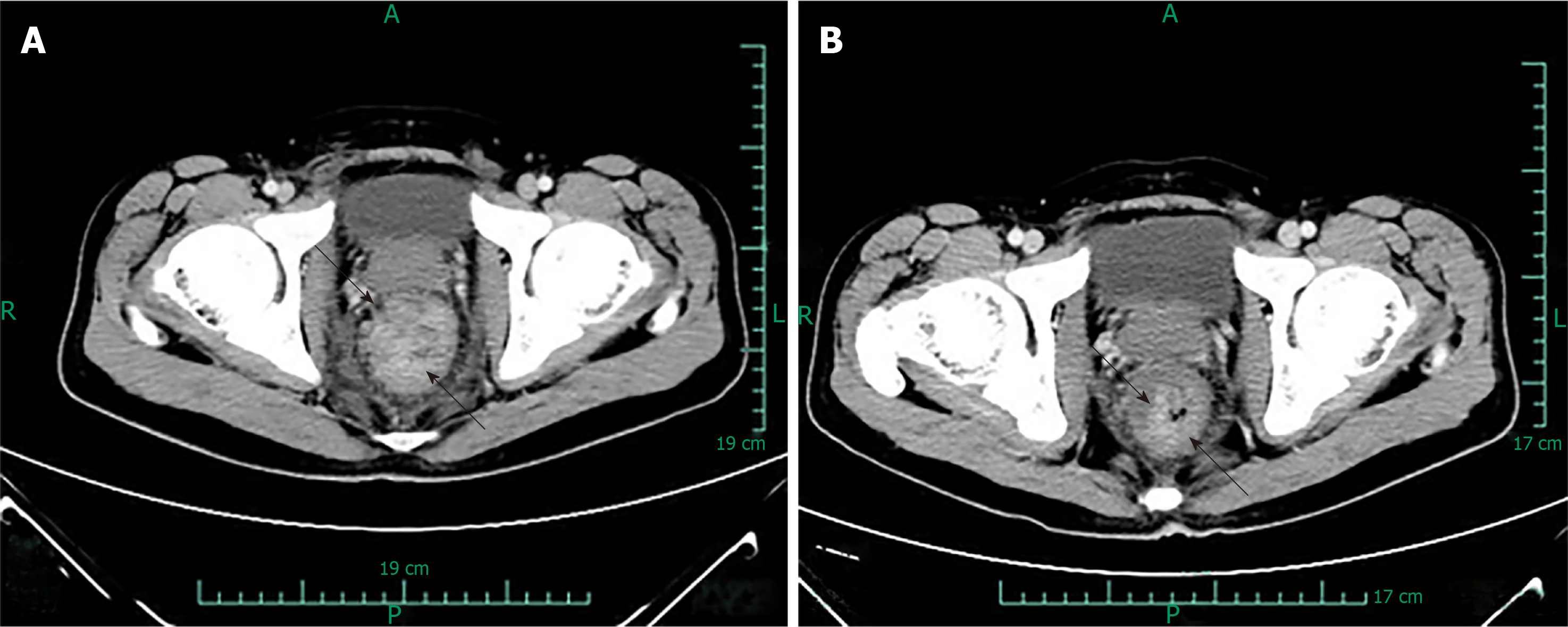
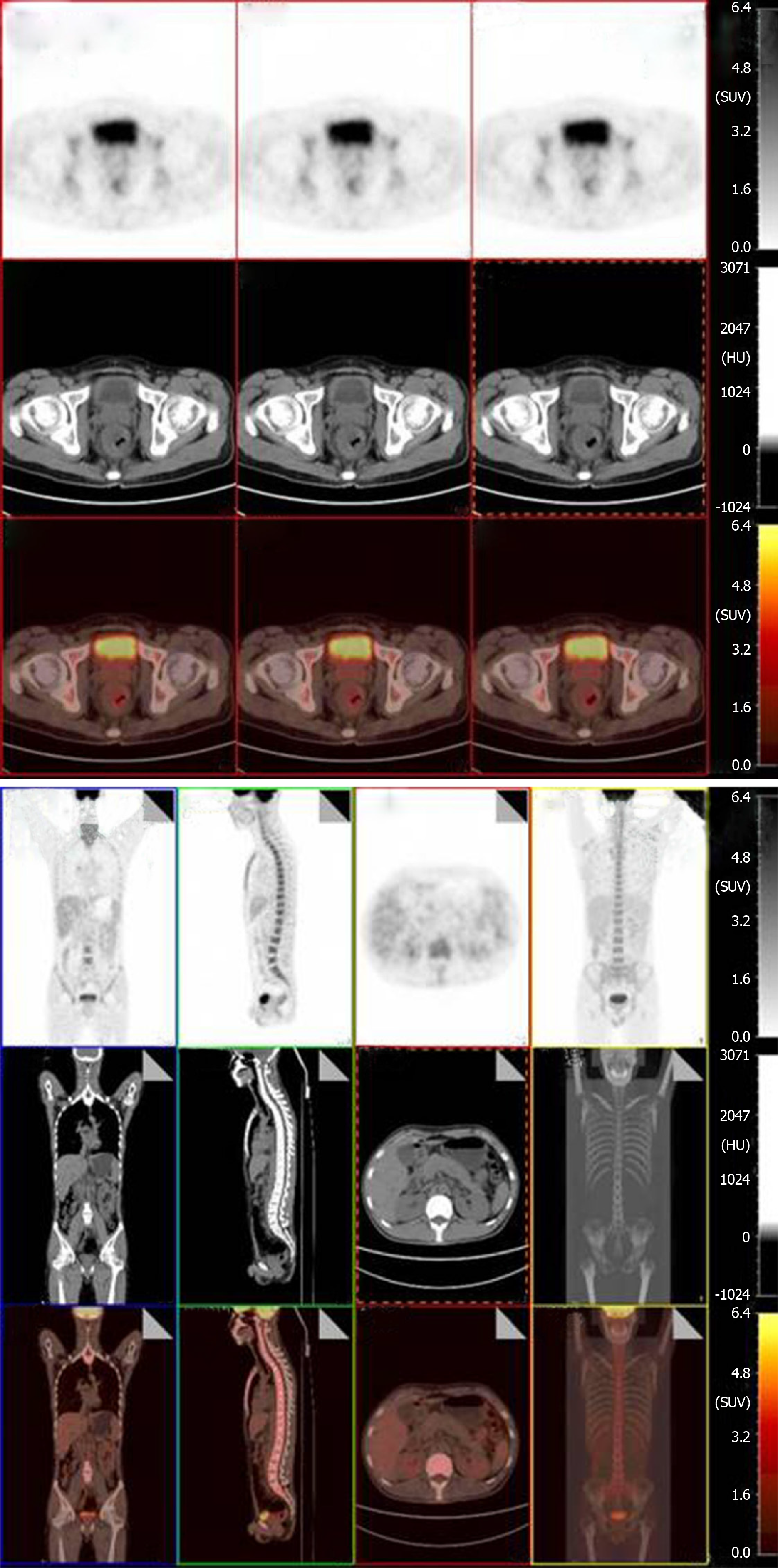
Prior to each chemotherapy treatment, he routinely underwent a CT scan (Figure 3). The results revealed that the focus had shrunk, indicating that the patient had palliated successfully. At his 6-mo follow-up visit, the patient’s viral load decreased to 7.39E + 01 copies/mL. He also underwent a positron emission tomography (PET) scan that showed great improvement in hypermetabolic activity in the rectum with mild residual activity (Figure 4). He completed six doses of liposomal doxorubicin and experienced no sign of lower GI bleeding again.
Currently, KS is one of the most common cancers in HIV-positive patients and leads to a high prevalence of morbidity and mortality. As previous reports have indicated, the incidence of KS has declined for more than 80%. It has been classified into four clinical variants: classic, endemic, HIV-related, and immunosuppression-related. Each type has unique characteristics[6]. The classic type, which is mainly presented as solitary cutaneous lesions on the lower extremities, affects elderly populations of Mediterranean or Eastern European descent. The endemic type shows familiar cutaneous manifestations that are more aggressive during the course of lymphadenopathy and has been found in South African children. The transplantation-related type often occurs in immunocompromised patients who receive immunosuppressive medications. HIV-related KS is the most common malignancy that affects HIV-infected patients[4]. As a previous retrospective study discussed, it is predominantly found among homosexual men with low CD4 cell counts (usually < 100 cells/µL) and a high viral load (> 10000 copies/mL)[1,4,7]. KS is a low-degree tumour consisting of multicentric vascular nodules. HHV8, also known as KSHV, is an aetiological agent of KS[1,8,9]. KS can occur in patients with or without HIV infection as well as in patients with or without normal CD4 cell counts[5].
KS usually appears as cutaneous or mucous lesions, but this does not indicate that KS in the viscera, which accounts for 3.5% of cases, can be excluded[10,11]. A majority of patients with visceral KS are asymptomatic and clinically silent[1]. As the disease advances, patients may progress from a normal condition to exhibiting severe symptoms including anaemia, abdominal pain, diarrhoea, dyspepsia, vomiting, and even complications that are rarely presented such as intestinal obstruction, perforation, and weight loss[12,13]. In this unusual case, the patient was hospitalised with GI bleeding, fever, and pain at the waist. The initial examination found hepatitis, cirrhosis, and septicaemia, which were consistent with the chief complaint. However, treatment for cirrhosis and septicaemia was not effective. At that time, other causes were considered, and further examinations such as colonoscopy were conducted. Pathologic diagnosis remains the gold diagnostic standard. The HIV-positive patient presented with lower GI bleeding without skin lesions.
Diagnosis of this type of primary KS without a cutaneous manifestation is a challenging problem for physicians. Imaging as a reliable, non-invasive method plays an important role in the diagnosis and prognosis of acquired immunodeficiency syndrome-related KS. Razek et al[14] showed that apparent diffusion coefficient of the spleen could act as a simple predictor of oesophageal varices in cirrhotic patients through diffusion-weighted magnetic resonance imaging. In our case, at the very beginning, abdominal CT showed us morphologic characteristics, location and number of lesions, as well as enhancement pattern, but it was not enough for a tissue-specific diagnosis. It also presented us decreased size of tumour in CT and great improvement in hypermetabolic activity in PET-CT after treatments of HAART and chemotherapy. PET-CT is generally used for differentiating between benign and malignant tumours, grading and staging of tumours, as well as evaluating the effects of treatments. Imaging techniques and findings help a lot in complementing to discriminate nature of tumours[15]. Nevertheless, the golden standard of diagnosis should rely on colonoscopy and biopsy findings[10]. The endoscopic appearance often features submucosal nodules with a polypoid formation and volcano-like mass lesions[10]. Its severity depends on its endoscopic appearance including the size, ulceration, and number of lesions. As previously reported, multiple lesions are more closely related to a high viral load (i.e. > 10000 copies/µL), while massive lesions are associated with low CD4 cell counts (i.e. < 100 cells/µL)[7]. The pathological results showed that proliferating spindle cells were positive for CD31, CD34, and latent nuclear antigen, suggestive of HHV8[9]. The differential diagnoses should exclude diseases such as leiomyomas, rhabdomyosarcomas, GI stromal tumours, cytomegalovirus infections and non-Hodgkin lymphomas that also occur in the GI tract of HIV-positive patients[10,16]. Among HIV-negative patients, angiosarcoma and GI stromal tumours have the same spindle-shaped cells as KS. The diagnosis should be based on a complete medical history and physical examination.
In addition to inhibiting virus production, HAART management also repairs the immune system, which may be a prerequisite for other treatments. Radiation, surgical excision, sclerotherapy, and liquid nitrogen cryotherapy are used in cases of limited lesions[4]. Therapies targeting KS, including ganciclovir and foscarnet, have been proven useful. Cytotoxic chemotherapy, including liposomal doxorubicin, can be used as a first-line treatment; paclitaxel or oral etoposide are second-line treatments. In one study, combined HAART and liposomal doxorubicin treatment outperformed HAART therapy alone[17]. Two clinical trials are currently investigating whether administering HAART and chemotherapy together or sequentially is the better mode of therapy[18]. Other treatments used for anti-angiogenesis-like vascular endothelial growth factor neutralizing antibody bevacizumab, tyrosine kinase inhibitor imatinib, and interferon-α are being formulated[10,19]. The prognosis of HIV-related KS depends on CD4 count and opportunistic infections. KS occurring in the viscera indicates a worse prognosis.
There have only been a few reports of KS manifested as lower GI bleeding in a HIV/HBV-co-infected liver cirrhosis patient. Prompt diagnosis and treatment are indispensable for symptomatic patients with GI bleeding. Any delay may worsen the condition, lower the quality of life, and increase the mortality and morbidity.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: EI-Razek AA S-Editor: Dou Y L-Editor: Filipodia E-Editor: Li X
| 1. | Yarchoan R, Uldrick TS. HIV-Associated Cancers and Related Diseases. N Engl J Med. 2018;378:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 2. | Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. 2013;23:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Scadden DT. AIDS-related malignancies. Annu Rev Med. 2003;54:285-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, Seeber S. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, Seeber S. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 2: pathogenesis, Castleman's disease, and pleural effusion lymphoma. Lancet Infect Dis. 2002;2:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Mansfield SA, Stawicki SP, Forbes RC, Papadimos TJ, Lindsey DE. Acute upper gastrointestinal bleeding secondary to Kaposi sarcoma as initial presentation of HIV infection. J Gastrointestin Liver Dis. 2013;22:441-445. [PubMed] |
| 7. | Nagata N, Shimbo T, Yazaki H, Asayama N, Akiyama J, Teruya K, Igari T, Ohmagari N, Oka S, Uemura N. Predictive clinical factors in the diagnosis of gastrointestinal Kaposi's sarcoma and its endoscopic severity. PLoS One. 2012;7:e46967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Lee AJ, Brenner L, Mourad B, Monteiro C, Vega KJ, Munoz JC. Gastrointestinal Kaposi's sarcoma: Case report and review of the literature. World J Gastrointest Pharmacol Ther. 2015;6:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Wang B, Song B, Oster C, Cao J, Raza A, Wang J. Coexistence of intestinal Kaposi sarcoma and plasmablastic lymphoma in an HIV/AIDS patient: case report and review of the literature. J Gastrointest Oncol. 2016;7:S88-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Nidimusili AJ, Eisa N, Shaheen K. Gastrointestinal Kaposi's Sarcoma Presenting as Ileocolic Intussusception. N Am J Med Sci. 2013;5:666-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Goncalves PH, Ziegelbauer J, Uldrick TS, Yarchoan R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Curr Opin HIV AIDS. 2017;12:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Akanbi O, Saleem N, Maddika S, Saba R. Kaposi sarcoma: an unusual cause of gastrointestinal bleeding. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Ling J, Coron R, Basak P, Jesmajian S. Recurrent lower gastrointestinal bleeding due to primary colonic Kaposi's sarcoma in a patient with AIDS. Int J STD AIDS. 2013;24:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging. 2015;40:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics. 2011;31:1923-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Chalasani N, Wilcox CM. Gastrointestinal hemorrhage in patients with AIDS. AIDS Patient Care STDS. 1999;13:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ferrer E, Santamariña E, Domingo P, Fumero E, Ribera E, Knobel H, Lopez JC, Barrios A, Podzamczer D. Nevirapine-containing regimens in HIV-infected naive patients with CD4 cell counts of 200 cells/microl or less. AIDS. 2004;18:1727-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest. 2016;126:3165-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Ayoola R, Guha A, Daram S. GI bleeding as the initial presentation of HIV/AIDS. Gastrointest Endosc. 2015;82:577; discussion 577-577; discussion 578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |