Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3062
Peer-review started: May 10, 2019
First decision: August 1, 2019
Revised: September 5, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 145 Days and 11.6 Hours
Acute myocardial infarction (AMI) is characterized by chest pain as well as cardiac troponin I (cTnI) and electrocardiography (ECG) changes. Recently, clinical researchers have used the term “MINOCA” to indicate myocardial infarction with nonobstructive coronary arteries. To the best of our knowledge, no report has documented MINOCA in a young patient after choledocholithiasis by endoscopic retrograde cholangiopancreatography (ERCP).
An 18-year-old Chinese man presented to the cardiac intensive care unit with chest pain radiating to the left shoulder for 1 h after choledocholithiasis by ERCP and the following treatment. ECG showed a sinus rhythm with ST-segment elevation in the II, III, and aVF leads compared with the baseline. Laboratory data revealed cTnI levels of 67.55 ng/mL and 80 ng/mL at the peak (relative index below 0.034 ng/mL) and creatine kinase-MB levels of 56 U/L and 543 U/L at the peak (relative index below 24 U/L). AMI was suspected, and coronary angiography was performed the second day. The results revealed a smooth angiographic appearance of all arteries. The patient had been diagnosed with gallstones and cholecystitis for four years but had not accepted treatment. He had abdominal pain and bloating a week previously and underwent ERCP and subsequent treatments on the second day of admission; 1.4 cm × 1.6 cm of stones were removed from his common bile duct during surgery. The results of his laboratory tests at admission revealed abnormal alanine aminotransferase, aspartate aminotransferase, glutamyl transpeptidase, total bile acid, total bilirubin, direct bilirubin, and indirect bilirubin levels. His temperature, heart rate, blood pressure, and body mass index were normal. His echocardiographic examination revealed no obvious abnormalities in the structure and movement of the ventricular wall and an estimated left ventricular ejection fraction of 57% after the heart attack. His cholesterol and triglycerides were within normal ranges, and his low-density lipoprotein cholesterol was 2.23 mmol/L (normal range 2.03-3.34 mmol). Further testing after AMI revealed nothing remarkable in his erythrocyte sedimentation rate, thyroid function, and tumour markers.
We ultimately made a diagnosis of MINOCA caused by coronary artery spasm, which seemed to be the most suitable diagnosis of this young patient. We are concerned that the heart attack may have been induced by the ERCP rather than occurred coincidentally afterward, so we should investigate the timing of the event further. Additional studies are needed to unravel the underlying pathophysiology.
Core tip: This is a case report about a young man who were ultimately diagnosed with myocardial infarction with nonobstructive coronary arteries, but initially diagnosed with acute myocardial infarction (AMI). AMI is characterized by chest pain as well as cardiac troponin I and electrocardiography changes. In recent years, clinical researchers have used “MINOCA” to characterize myocardial infarction with non-obstructive coronary arteries. As far as we know, no report has documented this type in a young patient after choledocholithiasis by endoscopic retrograde cholangiopancreatography.
- Citation: Li D, Li Y, Wang X, Wu Y, Cui XY, Hu JQ, Li B, Lin Q. Diagnosis of myocardial infarction with nonobstructive coronary arteries in a young man in the setting of acute myocardial infarction after endoscopic retrograde cholangiopancreatography: A case report. World J Clin Cases 2019; 7(19): 3062-3068
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3062.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3062
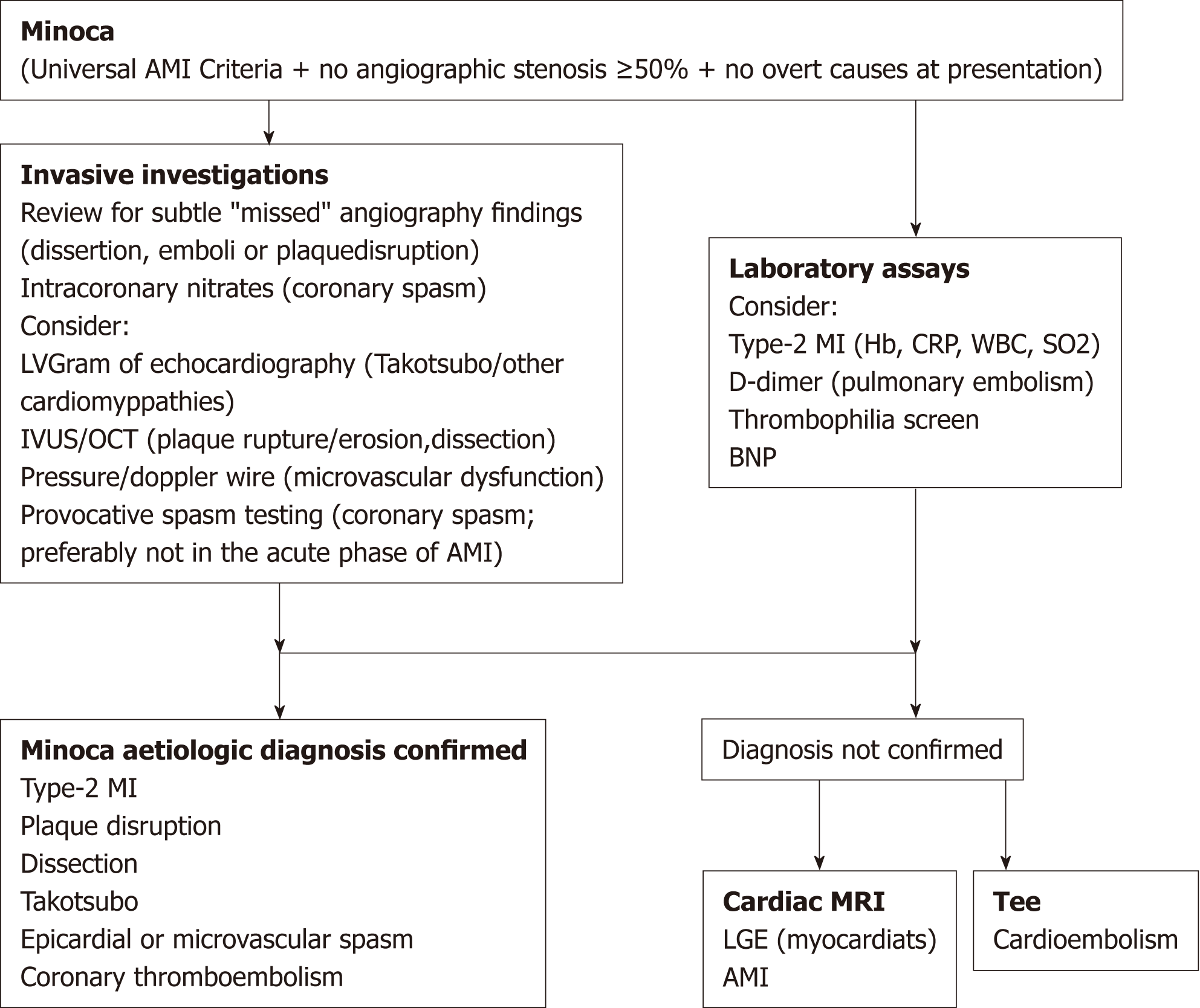
Acute myocardial infarction (AMI) is characterized by chest pain as well as cardiac troponin I (cTnI) and electrocardiography (ECG) changes. Recently, clinical researchers have used the term “MINOCA” to refer to myocardial infarction with nonobstructive coronary arteries. This condition has been confirmed in several large AMI registries in which 1%-13% of AMI cases occurred in the absence of obstructive coronary artery disease (CAD), prompting clinical researchers to coin the term MINOCA. The European Society of Cardiology working group on MINOCA has produced a position paper describing the associated clinical features and mechanisms, detailing an assessment pathway for its evaluation and stimulating research into its mechanisms and treatment[1] (Figure 1).
MINOCA has various causes. In this report, we present the case of a young patient who was first diagnosed with AMI after choledocholithiasis by endoscopic retrograde cholangiopancreatography (ERCP).
An 18-year-old Chinese man presented to the cardiac intensive care unit with chest pain radiating to the left shoulder for 1 h after choledocholithiasis by ERCP and the subsequent treatment.
The patient had abdominal pain and bloating accompanied by nausea a week prior to his admission to the hospital.
The patient had been diagnosed with gallstones and cholecystitis for four years but had not accepted treatment.
The young man reported no history of smoking, alcohol consumption, or use of medications. There was no personal or family history of CAD, hypertension, or obesity.
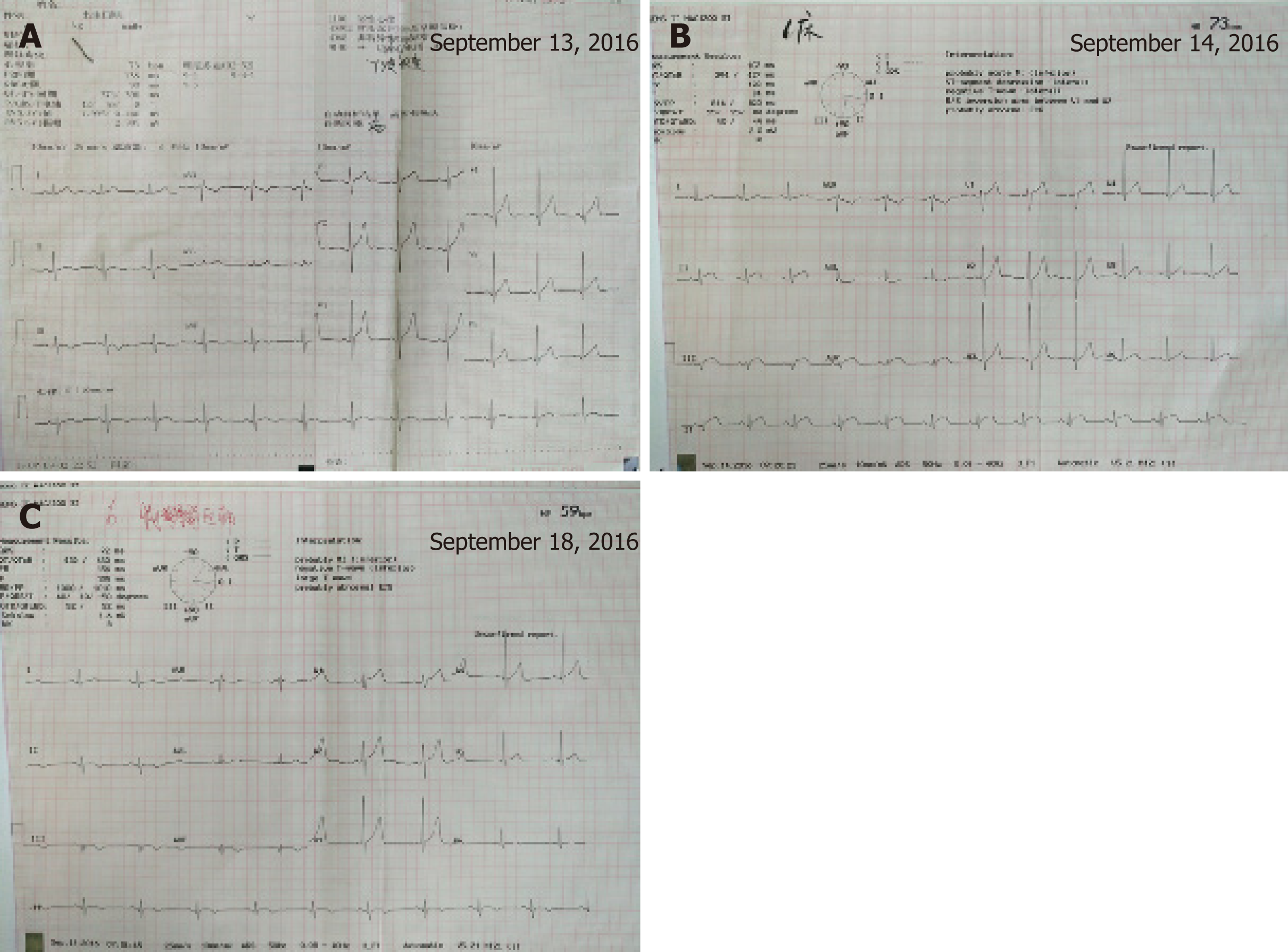
His temperature, heart rate, blood pressure, and body mass index were normal. His blood pressure was 130/70 mmHg symmetrically in both arms, and his pulse was 60 beats per minute. His physical examination was normal. ECG showed a sinus rhythm with ST-segment elevation in the II, III, and aVF leads compared with the baseline (Figure 2).
The laboratory data showed cTnI levels of 67.55 ng/mL and 80 ng/mL at the peak (relative index below 0.034 ng/mL) and creatine kinase-MB (CK-MB) levels of 56 U/L and 183.6 U/L at the peak (relative index below 24 U/L). The results of his laboratory tests at baseline, including routine blood and urine tests, blood lipid and glucose levels, renal function tests, coagulation factors, and electrolytes, were within the normal ranges. His cholesterol and triglyceride levels were within the normal ranges, and his low-density lipoprotein cholesterol level was 2.23 mmol/L (normal range 2.03-3.34 mmol). Further tests after the AMI revealed nothing remarkable in the erythrocyte sedimentation rate, thyroid function, or tumour markers.
The chest X-ray and abdominal and reproductive organ ultrasound examinations were normal. His echocardiographic examination showed no obvious abnormalities in the structure or movement of the ventricular wall and an estimated left ventricular ejection fraction of 57%.
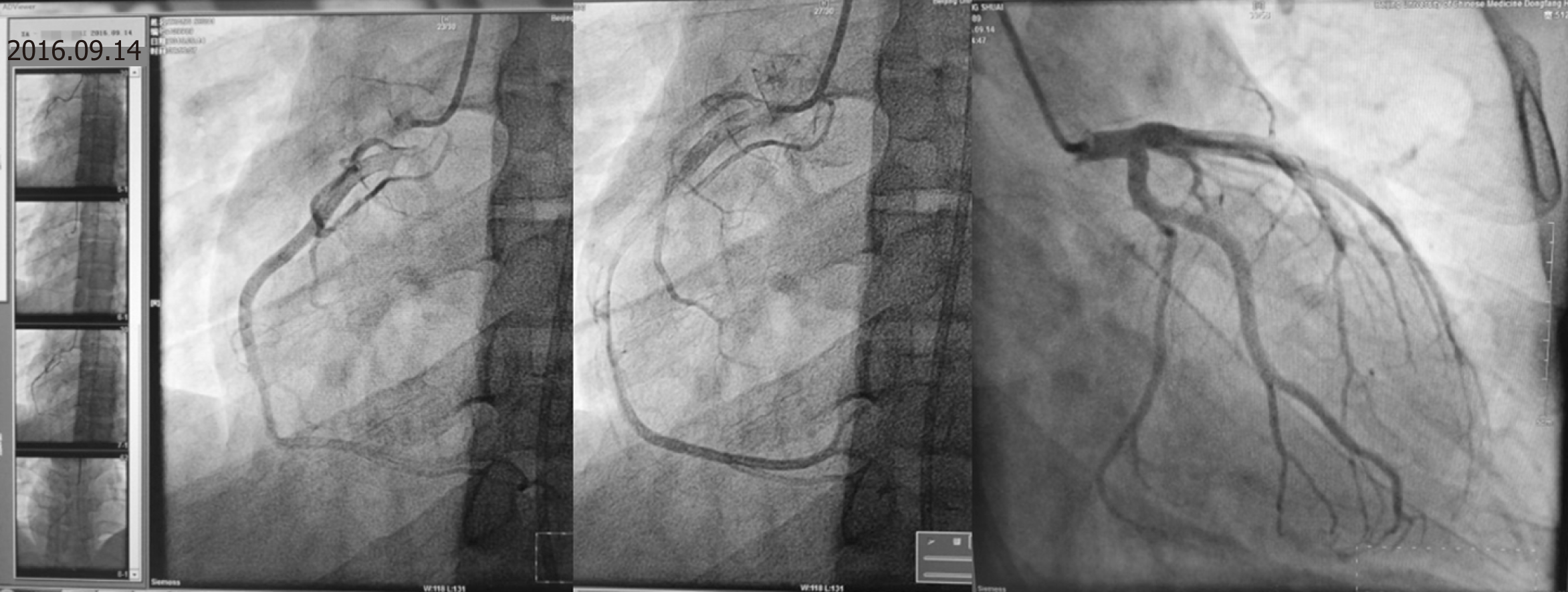
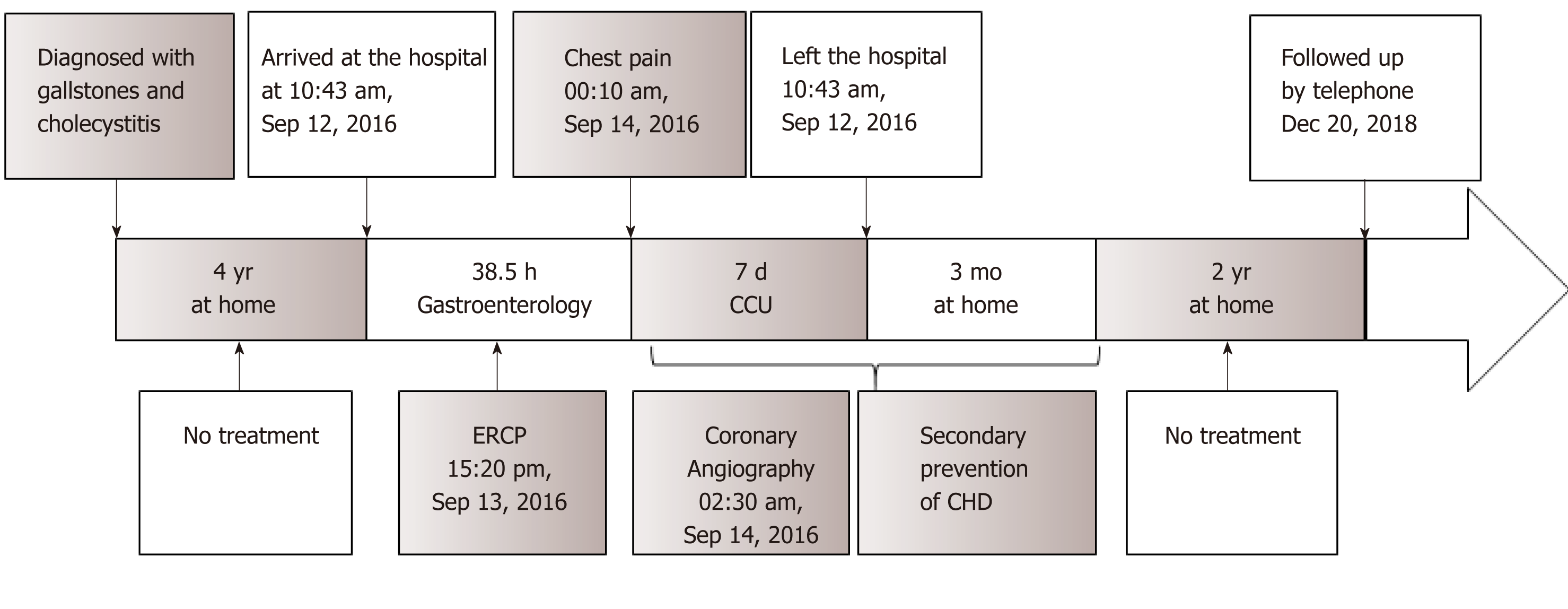
He had been diagnosed with gallstones and cholecystitis for the previous four years. Despite the low risk of cardiovascular disease in his history, AMI was suspected, and coronary angiography was performed the second day (the chest pain stopped 1 h later and lasted for 2 h in all). The results revealed a smooth angiographic appearance of all arteries (Figure 3). The patient was transferred to the cardiac intensive care unit and treated accordingly. He received secondary preventive treatment for coronary heart disease during the following week and left the coronary care unit after all his laboratory parameters returned to within the normal ranges seven days later (Figure 4).
We ultimately made a diagnosis of MINOCA caused by coronary artery spasm, as it seemed to be the most suitable diagnosis for this young patient.
The young man was treated with aspirin, angiotensin converting enzyme (ACE) inhibitors, and statins in hospital. He underwent treatments including ERCP, endoscopic sphincterotomy, pancreaticobiliary dilatation, removal of stones, and endoscopic nasobiliary drainage (ENBD) on the second day after his admission to the hospital, and 1.4 cm × 1.6 cm of stones were removed from his common bile duct during surgery. He received lidocaine anaesthesia in the throat during treatment, without any discomfort during or after the treatment. The results of his laboratory tests at admission revealed an alanine aminotransferase level of 459.6 U/L (normal range 9-50 U/L), aspartate aminotransferase level of 257.2 U/L (normal range 15-40 U/L), glutamyl transpeptidase level of 421.7 U/L (normal range 10-60 U/L), total bile acid level of 101.86 µmol (normal range 0-10 µmol), total bilirubin level of 182.21 μmol (normal range 5-21 μmol), direct bilirubin level of 99.10 µmol (normal range 0.2-6.0 µmol), and indirect bilirubin level of 83.1 µmol (normal range 3.4-15.5 µmol).
We telephoned the patient two years later, and he told us that he felt normal and did not take any medicine after the third month after discharge.
The young man had no conventional risk factors for AMI, making this case unique. All common risk factors, such as smoking, hyperlipidaemia, a family history of CAD, hypertension, diabetes mellitus, and obesity, were excluded[2,3]. More importantly, coronary angiography in this young patient did not reveal signs of occlusion, stenosis, or thrombosis, so we believe that he did not experience a classic myocardial infarction. This phenomenon has been confirmed in several large AMI registries in which 1%-13% of AMI cases occurred in the absence of obstructive CAD, prompting clinical researchers to coin the term MINOCA. The diagnostic criteria for MINOCA include: (A) The universal definition of AMI; (B) Nonobstructive coronary arteries on angiography (no stenosis ≥ 50%); and (C) No clinically overt cause of the acute clinical presentation[1]. The apparent increase in cTnI in this young patient needs to be explained in the absence of a diagnosis of AMI. The potential aetiologies include coronary causes, noncoronary causes, and extracardiac disorders[1]. The coronary angiography revealed a smooth angiographic appearance of all the arteries, and the laboratory data were nearly within the normal ranges; thus, we can exclude coronary causes and those associated with extracardiac disorders. Among all the noncoronary causes, Takotsubo cardiomyopathy and myocarditis have been reported in some cases.
This young patient had been diagnosed with gallstones and cholecystitis for four years, but we do not have evidence to confirm a relationship between AMI and common bile duct stones. His AMI occurred early during the morning following the ERCP and subsequent treatments, so we attempted to determine whether there was a relationship between the treatments and AMI. ERCP and the following treatments are primary tools for the treatment of biliary and pancreatic ductal diseases. The common adverse events are post-ERCP pancreatitis, cholangitis, post-sphincterotomy bleeding, post-sphincterotomy perforation, and sedation-related cardiopulmonary compromises. There were no related reports regarding a relationship between ERCP and AMI[4].
In our further research, we found some case reports on AMI after ERCP and the subsequent treatments, as well as after surgery to remove gallstones. One case was an 85-year-old woman who was admitted for obstructive jaundice and underwent ERCP, ENBD, and then surgery for common bile duct stones; that patient experienced Takotsubo cardiomyopathy after the surgery[5]. The other case was an 86-year-old woman diagnosed with Takotsubo cardiomyopathy following cholecystectomy at Mayo Clinic, which was the second case of Takotsubo cardiomyopathy reported to be associated with cholecystectomy[6]. One case-review series reported patients with Takotsubo cardiomyopathy. The syndrome more often affects postmenopausal women (82% to 100%; mean age, 62 to 75 years). Patients commonly present with ST-segment elevation in the precordial leads, chest pain, relatively minor elevation of cardiac enzyme and biomarker levels, and transient apical systolic left ventricular dysfunction despite the absence of obstructive epicardial coronary disease[7]. In our young patient, his echocardiographic and ECG examinations did not show changes typical of Takotsubo cardiomyopathy or myocarditis. Therefore, we did not diagnose him with either of those conditions.
The patient’s heart attack occurred at midnight, so we should consider coronary artery spasm. Coronary artery spasm may potentially contribute to the pathogenesis of AMI in patients with obstructive CAD and particularly warrants close consideration in those with MINOCA. A diagnosis of vasospastic angina can be made if spontaneous episodes of resting angina are associated with ST-segment changes that respond promptly to short-acting nitrates. However, provocative spasm testing should generally be avoided in the acute phase of AMI. Another reason might be coronary thromboembolism, which may be a contributory mechanism underlying AMI in the context of plaque disruption or coronary artery spasm or may be the cause of MI in the absence of these factors. Coronary emboli may occur as a result of iatrogenic air emboli[1]. However, coronary thromboembolism has not been reported with ERCP treatments, making this aetiology unlikely[4].
We ultimately made a diagnosis of MINOCA caused by coronary artery spasm, as it seemed to be the most suitable diagnosis for this young patient. A coronary artery spasm usually leads to changes in the ECG, and we observed ST-segment elevations in the II, III, and aVF leads during the angina. However, we still have some questions that could not be resolved. The chest pain lasted for two hours, but what caused the elevations in cTnI and CK-MB? If the myocardium was severely injured, why could we not observe that on echocardiography? We telephoned the patient two years later, and he told us that he felt normal and did not take any medicine after the third month after discharge. There are small trials that suggest the benefit of aspirin, ACE inhibitors, and statins, but there is a lack of appropriately designed clinical outcome trials to inform management decisions. The management and treatment of MINOCA patients should follow the cause of AMI in each individual case. Further studies are still needed to unravel the underlying pathophysiology.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tabibian JH S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 2. | Huang J, Qian HY, Li ZZ, Zhang JM. Comparison of clinical features and outcomes of patients with acute myocardial infarction younger than 35 years with those older than 65 years. Am J Med Sci. 2013;346:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Wong CP, Loh SY, Loh KK, Ong PJ, Foo D, Ho HH. Acute myocardial infarction: Clinical features and outcomes in young adults in Singapore. World J Cardiol. 2012;4:206-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Chavalitdhamrong D, Donepudi S, Pu L, Draganov PV. Uncommon and rarely reported adverse events of endoscopic retrograde cholangiopancreatography. Dig Endosc. 2014;26:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Tori M, Ueshima S, Nakahara M. A case of takotsubo cardiomyopathy after surgery for common bile duct stones. Case Rep Gastroenterol. 2008;2:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Jensen JB, Malouf JF. Takotsubo cardiomyopathy following cholecystectomy: a poorly recognized cause of acute reversible left ventricular dysfunction. Int J Cardiol. 2006;106:390-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |