Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3074
Peer-review started: May 27, 2019
First decision: August 1, 2019
Revised: August 25, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 127 Days and 4 Hours
Alopecia areata is a hair loss disease associated with genetics, autoimmunity, and other factors. There is an intriguing link between alopecia areata and gut dysbiosis. Fecal microbiota transplantation (FMT) has been recommended to treat Clostridium difficile (previously known as Clostridioides difficile) infection, and has also shown potentials in the treatment of inflammatory bowel disease, irritable bowel syndrome, and non-alcohol fatty liver disease.
An 86-year-old man, with a history of sigmoid colon carcinoma, suffered from recurrent abdominal pain and distension, and diarrhea for six months, with inappetence. At admission, he was also diagnosed with depression. Upon physical examination, the patient presented with a 1.5 cm × 2.0 cm alopecia areata on his right occiput. Due to the negative results of laboratory testing, capsule endoscopy, and colonoscopy, the patient was diagnosed with noninfectious diarrhea, depressive disorder, and patchy alopecia areata. Considering that noninfectious diarrhea in the elderly patient was mainly caused by gut dysbiosis, he was given six rounds of FMT. His diarrhea improved remarkably one month after FMT, with improved appetite and disappearance of abdominal pain, distension, and depressive symptoms. Surprisingly, he reported new hair growth on the affected region of his scalp, with some of his white hair gradually turning to black, without taking any other therapies for alopecia areata before and after FMT.
FMT might act as a potential therapy for patients who suffer from alopecia areata. Large and well-designed studies are required to confirm the role of FMT in alopecia areata.
Core tip: Fecal microbiota transplantation (FMT) has been recommended to treat Clostridium difficile (previously known as Clostridioides difficile) infection, and has shown its potential role in the treatment of inflammatory bowel disease, irritable bowel syndrome, liver disease, and other disorders. This case report describes an elderly Chinese patient with alopecia areata who experienced restored hair growth and pigmentation after receiving FMT for his noninfectious diarrhea. Concurrently, the senile plaques on his face disappeared and his depressive symptoms improved.
- Citation: Xie WR, Yang XY, Xia HHX, Wu LH, He XX. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J Clin Cases 2019; 7(19): 3074-3081
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3074.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3074
Alopecia areata involves a chronic inflammation that impacts on hair follicles and results in hair loss, and its etiology is associated with genetics, autoimmunity, and environmental factors[1]. Recent studies show that there is a close link between alopecia areata and gut dysbiosis[2,3]. In recent years, fecal microbiota transplantation (FMT), a strategy to treat gut dysbiosis through restoring a healthy gut microbiome, has been strongly recommended to treat Clostridium difficile (previously known as Clostridioides difficile) infection (CDI) in clinical practice[4], and it has also been employed to treat patients with other gut dysbiosis-related diseases, such as inflammatory bowel disease (IBD, including ulcerative colitis and Crohn’s disease), irritable bowel syndrome (IBS), liver disease, and constipation[5-9]. Here we present a case of alopecia areata in whom hair regrew following FMT.
On April 5, 2017, an 86-year-old man, suffering from recurrent abdominal pain and distension, and diarrhea, was seen at the Outpatient Department of Gastroenterology, the First Affiliated Hospital of Guangdong Pharmaceutical University.
The patient’s symptoms had sustained for six months, with inappetence. His stool frequency was 4–5 times per day, without fever or bloody purulent stool. At admission, he was also diagnosed with depression and treated with Deanxit (flupentixol and melitracen) tablets.
The patient had a history of sigmoid colon carcinoma and underwent surgical operation and sigmoidostomy for sigmoid colon carcinoma 6 years ago.
The patient had a free personal and family history.
At admission, the patient’s temperature was 36.6 °C, heart rate was 78 bpm, respiratory rate was 20 breaths per minute, blood pressure was 123/72 mmHg, and body mass index (BMI) was 17.2 kg/m2. Upon physical examination, the patient presented with a 1.5 cm × 2.0 cm alopecia areata on his right occiput (no photo was taken). The abdomen was soft with no tenderness or rebound tenderness.
Serum albumin was decreased at 35 g/L (normal range 40–55 g/L). Blood routine examination, stool routine examination, and fecal occult blood test were all negative.
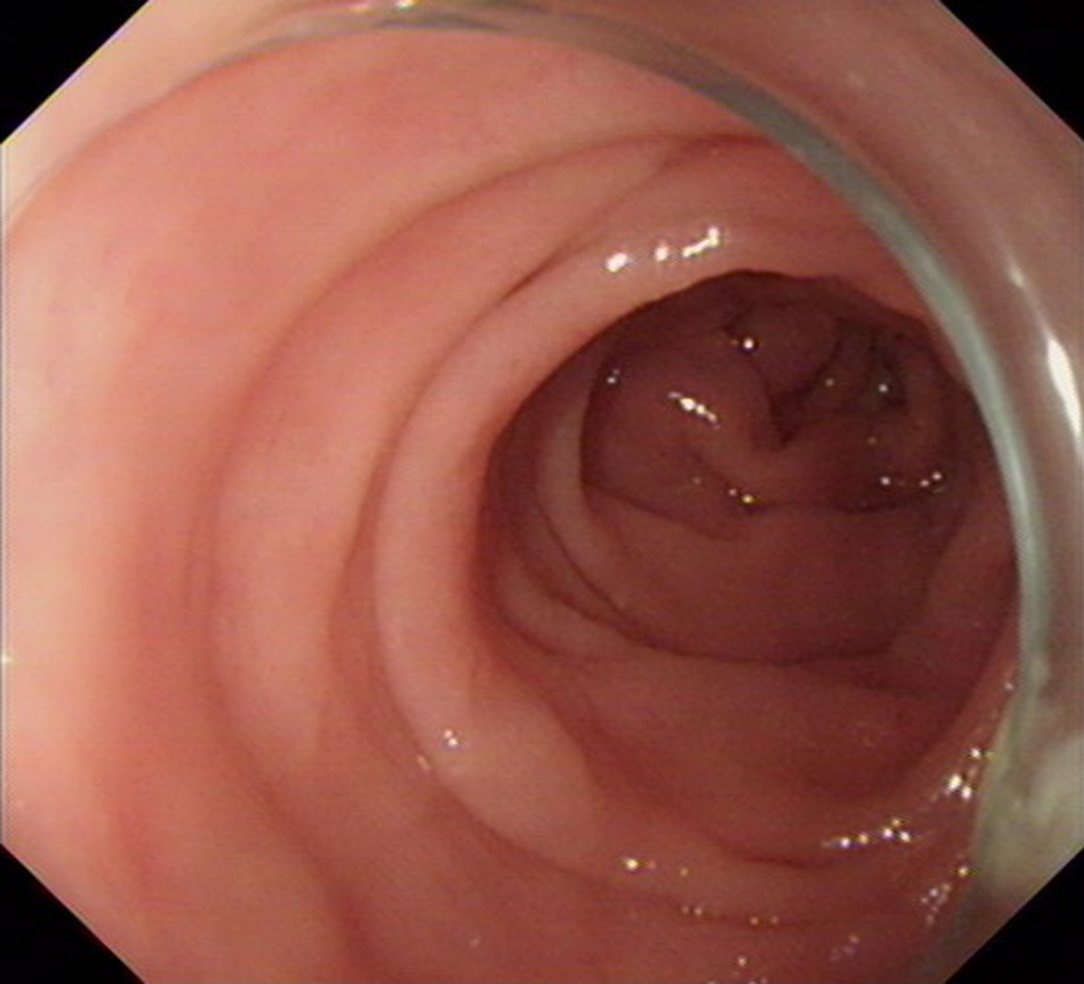
The normal mucosae of the stomach and small intestine were observed on capsule endoscopy. Colonoscopy was conducted, which showed that the mucosa of the colon appeared to be normal (Figure 1).
According to the above examinations, we excluded infectious diarrhea on the basis of the negative results of laboratory testing, capsule endoscopy, and colonoscopy. The patient was diagnosed with noninfectious diarrhea, depressive disorder, and patchy alopecia areata.
Due to the negative results of laboratory testing, capsule endoscopy, and colonoscopy, and no usage of antibiotic, we considered that noninfectious diarrhea in the elderly patient was mainly caused by gut dysbiosis[10-12], but not by diseases (including pancreatic exocrine insufficiency, bile acid malabsorption, gastrointestinal tumor, and Crohn’s disease) and antibiotic-associated colitis. Thus, the patient was given six rounds of FMT for noninfectious diarrhea on April 10, April 12, April 14, May 17, May 19, and May 22, 2017, respectively. The stool for FMT was obtained from a 22-year-old healthy male donor, in whom we conducted the routine screening for potential pathogens according to the selection criteria formulated by Zhang’s group[13,14]. The fresh fecal microbiota suspension, prepared with the automatic purification system (GenFMTer; FMT Medical, Nanjing, China), was administered through the lower digestive tract with a colonoscope, following the laboratory protocol formulated by Zhang’s group[13].
During the 18-mo follow-up after six rounds of FMT, with the last follow-up visit on November 22, 2018, his stool frequency was reduced to 1-2 times per day, with the improved appetite and no abdominal pain or distension. Specifically, his diarrhea improved remarkably one month after FMT. His depressive symptoms were also improved; his score on the Hamilton Depression Scale (HAM-D17)[15] was reduced from 30 points at administration to 13 points at the last visit. His BMI rose to 18.3 kg/m2, and serum albumin increased slightly to 38 g/L.
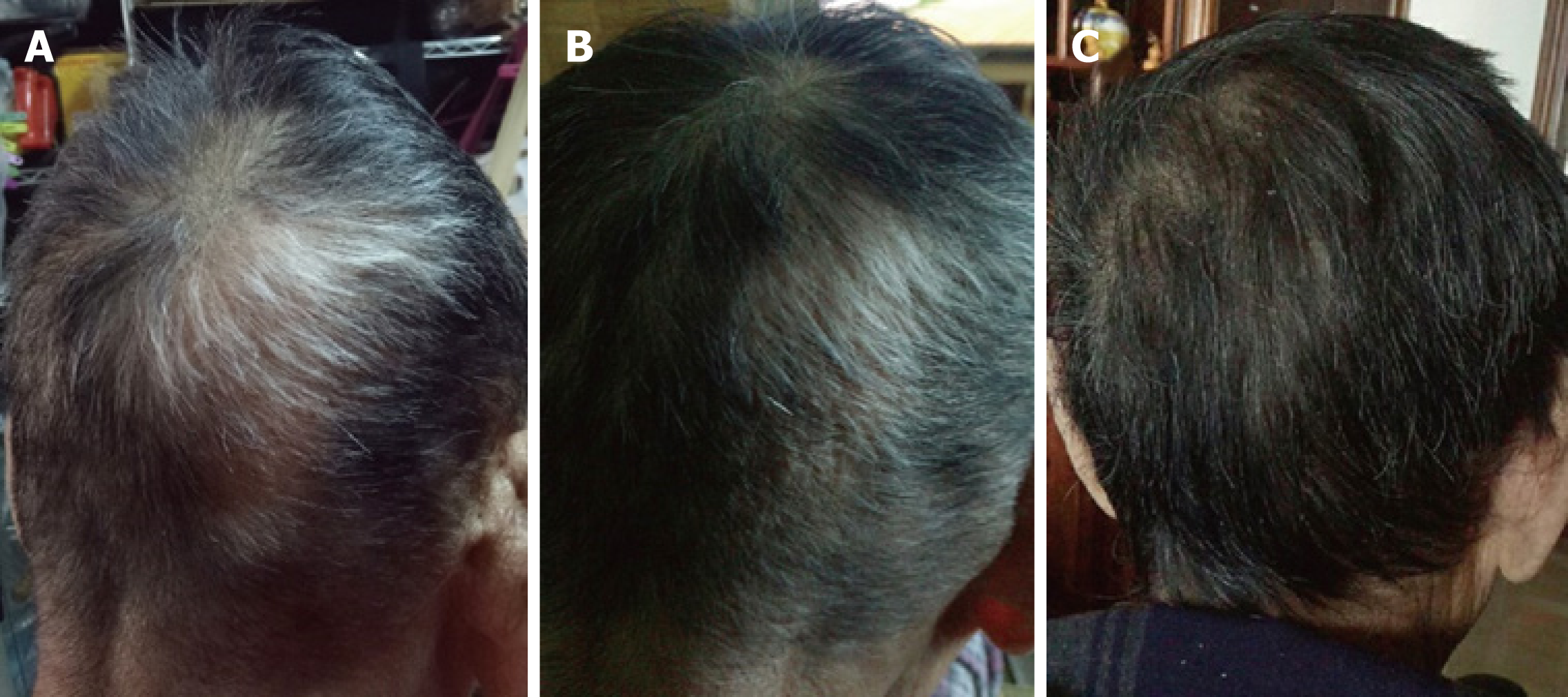
Surprisingly, at the follow-up 4 wk after FMT, he reported new hair growth on the affected region of his scalp, with some of his white hair gradually turning to black, without taking any other therapies for alopecia areata before and after FMT. His hair growth maintained at the last visit (Figure 2). In addition, the senile plaques on his face disappeared at the same time (no photo was taken).
We present the case of an elderly Chinese patient who experienced new hair growth on the alopecia areata-affected regions of his scalp, with restoration of hair pigmentation, after receiving FMT, which was indicated for noninfectious diarrhea. Furthermore, his diarrhea and depressive symptoms improved and the senile plaques on his face disappeared at the same time. We speculate that all of these changes are related to altered gut microbiota after FMT.
Gut dysbiosis plays a critical role in infectious diarrhea[16,17]. FMT has been performed to treat Clostridium difficile-associated diarrhea, which was more effective than vancomycin or placebo treatment[18]. Beyond infectious diarrhea, gut dysbiosis also plays an important role in noninfectious diarrhea[10-12]. A double-blind, random-ized, placebo-controlled, parallel-group, single-center trial revealed that symptoms of IBS, including diarrhea, got improved significantly after receiving FMT[7]. In our FMT trial center at the First Affiliated Hospital of Guangdong Pharmaceutical University, we have treated about 40 patients with diarrhea using FMT, showing significant symptom improvement (data not published). In this case, we conducted FMT in the elderly patient for his noninfectious diarrhea. During the treatment, we observed hair regrowth on the alopecia areata-affected regions of his scalp surprisingly.
Clinical patterns of alopecia areata comprise patchy alopecia areata, alopecia totalis, and alopecia universalis[19]. The following factors contribute to the pathogenesis of alopecia areata: (1) Genetics: 62 genes have been identified to be involved in the pathogenesis of alopecia areata[19]; (2) Immune response: The loss of the immune privilege with the subsequent attack of autoreactive infiltrates on the hair follicle is considered to be the predominant cause of alopecia areata[1,19,20]; and (3) Other factors: Oxidative stress and infectious agents could lead to breakdown of the immune privilege and initiation of alopecia areata[19,20]. In addition, psychological stress may act as an aggravating factor in initiation and progression of alopecia areata, although this is controversial[20-23].
Several treatments do result in hair growth in patients with alopecia areata. First, application of local corticosteroids shows a positive curative effect in patchy alopecia areata. But this treatment cannot inhibit the development of alopecia areata at other sites[1,19]. Second, patients with extensive and rapidly progressive alopecia areata got benefits from application of systemic corticosteroids[19,24,25]. However, continued application is required to maintain hair growth in most cases[19]. Third, contact immunotherapy is effective for patients with patchy alopecia areata, not for those with alopecia totalis and alopecia universalis[19]. But a high relapse rate (62%) of this management becomes a disturbing issue[19]. Therefore, none of the treatments mentioned above has been ratified by the US FDA, indicating that a new and more effective therapeutic intervention aiming at new targets is needed.
Recently, several studies demonstrated that gut dysbiosis plays critical roles in the onset of skin diseases, including atopic dermatitis and psoriasis[26-29]. However, the association between alopecia areata and gut dysbiosis awaits to be elucidated. Alopecia areata may link with other autoimmune diseases, especially with IBD[30]. A series of clinical cases reported that hair loss was found in patients with IBD[31-34], with little knowledge of its causes. Since gut dysbiosis is one of the major pathogenesis of IBD[35-37], gut dysbiosis may act as a common pathway coexisting with alopecia areata and IBD. Biotin (vitamin B7), a water-soluble vitamin, has the heavy reliance on bacterial production in guts. Hair loss is one of the symptoms of biotin deficiency[38], which is induced by a variety of factors, including IBD[39]. Lately recently, Hayashi et al[2] reported that alopecia was developed in biotin-deficient germ-free mice, with overgrowth of Lactobacillus murinus. The alopecia mice showed hair regrowth after supplementation of biotin, with reduction of Lactobacillus murinus, indicating that alopecia was caused mainly by gut dysbiosis, particularly overgrowth of Lactobacillus murinus, which consequently led to biotin deficiency[2]. Thus, it is not implausible that there exists a close link between gut dysbiosis and alopecia areata.
Short-chain fatty acids (SCFAs), produced by gut microbiota, contribute to maintaining immunological homeostasis[40], via modulating the numbers and function of regulatory T cells (Tregs), which play critical role in the induction of alopecia areata[3,30]. Borde et al[3] hypothesized that propionate, one of SCFAs, would induce more tolerogenic Tregs through stimulating G protein-coupled receptors to protect the hair follicles against the immunological attack. They observed that hair regrowth in five out of five C3H/He J mice (a kind of mouse developing alopecia areata spontaneously) after 11 wk of propionate treatment, with an increased Treg/CD4+ ratio. Unfortunately, they failed to reproduce the positive results of hair regrowth when they repeated the study. Due to the vast varieties of gut microbiota, it is insufficient to restore the normal gut microbiota merely through supplying one of SCFAs, which leads to the inconclusive results. Nevertheless, the intriguing link between gut dysbiosis and alopecia areata does exist, and restoring a healthy gut, instead of supplying one to several kinds of SCFAs, will perform more effectively in treating alopecia areata.
Vitamin D, one of the micronutrients in our daily diet, is well-known for the effect of maintaining the normal levels of calcium and phosphorus in the blood[41]. Vitamin D exerts its biological functions through conversion into its active form of 1,25(OH)2D3 by the activating enzyme Cyp27B1[42]. 1,25(OH)2D3 binds to vitamin D receptor (VDR) to possess biological activities[43]. Growing evidence demonstrates that 1,25 (OH)2D3 deficiency is associated with alopecia areata[44-46], in which VDR deficiency plays a vital role[47-49]. On one hand, a recent study indicated that 1,25(OH)2D3 deficiency induced gut dysbiosis, leading to a reduction in SCFAs production[50]. On the other hand, several studies have shown that the expression of VDR and Cyp27B1 is regulated by gut microbiota[51-53]. Thus, it is unclear which takes the main responsi-bility for the onset of alopecia areata, although it is likely that there exists a bidirectional relationship between 1,25(OH)2D3 and gut microbiota and between 1,25(OH)2D3 deficiency and gut dysbiosis [43].
FMT is considered a safe and effective method to restore a healthy gut microbial environment, and displays astonishing clinical efficacy for recurrent CDI, IBD, IBS, liver disease, and other disorders[54-57]. The rationale for FMT restoring health is still unclear. Ooijevaar et al[58] proposed that there could be two pathways, the direct and indirect pathways. In the direct pathway, some beneficial bacteria and nutrients are transferred directly with FMT to compete with the pathogenic bacteria and replenish the missing nutrients, the process of which is unrelated to the host. In the indirect pathway, host-related factors, including immunomodulation and mechanical barrier function, are involved.
This widespread efficacy of FMT provides a clue that FMT might also serve as a potential therapy for alopecia areata via the restoration of gut microbiota balance[3,30]. In 2017, Rebello et al[59] reported that two patients with alopecia universalis experienced hair regrowth after FMT used for recurrent CDI. Consistent with these two cases, the elderly patient with patchy alopecia areata in this case demonstrated long-term hair growth after FMT for noninfectious diarrhea (Table 1). These observations suggest that FMT contributes to hair regrowth by replenishing the gut microbiota. Restoration of a healthy gut microbial environment might lead to improvement of the absorption and synthesis of nutrients, including amino acid/proteins, biotin, SCFAs, and vitamin D, by immunomodulation, which ultimately results in hair regrowth. In this case, the elderly patient got his nutritional status improved after FMT, as his BMI and serum albumin increased slightly. Unfortunately, we could not provide the levels of biotin and vitamin D of this patient before and after FMT; acquisition of this information might help to elucidate the mechanism by which FMT results in hair regrowth.
| Reference | Age | Sex | Disease | Pattern of alopecia | Previous therapies for alopecia | Other therapies for alopecia during FMT | Hair regrowth after FMT |
| Rebello et al[59], 2017 | 38 | Male | CDI | Alopecia universalis | Steroid injection | No | 8 wk |
| Rebello et al[59], 2017 | 20 | Male | CDI | Alopecia universalis | Intralesional corticosteroid injections | 2 additional intralesional corticosteroid injections after FMT | A few months |
| 86 | Male | Noninfectious diarrhea | Alopecia areata | No | No | 4 wk |
In addition to hair regrowth, the elderly patient we report here reported that his hair color returned and the senile plaques on his face disappeared. The pathogenesis of canities has not been completely understood yet. Certain organ-specific autoimmune disorders may contribute to the process of canities[60], which gives us a hint that the gut microbiota may be associated with the process of canities. Hence, our elderly patient could get his hair color returned after restoration of the balance of the gut microbial environment.
As the gut microbiota acts as a direct mediator of psychopathology through the gut–brain axis[61,62], the patient’s depressive symptoms were also improved after FMT.
In conclusion, despite the astonishing therapeutic effect described in this report, as well as in the report by Rebello et al[59], further investigations on the role of the gut microbiota in alopecia areata through large and well-designed clinical trials are required to support the clinical application of FMT as a treatment option for this distressing disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abadi TAB, Gonzalez F S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Trüeb RM, Dias MFRG. Alopecia Areata: a Comprehensive Review of Pathogenesis and Management. Clin Rev Allergy Immunol. 2018;54:68-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Hayashi A, Mikami Y, Miyamoto K, Kamada N, Sato T, Mizuno S, Naganuma M, Teratani T, Aoki R, Fukuda S, Suda W, Hattori M, Amagai M, Ohyama M, Kanai T. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. 2017;20:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Borde A, Åstrand A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert Opin Ther Targets. 2018;22:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 785] [Article Influence: 98.1] [Reference Citation Analysis (1)] |
| 5. | Millan B, Laffin M, Madsen K. Fecal Microbiota Transplantation: Beyond Clostridium difficile. Curr Infect Dis Rep. 2017;19:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, Petersen AM. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 7. | Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, Goll R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 9. | Tian H, Ding C, Gong J, Ge X, McFarland LV, Gu L, Wei Y, Chen Q, Zhu W, Li J, Li N. Treatment of Slow Transit Constipation With Fecal Microbiota Transplantation: A Pilot Study. J Clin Gastroenterol. 2016;50:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Lee JR, Magruder M, Zhang L, Westblade LF, Satlin MJ, Robertson A, Edusei E, Crawford C, Ling L, Taur Y, Schluter J, Lubetzky M, Dadhania D, Pamer E, Suthanthiran M. Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am J Transplant. 2019;19:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Logan C, Beadsworth MB, Beeching NJ. HIV and diarrhoea: what is new? Curr Opin Infect Dis. 2016;29:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R, Abreu MT, Koren O, Kashi Y, Chowers Y. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018;67:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 13. | Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z, Xu H, Xiang J, He Z, Zhang T, Nie Y, Wu K, Fan D, Ji G, Zhang F. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, Huang G, Liu Z, Wu P, Fan Z, Ji G, Wang X, Wu K, Fan D, Zhang F. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 15. | Bech P, Kastrup M, Rafaelsen OJ. Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl. 1986;326:1-37. [PubMed] |
| 16. | Sarker SA, Ahmed T, Brüssow H. Persistent diarrhea: a persistent infection with enteropathogens or a gut commensal dysbiosis? Environ Microbiol. 2017;19:3789-3801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Battaglioli EJ, Hale VL, Chen J, Jeraldo P, Ruiz-Mojica C, Schmidt BA, Rekdal VM, Till LM, Huq L, Smits SA, Moor WJ, Jones-Hall Y, Smyrk T, Khanna S, Pardi DS, Grover M, Patel R, Chia N, Nelson H, Sonnenburg JL, Farrugia G, Kashyap PC. Clostridioides difficile uses amino acids associated with gut microbial dysbiosis in a subset of patients with diarrhea. Sci Transl Med. 2018;10:eaam7019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 18. | Moayyedi P, Yuan Y, Baharith H, Ford AC. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust. 2017;207:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 20. | Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: A multifactorial autoimmune condition. J Autoimmun. 2019;98:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and relationship with stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Picardi A, Pasquini P, Cattaruzza MS, Gaetano P, Baliva G, Melchi CF, Papi M, Camaioni D, Tiago A, Gobello T, Biondi M. Psychosomatic factors in first-onset alopecia areata. Psychosomatics. 2003;44:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Nakajima T, Inui S, Itami S. Pulse corticosteroid therapy for alopecia areata: study of 139 patients. Dermatology. 2007;215:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Yang CC, Lee CT, Hsu CK, Lee YP, Wong TW, Chao SC, Lee JY, Sheu HM, Chen W. Early intervention with high-dose steroid pulse therapy prolongs disease-free interval of severe alopecia areata: a retrospective study. Ann Dermatol. 2013;25:471-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 301] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, Manasson J, Pamer EG, Littman DR, Abramson SB. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 573] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 28. | Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 29. | Visser MJE, Kell DB, Pretorius E. Bacterial Dysbiosis and Translocation in Psoriasis Vulgaris. Front Cell Infect Microbiol. 2019;9:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Skogberg G, Jackson S, Åstrand A. Mechanisms of tolerance and potential therapeutic interventions in Alopecia Areata. Pharmacol Ther. 2017;179:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Safina DD, Abdulkhakov RA, Abdulkhakov SR, Odintsova AKh, Cheremina NA. [Clinical case of a combination of ulcerative colitis and alopecia areata]. Eksp Klin Gastroenterol. 2013;92-96. [PubMed] |
| 32. | Treem WR, Veligati LN, Rotter JI, Targan SR, Hyams JS. Ulcerative colitis and total alopecia in a mother and her son. Gastroenterology. 1993;104:1187-1191. [PubMed] |
| 33. | Patel KV, Farrant P, Sanderson JD, Irving PM. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Sobolewska-Włodarczyk A, Włodarczyk M, Fichna J, Wiśniewska-Jarosińska M. Alopecia areata in patients with inflammatory bowel disease: an overview. Folia Med Cracov. 2016;56:5-12. [PubMed] |
| 35. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 646] [Article Influence: 107.7] [Reference Citation Analysis (2)] |
| 36. | Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152:327-339.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 611] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 37. | Rapozo DC, Bernardazzi C, de Souza HS. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J Gastroenterol. 2017;23:2124-2140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (3)] |
| 38. | Zempleni J, Hassan YI, Wijeratne SS. Biotin and biotinidase deficiency. Expert Rev Endocrinol Metab. 2008;3:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748. [PubMed] |
| 40. | Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 41. | Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone. 2013;54:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 860] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Singh P, Kumar M, Al Khodor S. Vitamin D Deficiency in the Gulf Cooperation Council: Exploring the Triad of Genetic Predisposition, the Gut Microbiome and the Immune System. Front Immunol. 2019;10:1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Thompson JM, Mirza MA, Park MK, Qureshi AA, Cho E. The Role of Micronutrients in Alopecia Areata: A Review. Am J Clin Dermatol. 2017;18:663-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;78:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Chen CH, Sakai Y, Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology. 2001;142:5386-5389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Fawzi MM, Mahmoud SB, Ahmed SF, Shaker OG. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J Cosmet Dermatol. 2016;15:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Zhu W, Yan J, Zhi C, Zhou Q, Yuan X. 1,25(OH) 2D3 deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. 2019;11:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. 2009;1173:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004-G1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 54. | Hvas CL, Dahl Jørgensen SM, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, Erikstrup C, Dahlerup JF. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology. 2019;156:1324-1332.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 55. | Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer. 2019;145:2021-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 56. | Borody T, Fischer M, Mitchell S, Campbell J. Fecal microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev Gastroenterol Hepatol. 2015;9:1379-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Xie WR, Yang XX, Xia HHX, He XX. Fecal microbiota transplantation for treating hepatic encephalopathy: Experimental and clinical evidence and possible underlying mechanisms. J Explor Res Pharmacol. 2018;3:119-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med. 2019;70:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 59. | Rebello D, Wang E, Yen E, Lio PA, Kelly CR. Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG Case Rep J. 2017;4:e107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol. 2013;79:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Kuty-Pachecka M. Psychological and psychopathological factors in alopecia areata. Psychiatr Pol. 2015;49:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Groen RN, de Clercq NC, Nieuwdorp M, Hoenders HJR, Groen AK. Gut microbiota, metabolism and psychopathology: A critical review and novel perspectives. Crit Rev Clin Lab Sci. 2018;55:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |