Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.2976
Peer-review started: May 8, 2019
First decision: August 1, 2019
Revised: August 27, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: October 6, 2019
Processing time: 159 Days and 1.6 Hours
De-afferentation or non-weight bearing induces rapid cortical and spinal α-motor neuron excitability. Author supposed that an end-effector type gait robot (EEGR) could provide patients with a training condition that was specific enough to activate rapid cortical/spinal neuroplasticity, leading to immediate muscle strengthening. The electromyographic and biomechanical comparisons were conducted.
To compare the electromyographic activities of the thigh and shank muscles and isometric peak torque (PT) before and after walking training on a floor or in the end-effector gait robot.
Twelve outpatients without ambulatory dysfunction were recruited. Order of two interventions (5-min training on a floor at a comfortable pace or training in an EEGR with non-weight bearing on their feet and 100% guidance force at 2.1 km/h) were randomly chosen. Isometric PT, maximal ratio of torque development, amplitude of compound motor action potential (CMAP), and area under the curve (AUC) were evaluated before and 10 min after both interventions.
The degree of PT improvement of the dominant knee flexors was larger in the EEGR than on the floor (9.6 ± 22.4 Nm/BW, P < 0.01). The EEGR-trained patients had greater PT improvement of the dominant knee extensors than those who trained on the floor (4.5 ± 28.1 Nm/BW, P < 0.01). However, all electromyographic activities of the thigh and shank muscles (peak CMAP, mean and peak AUC) were significantly lower for the use of the EEGR than walking on the floor.
Immediate strengthening of the knee flexors and extensors was induced after the 5-min EEGR training, despite reduced muscular use.
Core tip: Just five-minute end-effector type robot-assisted gait training with non-weight bearing on their feet and 100% guidance force may induce immediate strengthening of the knee flexor and extensor muscles. Moreover, it may even reduce the real-time use of the thigh and calf muscles. It may be a useful tool to strengthen the leg muscles in the elderly or in patients with musculoskeletal injuries. As for its underlying mechanism, author supposes the rapid brain and spinal plasticity in theory.
- Citation: Hwang CH. Immediate muscle strengthening by an end-effector type gait robot with reduced real-time use of leg muscles: A case series and review of literature. World J Clin Cases 2019; 7(19): 2976-2985
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/2976.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.2976
Various kinds of gait robots have been developed for rehabilitation of various conditions. Their clinical application has been mostly focused on stroke[1] and spinal cord injury patients[2]. In case that patients cannot walk due to paralysis of the lower legs, long-term brain plasticity can be evoked if patients with injuries of the central nervous system (CNS) can walk repeatedly with the help of gait robots[3]. Meanwhile, a significant number of patients who cannot walk themselves show different underlying causes such as deconditioning in the very elderly, restricted weight bearing of the legs due to musculoskeletal injuries, or poor muscular activation from pain rather than paraparesis. Gait robots can control patients’ weight bearing and leg movement as well as provide body support for the deconditioned elderly patients to stand. However, there are few reports on whether gait robots could contribute to strengthening the muscles of the lower legs in able-bodied patients, who are different from those with CNS injuries.
Cortical plasticity of the brain can be induced very rapidly, even with five-minute repeated, stereotyped movements of a specific body part in healthy individuals[4]. Rapid plasticity of the brain can also be evoked by 10-min ambulation using a gait robot in healthy humans[5]. In terms of durability and repeatability, end-effector type gait robots may be reliable therapeutic tools to allow patients to perform constant, stereotyped movements. In addition, changes of efferent sensory feedback alter the cortical motor pathway excitability[6] so much that exercise after deafferentation can influence cortical activation in healthy people[7,8]. Weight bearing on the feet during exercise can modulate spinal motor neuron excitability, and the activation of which depends on the degree of weight bearing[9-12]. Considering that end-effector type gait robots can simulate deafferentation and control the degree of weight bearing on the leg, this kind of robot may have an added effect on rapid plasticity of the brain.
Prior to an electrophysiology- and biomechanics-based randomized, controlled trial for rapid brain and spinal plasticity induction by end-effector type gait robots, author prepared a trial for concept approval. Author supposed that an end-effector type gait robot could provide patients with a training condition that was specific enough to activate rapid cortical plasticity and spinal motor neuron excitability, leading to immediate muscle strengthening throughout the legs. Author therefore compared the electromyographic (EMG) activities of the thigh and shank muscles and isometric peak torque (PT) before and after walking training on a floor or in the end-effector gait robot.
A prospective, cross-sectional trial was conducted at a tertiary medical center/ university teaching hospital between December 2015 and May 2016 after approval of the institutional review board (UUH-2016-01-007) and registration in a clinical trial registry (NCT02962453). After providing written, informed consent, outpatients who visited the department of physical medicine and rehabilitation and had no functional impairment of ambulatory ability were included. Patients who had musculo-skeletal or neurological diseases involving their spine or lower extremities, altered consciousness (Mini-Mental Status Exam score < 23), showed ambulatory dysfunction due to systemic diseases or declined to participate were excluded.
The demographic information, PT, and maximal ratio of torque development (MRTD) of each patient were preemptively evaluated. The real-time amplitude of compound motor action potential (CMAP) and area under the curve (AUC) were constantly recorded, with the help of 2.46 GHz Industrial, Scientific, Medical band, during the intervention [five-minute walking training on an even floor at a comfortable pace (control) or in the end-effector type gait robot (Morning Walk®, Curexo, Inc, South Korea) with non-weight bearing, with 100% guidance force at the speed of 2.1 km/h (case)]. Each order of the two consecutive interventions was determined randomly and then both interventions were provided to the same patients. After a 10-min break following each intervention, data on PT and MRTD were recollected. Any side effects such as pain, dizziness, palpitation, false step, or falling down were monitored before, during, and five minutes after using the robot.
PT and MRTD of the dominant knee flexors and extensors were collected using Biodex® (Biodex Medical Systems, New York, NY, USA) according to the method of Pamukof et al[13] The patients were allowed to warm up for three minutes, and performed voluntary flexion and extension of the knee at the intensity of 25%, 50%, and 75% of the maximum force in stages. The trunk, hips, and thighs were secured tightly using a chest strap and Velcro®, a lever arm was positioned at 60º of knee flexion, and an ankle strap was fastened two fingers-breadth above the medial malleolus. The patient was asked to perform maximal isometric contractions for five seconds. After a one-minute break, the same evaluation was conducted again. Then, the mean value of the PTs was normalized into body mass (Nm/BW). MRTD was defined as the slope from the onset to the PT over torque versus time curve, and dominance of the leg was determined based on which leg was used for kicking a soccer ball.
The mean and peak values of CMAP amplitude, and mean and total values of AUC of the dominant vastus medialis (VM), biceps femoris long head (BF), tibialis anterior (TA), and gastrocnemius medial head (GC) were recorded three times using eight-channel surface electromyography (WEMG-8, LAXTHA, Republic of Korea). The moving average of smoothed electrical signals that lasted for at least one second was determined and divided by the baseline amplitude of CMAP to obtain the standard value. According to the method by Hermens et al[14] surface electrodes were affixed to the corresponding belly-tendon montage for each muscle, and a ground electrode was affixed to the patellar tendon. A wireless communications system for electrical signals was securely positioned at the posterior of the participant’s waist with a strap, and every variable was constantly recorded during five minutes of training on the floor or in the robot. The same evaluation was conducted twice more after a one-minute break, and the mean value for each variable was calculated.
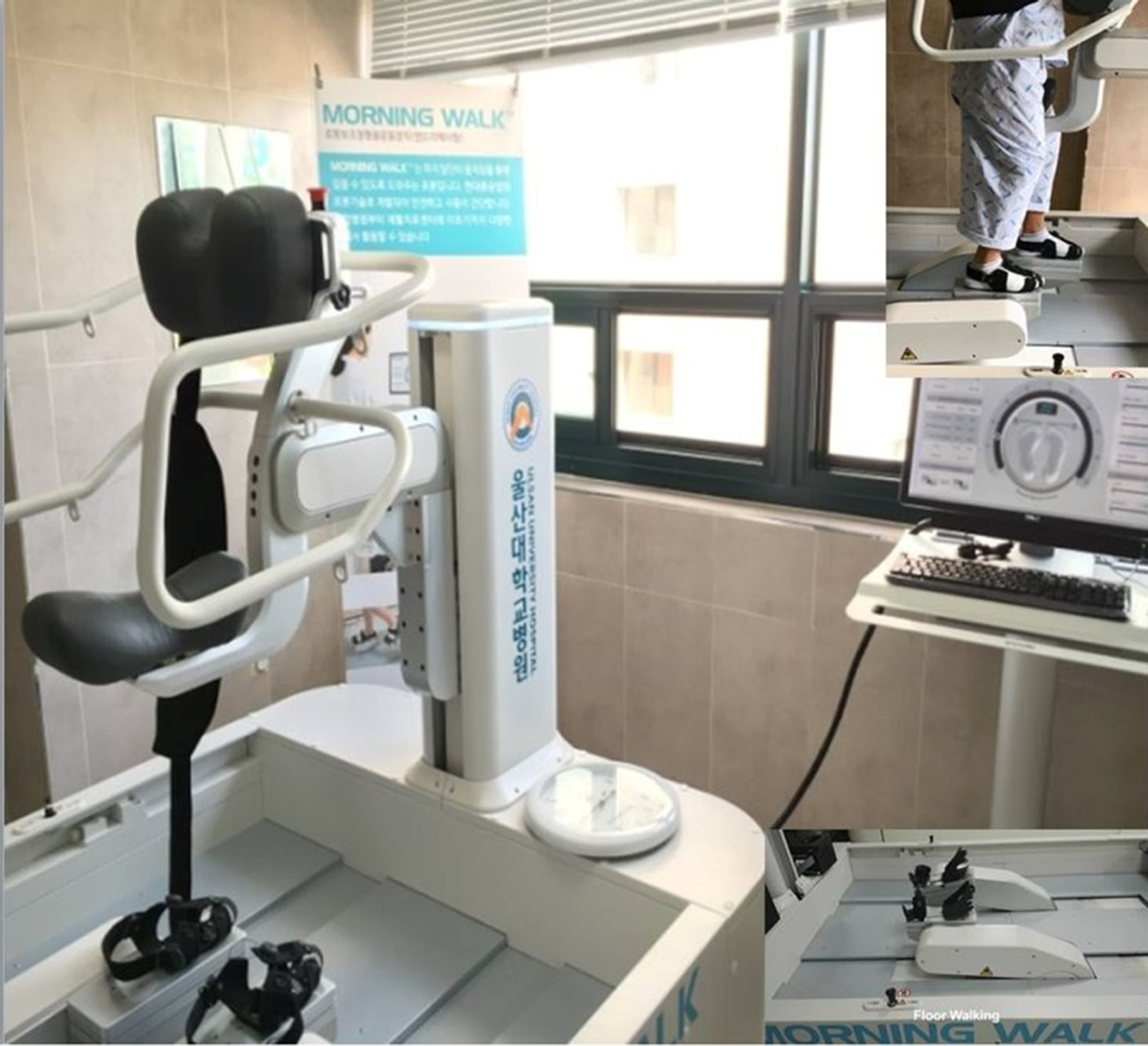
The end-effector type gait robot (Morning Walk®, Curexo, Inc, Republic of Korea) was equipped with a saddle for pelvic weight bearing, an anterior chest support with a strap to secure the trunk, a surrounding safety bar for manual holding, and a pair of end-effectors. The saddle part could sense upward or downward trajectory of the pelvis during training and adapt to its fluctuation to support the predetermined weight. The end-effector totally contacted the soles in the standing position and was secured with forefoot, mid-foot, and ankle straps, but free movement of the forefeet and ankles was allowed (Figure 1). If the foot sensors detected excessive change of symmetry between the feet or of the desired trajectory of the end-effectors on ground reaction force, the robot stopped immediately. A physical therapist could also terminate its action by pushing a button.
Referring to the data of Kang et al[15] author supposed that the smallest effects of interest were 0.26 Nm/kg, 0.05 Nm/kg of standard deviation, 10 of the standardized difference, 0.05 of the α value, 0.9 of power, and 20% of dropping ratio, and data of the 12 patients were calculated using Altman’s nomogram. Statistical Package for the Social Sciences 21 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analyses. PT and MRTD were compared using a Kruskal Wallis test and Mann-Whitney test with Bonferroni’s method for post-hoc comparison, and the AUC was analyzed using the Wilcoxon sign rank test.
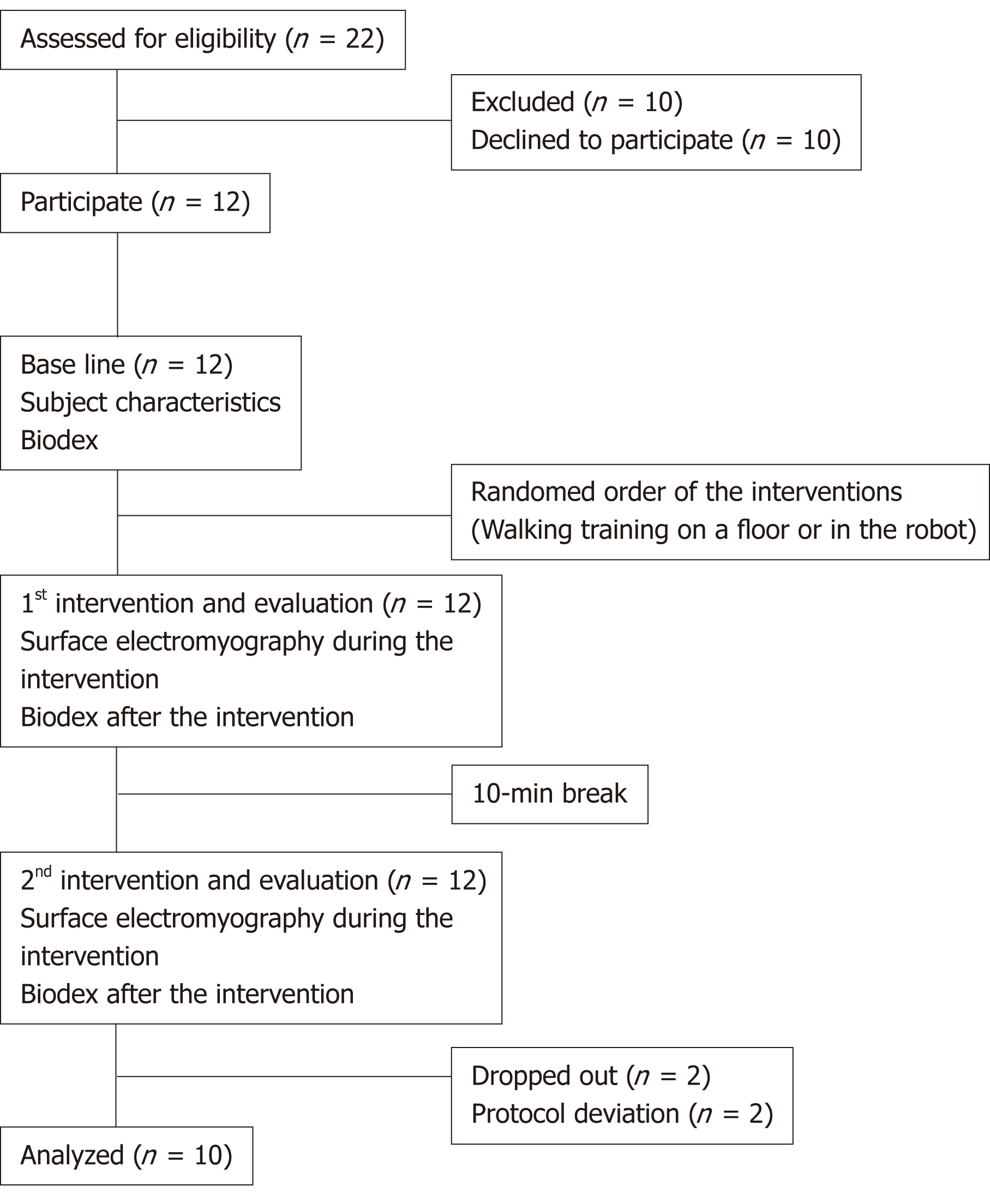
Twenty-two adults were assessed for eligibility, 10 individuals rejected the participation, and 12 persons were participated. During the trial, two patients dropped out due to protocol deviation (failure of surface EMG recording and incorrect protocol training); therefore, data on 10 patients were analyzed (Figure 2). The mean age, weight, height, and gait speed on the floor of the participants were 32.5 years, 70.1 kg, 172.5 cm, and 2.5 km/h respectively (Table 1).
| Subjects | Age (yr) | Sex | Weight (kg) | Height (cm) | Evaluated side | Walking speed on a floor (km / h) | Protocol deviation |
| 001 | 34 | M | 81.3 | 176.8 | Right | 2.3 | + |
| 002 | 38 | F | 70.0 | 163.1 | Right | 2.4 | |
| 003 | 27 | M | 78.0 | 177.9 | Right | 2.1 | |
| 004 | 34 | M | 85.0 | 172.9 | Right | 2.8 | |
| 005 | 28 | M | 85.0 | 186.1 | Right | 2.6 | |
| 006 | 30 | F | 50.3 | 160.5 | Right | 2.1 | |
| 007 | 22 | F | 54.0 | 156.2 | Right | 2.5 | |
| 008 | 24 | F | 70.0 | 169.8 | Right | 2.4 | |
| 009 | 35 | M | 74.0 | 178.0 | Left | 2.9 | |
| 010 | 24 | M | 70.0 | 180.1 | Right | 3.1 | |
| 011 | 49 | M | 60.0 | 174.9 | Left | 2.8 | |
| 012 | 45 | F | 63.0 | 174.0 | Right | 2.0 | + |
The PT of the dominant knee flexors was significantly improved 10 min after five minutes of walking training on the floor and in the robot (42.2 ± 15.7 Nm/BW versus 51.4 ± 20.5 Nm/BW, respectively, 61.0 ± 24.2 Nm/BW, P < 0.01), and the degree of improvement was significantly higher in the robot than training on the floor (18.8 ± 19.9 Nm/BW versus 9.2 ± 18.1 Nm/BW, P < 0.01). The PT of the dominant knee extensors was significantly improved 10 minutes after five minutes of training on the floor and in the robot (85.6 ± 23.5 Nm/BW vs 100.0 ± 29.8 Nm/BW, respectively, 104.4 ± 32.5 Nm/BW, P < 0.01), and the degree of improvement was significantly larger in the robot than it was on the floor (18.9 ± 28.0 Nm/BW versus 14.4 ± 26.7 Nm/BW, P < 0.01). The MRTD of the dominant knee flexors and extensors showed no significant change 10 minutes after five minutes of walking training on the floor and in the robot (P = 0.08, P = 0.13) (Table 2).
| Dominant side | Peak torque (Nm / kg) | Maximal ratio of torque development(Nm / s· kg) | |
| mean ± SE | 95%CI | ||
| Knee flexors | |||
| Before intervention | 42.2 ± 15.7 | 10.9 - 73.5 | 71.2 ± 32.3 |
| After training on the floor | 51.4 ± 20.5a | 10.5 – 92.3 | 65.3 ± 19.7 |
| After training in the robot | 61.0 ± 24.2ac | 12.7 - 109.4 | 68.0 ± 24.0 |
| Knee extensors | |||
| Before intervention | 85.6 ± 23.5 | 38.7 – 132.6 | 68.3 ± 6.2 |
| After training on the floor | 100.0 ± 29.8a | 40.5 - 159.5 | 70.5 ± 6.5 |
| After training in the robot | 104.4 ± 32.5ac | 39.4 – 169.4 | 70.5 ± 8.8 |
The peak amplitude of CMAP of the dominant BF was more than five times reduced during five-minute training in the robot compared with training on the floor (89.3 ± 8.5 mV vs 473.3 ± 139.8 mV, P < 0.01) and that of the dominant VM CMAP was reduced more than twice (192.5 ± 23.6 mV vs 517.2 ± 115.4 mV, P < 0.01). The peak amplitude of the dominant TA CMAP was more than eight times reduced during training in the robot compared with that on the floor (92.0 ± 19.5 mV vs 812.8 ± 174.5 mV, P < 0.01) and that of the dominant GC CMAP was more than four times reduced (142.9 ± 23.5 mV vs 599.5 ± 124.4 mV, P < 0.01). However, no significance was found in the mean amplitude among the four muscles (P = 0.35) (Table 3).
| Dominant side | Intervention | Real-time mean amplitude (mV) | Real-time peak amplitude (mV) | Real-time mean value of AUC (cm2 / s) | Real-time total value of AUC (cm2 / 5 min) |
| Biceps femoris long head | On the floor | 90.7 ± 0.3 | 473.3 ± 139.8 | 75.6 ± 11.6 | 13933489 ± 2142999 |
| In the robot | 90.1 ± 0.8 | 89.3 ± 8.5a | 16.4 ± 1.6a | 3028676 ± 296582a | |
| Vastus medialis | On the floor | 90.2 ± 0.6 | 517.2 ± 115.4 | 68.5 ± 12.2 | 12631555 ± 2240388 |
| In the robot | 89.8 ± 0.3 | 192.5 ± 23.6a | 42.2 ±3.1a | 7781851 ± 579361a | |
| Gastrocnemius medial head | On the floor | 90.1 ± 0.3 | 599.5 ± 124.4 | 89.2 ± 11.2 | 16444668 ± 2059635 |
| In the robot | 90.6 ± 1.1 | 142.9 ± 23.5a | 25.9 ± 4.8a | 4764851 ± 885854a | |
| Tibialis anterior | On the floor | 90.8 ± 0.5 | 812.8 ± 174.5 | 115.3 ± 19.6 | 21254,406 ± 3610903 |
| In the robot | 89.9 ± 0.4 | 92.0 ± 19.5a | 13.9 ± 2.2a | 2568851 ± 404428a |
The mean area of the dominant BF CMAP was more than four times reduced during five-minute training in the robot than that on the floor (16.4 ± 1.6 cm2/s vs 75.6 ± 11.6 cm2/s, P < 0.01) and that of the dominant VM CMAP was reduced compared with walking training on the floor (42.2 ± 3.1 cm2/s vs 68.5 ± 12.2 cm2/s, P < 0.01). The mean area of the dominant TA CMAP was more than eight times reduced during training in the robot compared with training on the floor (13.9 ± 2.2 cm2/s vs 115.3 ± 19.6 cm2/s, P < 0.01), and that of the dominant GC CMAP was more than three times reduced during training in the robot compared with training on the floor (25.9 ± 4.8 cm2/s vs 89.2 ± 11.2 cm2/s, P < 0.01). The total area of the dominant BF CMAP was more than four times reduced during five-minute walking training in the robot than it was on the floor (3028676 ± 296582 cm2/5 min vs 13933489 ± 2142999 cm2/5 min, P < 0.01) and that of the dominant VM CMAP was reduced (7781851 ± 579361 cm2/5 min vs 12631555 ± 2240388 cm2/5 min, P < 0.01). The total area of the dominant TA CMAP was more than eight times reduced in the robot compared with on the floor (2568851 ± 404428 cm2/5 min vs 21254406 ± 3610903 cm2/5 min, P < 0.01), and that of the dominant GC CMAP was more than four times reduced (4764851 ± 885854 cm2/5 min vs 21254406 ± 3610903 cm2/5 min, P < 0.01) (Table 3).
No side effects such as pain, dizziness, palpitation, false step, or falling down occurred during or five minutes after the intervention.
Author conducted a prospective, cross-sectional study to evaluate whether an end-effector type gait robot could lead to prompt strengthening of the lower extremities. The patients were provided with a random sequence of walking training on the floor and in the gait robot, and the EMG activities of the dominant thigh and shank muscles and the PT and MRTD of the thigh muscles were compared. The keys findings were:(1) PT of the dominant knee flexor and extensor muscles significantly improved 10 min after both of the five-minute interventions, and the degree of improvement was significantly larger in the gait robot than on the floor; and (2) The real-time EMG activities of the dominant thigh and shank muscles were much more lower in the gait robot than those for the floor.
In the current trial, only five-minute walking training on the floor and in the robot induced immediate strengthening in adults with healthy legs. Just short term training provoked such significant improvement of strength that it might not be as usual. This result can be explainable with Kantak et al’s[5] finding that rapid brain plasticity occurred after 10-min reaching training in 22 healthy individuals[4]. In another report, similar plasticity was shown after robot-assisted treadmill training for 10 min in 39 healthy adults. Prompt GABAergic inhibition in the brain was proposed as the mechanisms of movement-dependent rapid plasticity[16].
However, in the current trial, the degree of the improvement of PT of the dominant knee flexors and extensors in the robot was significantly larger than it was on the floor. Levy et al[7] reported that deafferentation through complete ischemic nerve blocking could quickly reduce the GABA level to 59% in 12 healthy volunteers. Fifteen trials of ballistic exercises of the biceps brachii of six healthy individuals induced a significant increase (2.5-fold) of the motor-evoked potential of the biceps brachii immediately after deafferentation[8]. Afferent feedback from the muscles contributes as much as one third of the total central motor activation[17], and changes of this input alter the excitability of the cortico-spinal motor pathway[6]. Passive movement with 100% guidance force in a gait robot might let simultaneous afferent feedback be perceived differently from the self-directed action[18]. Blicher et al[18] reported that 20-min passive gait robot training with 100% guidance force induced a significant reduction (1.5-fold) of short interval intra-cortical inhibition in 13 healthy adults. Sixty-second robot gait training with 100% guidance force showed significant activation of the motor-sensory cortex compared with stepping or treadmill walking in 14 healthy individuals[19]. Similarly, rapid brain plasticity might be reinforced by deafferentation in the current trial.
In Dietz et al’s[9] review article, the contact force of the feet against gravity during ambulation modulated α-motor neuron excitability in the spinal cord, depending on the degree of weight bearing. Mazzocchio et al[11] proved that 16-min cycling on a bike immediately inhibited the amplitude of the Hoffmann-reflex (H-reflex), the most sensitive representative of spinal α-motor neuron excitability[10], of the soleus to 75% in 18 healthy people[11]. Furthermore, another study found that only 60% loading of body weight during ambulation could reduce the H-reflex amplitude of even the upper extremity muscles after 10-min gait training with Lokomat® in 11 healthy individuals[12]. Blicher et al[18] reported that passive training with body weight unloading to 33% led to no inhibition of spinal excitability in 13 healthy adults. In the current trial, non-weight bearing on the legs might add additional effect on the aforementioned deafferentation-reinforced rapid brain plasticity. However, author did not directly evaluate the change of rapid brain and spinal plasticity.
MRTD showed no significant differences before, after, and between both of the interventions in the current trial. RTD is defined as how efficiently and quickly peripheral motor units are activated during the force-time curve so that MRTD should be increased after the intervention. Because only healthy, young, socially active adults were recruited, motor unit firing of the knee flexor and extensor muscles might already be fully activated during the isometric contraction, leading to a potential ceiling effect. Therefore training in the gait robot or on the floor may enhance the neuro-muscular pathway through up-regulated motor unit recruitment, but it showed an imperceptible effect on the recruitment ratio; therefore there was not a gain in MRTD. A discrepancy between MRTD quantification and different time intervals was also found in healthy people[20]; therefore evaluation of the whole signal period in the current trial may have influence on the recorded MRTD results.
In the current trial, the peak amplitude of CMAP and mean and total area of AUC of the dominant VM, BF, TA, and GC during the training in the robot were significantly reduced compared with those during the training on the floor. Krishnan et al[21] found that treadmill training in Lokomat® inceased the EMG activities of the medial hamstring, BF, TA, and soleus in 6 healthy adults. Hider et al[22] reported similar results in 7 healthy individuals using the same robot, except for decreased activities of the TA and GC. However, the control algorithm that was used for each robotic joint in the aforementioned two trials was either assist-as-needed or error augmentation[21], and the exoskeleton could create its own inertia or abnormal torque to exert a strong influence on the muscle activities[22]. EMG activation of the lower leg muscles in exoskeleton-type robots can depend on inertia and impedance, and have a limited degree of freedom[2,23]. Therefore, these findings may not be comparable to the current results. Although a similar situation could occur around the ankle in an end-effector type robot, it might be one of another advantages of end-effector type robots that activation of the TA and GC was unexpectedly reduced.
Contrary to exoskeleton-type robots, there are few reports on the EMG activities in end-effector type gait robots, except for one report on velocity and leg power in the DEGAS trial[24]. The end-effector robot called the G-EO system® showed a contrary result to the current trial; more prolonged, hightened activation of the VM, vastus lateralis (VL), and TA was noticed throughout the gait cycles. However, six patients in that study had hemiparetic limbs. Furthermore, the degree of body weight support and speed was not standardized, and a physical therapist simultaneously provided manual assistance for knee extension[25]. Hussein et al[26] conducted a trial with an end-effector robot called HapticWalker® in nine healthy subjects and found that activation of the VM, VL, and BF was increased, and that of TA and GC were reduced, but the degree of body weight support was uncontrolled. Future studies should be performed to clarify these in the manner of standardized paremeters.
Furthermore, it is well known that the degree of guidance force is an important factor of rapid brain plasiticy; the larger the guidance force, the more rapid the brain plasiticity is. Kammen et al[27] reported that gait training in Lokomat® reduced the EMG activities of the gluteus maximus, BF, and GC, depending on the guidance force (from 0% to 50% and 100%) in 10 healthy adults, and Moreno et al[28] discovered that a lower degree of guidance force (20% and 40%) significanly increased the EMG activities of the hamstring and quadriceps muscles compared with a higher degree of guidance force (70% and 100%) during Lokomat®-assisted treadmill training in eight healthy participants. In the current trial, 100% guidance forece might potentiate rapid brain plasticy so that the leg muscles were less activated. If active recruitment of the leg muscles for strengthening is prohibited in case of musculoskeletal injury, the end-effector robot may provide protective effects on the muscles of the knee joint as guidance force is raised, except for when knee joint instability is present.
It is well-known that muscle volume declines with aging or during prolonged bed rest. The decrease in muscle mass and the inappropriate muscle strength contribute to the deficient physical performance. Moreover, such age-related decline can occur in the region-specific manner as well as between muscles[29]. That kind of differential atrophy can be also noticed during medical deconditioning state[30]. Meanwhile, knee extensors are significantly related with leg performance[31], and the greatest atrophy is noticed in the calf musculature, followed by the knee extensors and flexors in deconditioning-induced atrophy[30]. Taken into consideration of two aforementioned reports, it may be clinically significant that increased muscle strength of knee flexor and extensor was noticed in the current trial. However, muscle strength is closely related to the muscle balance in frail or elderly people. Because no effects was found on other muscle groups, effective way to control a proper muscle balance should be provided. Resistance exercise training is known to alleviate aging-associaged type II myofiber atrophy through the increased muscle protein synthesis[32]. Functional electrical stimulation can modulate charicteristics of muscle fibers so that it can be an alternative, especially in those who unable to perform physical activities or elderly people[33].
This trial is a cross-sectional, non-randomized, single-center study. Therefore more studies are needed to be done and proved the effects. The recruited subjects were young to middle ages with relatively healthy status. In a further trial, some elderly patients should be recruited for study because they will be the target for potential application. It also needs to be applied in different groups of patients with musculoskeletal injuries in comparison to healthy adults. Even though preemptive calculation on the number of participants was performed, the small sample size is a major limitation in the present study. Instead of the within-patients comparison, an appropirate control group should be included. Regarding the study about the muscle strength, the data of body composition that is important for basic information, especially for such development of new device, should be included. Walking speed may affect the EMG activities of the lower leg muscles[34]. Cortical activation measured by spectroscopy and lower leg muscles activities measured by surface electromy-ography were proportinally increased as gait speed increased in the exoskeleton robot (from 1.5 to 3.0 km/h and 2.7 to 6.2 km/h) in healthy people[19,34]. Although no change of muscle activities was also reported in the range of 1.5 to 2.7 km/h in healthy people[22], it is uncertain if 2.1 km/h of gait speed could be ideal for the current evaluation. Additionally, the different number of steps and the different walking speed between the two interventions could affect the current findings. Although the ground reaction forces in end-effector robots are changeable in different gait conditions[35], this factor was not evaluated. Furthermore, rapid CNS plasticity was not evaluated in terms of electrical evoked potential, H-reflex, or spectroscopy.
In conclusion, five-minute end-effector type robot-assisted gait training with non-weight bearing on their feet and 100% guidance force might induce immediate strengthening of the dominant knee flexor and extensor muscles which was maintained for 10 min and accompanied by the simultaneous reduction of the usage of the thigh and calf muscles. It may be a useful tool to strengthen the leg muscles in the elderly or in patients with musculoskeletal injuries. To prove the rapid brain and spinal plasticity in theroy as it’s underlying mechanism, author has been conducting an electrophysiology-, biomechanics-, computer tomography-based randomized, controlled trial using the end-effector type gait robot, based on the literature review.
De-afferentation or non-weight bearing induces rapid cortical and spinal α-motor neuron excitability. The author supposed that an end-effector type gait robot (EEGR) could provide patients with a training condition that was specific enough to activate rapid cortical/spinal neuroplasticity, leading to immediate muscle strengthening.
The author aimed to compare the electromyographic activities of the thigh and shank muscles and isometric peak torque (PT) before and after walking training on a floor or in the end-effector gait robot.
Twelve outpatients without ambulatory dysfunction were recruited. Order of two interventions were randomly chosen. Isometric PT, maximal ratio of torque development, amplitude of compound motor action potential (CMAP), and area under the curve (AUC) were evaluated before and 10 min after both interventions.
The degree of PT improvement of the dominant knee flexors was larger in the EEGR than on the floor. The EEGR-trained patients had greater PT improvement of the dominant knee extensors than those who trained on the floor. However, all electromyographic activities of the thigh and shank muscles (peak CMAP, mean and peak AUC) were significantly lower for the use of the EEGR than walking on the floor.
Immediate strengthening of the knee flexors and extensors was induced after the 5-min EEGR training, despite reduced muscular use.
Author sincerely gives thanks to You Kyung Son and Na-Young Joo, medical assistants, for data collection and making spreadsheet, Eun Ji Park for analyzing the data, and Jae Myoung Ju, a physical therapist, for conducting gait robot training.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang RS S-Editor: Wang JL L-Editor: A E-Editor: Xing YX
| 1. | Coenen P, van Werven G, van Nunen MP, Van Dieën JH, Gerrits KH, Janssen TW. Robot-assisted walking vs overground walking in stroke patients: an evaluation of muscle activity. J Rehabil Med. 2012;44:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006;86:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Yang HE, Kyeong S, Lee SH, Lee WJ, Ha SW, Kim SM, Kang H, Lee WM, Kang CS, Kim DH. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci Lett. 2017;637:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kantak SS, Jones-Lush LM, Narayanan P, Judkins TN, Wittenberg GF. Rapid plasticity of motor corticospinal system with robotic reach training. Neuroscience. 2013;247:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kim SH, Banala SK, Brackbill EA, Agrawal SK, Krishnamoorthy V, Scholz JP. Robot-assisted modifications of gait in healthy individuals. Exp Brain Res. 2010;202:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Carson RG, Riek S, Mackey DC, Meichenbaum DP, Willms K, Forner M, Byblow WD. Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol. 2004;560:929-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 2002;52:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171-1181. [PubMed] |
| 9. | Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102-110. [PubMed] |
| 10. | Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Mazzocchio R, Kitago T, Liuzzi G, Wolpaw JR, Cohen LG. Plastic changes in the human H-reflex pathway at rest following skillful cycling training. Clin Neurophysiol. 2006;117:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Nakajima T, Kamibayashi K, Kitamura T, Komiyama T, Zehr EP, Nakazawa K. Short-Term Plasticity in a Monosynaptic Reflex Pathway to Forearm Muscles after Continuous Robot-Assisted Passive Stepping. Front Hum Neurosci. 2016;10:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Pamukoff DN, Pietrosimone B, Lewek MD, Ryan ED, Weinhold PS, Lee DR, Blackburn JT. Immediate effect of vibratory stimuli on quadriceps function in healthy adults. Muscle Nerve. 2016;54:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361-374. [PubMed] |
| 15. | Kang HC, Lee JH, Kim SM. Evaluation of joint moment patterns of a wearable walking assistant robot: Experimental and simulation analyses. Biomed Mater Eng. 2015;26 Suppl 1:S717-S727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993;471:429-443. [PubMed] |
| 18. | Blicher JU, Nielsen JF. Cortical and spinal excitability changes after robotic gait training in healthy participants. Neurorehabil Neural Repair. 2009;23:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kim HY, Yang SP, Park GL, Kim EJ, You JS. Best facilitated cortical activation during different stepping, treadmill, and robot-assisted walking training paradigms and speeds: A functional near-infrared spectroscopy neuroimaging study. NeuroRehabilitation. 2016;38:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Lamont HS, Cramer JT, Bemben DA, Shehab RL, Anderson MA, Bemben MG. Effects of adding whole body vibration to squat training on isometric force/time characteristics. J Strength Cond Res. 2010;24:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Krishnan C, Ranganathan R, Dhaher YY, Rymer WZ. A pilot study on the feasibility of robot-aided leg motor training to facilitate active participation. PLoS One. 2013;8:e77370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Hidler JM, Wall AE. Alterations in muscle activation patterns during robotic-assisted walking. Clin Biomech (Bristol, Avon). 2005;20:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Van Kammen K, Boonstra A, Reinders-Messelink H, den Otter R. The combined effects of body weight support and gait speed on gait related muscle activity: a comparison between walking in the Lokomat exoskeleton and regular treadmill walking. PLoS One. 2014;9:e107323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, Hoölig G, Koch R, Hesse S. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single-blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clin Rehabil. 2007;21:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Hesse S, Waldner A, Tomelleri C. Innovative gait robot for the repetitive practice of floor walking and stair climbing up and down in stroke patients. J Neuroeng Rehabil. 2010;7:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Hussein S, Schmidt H, Volkmar M, Werner C, Helmich I, Piorko F, Krüger J, Hesse S. Muscle coordination in healthy subjects during floor walking and stair climbing in robot assisted gait training. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1961-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | van Kammen K, Boonstra AM, van der Woude LH, Reinders-Messelink HA, den Otter R. The combined effects of guidance force, bodyweight support and gait speed on muscle activity during able-bodied walking in the Lokomat. Clin Biomech (Bristol, Avon). 2016;36:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Moreno JC, Barroso F, Farina D, Gizzi L, Santos C, Molinari M, Pons JL. Effects of robotic guidance on the coordination of locomotion. J Neuroeng Rehabil. 2013;10:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Watanabe K, Kouzaki M, Moritani T. Effect of aging on region-specific functional role and muscle geometry along human rectus femoris muscle. Muscle Nerve. 2017;56:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Miokovic T, Armbrecht G, Felsenberg D, Belavý DL. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol (1985). 2012;113:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, Harris TB. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456-461. [PubMed] |
| 32. | Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, Chen HC, Liou TH. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:1078-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 33. | Carraro U, Kern H, Gava P, Hofer C, Loefler S, Gargiulo P, Mosole S, Zampieri S, Gobbo V, Ravara B, Piccione F, Marcante A, Baba A, Schils S, Pond A, Gava F. Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur J Transl Myol. 2015;25:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Hof AL, Elzinga H, Grimmius W, Halbertsma JP. Speed dependence of averaged EMG profiles in walking. Gait Posture. 2002;16:78-86. [PubMed] |
| 35. | Tomelleri C, Waldner A, Werner C, Hesse S. Adaptive locomotor training on an end-effector gait robot: evaluation of the ground reaction forces in different training conditions. IEEE Int Conf Rehabil Robot. 2011;2011:5975492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |